To monitor autoclave effectiveness and conduct routine validation of sterilization cycles.
Material and methodsAn observational, cross-sectioned, qualitative and prospective study of three Sterilization and Packing Center autoclaves of the Technological University of Mexico of the School of Dentistry was conducted. The study included 96 surgical and non-surgical packs, and spanned the period from May 2012 to April 2015. Monthly assessments were conducted, according to Official Mexican Norm stipulation number NOM-013-SSA2-1994, modification 2006, using rapid lecture biological indicators (1292 3M Attest™). Sterilization efficiency was determined as biological annihilation elicited in these indicators. Effectiveness assessment was conducted after three hours with an automatic optic reader (3M Attest 290™). A positive witness was included in all readings.
ResultsNegative results were obtained in biological indicators readings used in autoclaves. A 100% effectiveness was achieved in sterilization processes of surgical and non surgical packs. All witnesses were positive.
ConclusionsThe effectiveness of Sterilization and Packing Center autoclaves of the Technological University of Mexico of the School of Dentistry was corroborated. The Norm established by the Mexican Health Ministry was complied with.
Monitorear la eficacia de las autoclaves y realizar la validación rutinaria de los ciclos de esterilización.
Material y métodosSe realizó un estudio observacional, transversal, cualitativo y prospectivo de tres autoclaves de la Central de Equipos y Esterilización de la Facultad de Odontología de la Universidad Tecnológica de México, incluyéndose 96 biocargas no quirúrgicas y quirúrgicas, de mayo del 2012 hasta abril del 2015. Se realizó una determinación mensual, según lo marca la NOM-013-SSA2-1994 modificación 2006, utilizando indicadores biológicos de rápida lectura (1292 3M Attest®). La eficacia de la esterilización se definió como la aniquilación biológica producida en estos indicadores, y se realizó a través de un lector óptico automático (3M Attest 290®), después de tres horas. En todas las lecturas se incluyó un testigo positivo.
ResultadosSe obtuvieron resultados negativos en las lecturas de los indicadores biológicos utilizados en las autoclaves, obteniéndose 100% de eficacia en el proceso de esterilización en las biocargas quirúrgicas y no quirúrgicas. Todos los testigos fueron positivos.
ConclusionesSe corroboró la eficacia de las autoclaves de la Central de Equipos y Estirilización de la Facultad de Odontología de la Universidad Tecnológica de México, cumpliendo con la Norma establecida por la Secretaría de Salud.
Dentistry-related activities take place in a highly contaminated atmosphere. The mouth contains one of the highest microbial concentrations of the body. It has been calculated that a drop of saliva might contain up to 600,000 bacteriae.1
Frequently, hospital infection reports can be concealed or overlooked in hospital medical units. This situation is harder to ascertain in the dental environment, where these problems have not been object of great concern, notwithstanding the fact that in other countries great importance has been granted to the presence of pathogens in the dental environment.
It is therefore essential to increase bio-security levels and to adhere to norms and procedures for suitable infection control.
Dentists must bear in mind the fact that all sterilization techniques might be fallible, and that in fact, they often fail. Many factors might lead to sterilization process failures: there can be procedural errors such as overload, which might be easily solved, up to mechanical errors which might disable sterilizing units until their repair. Due to the fact that these factors directly affect sterilization processes’ success, and with the aim of guaranteeing their reliability, international organisms recommend process monitoring with biological indicators (BI's).2
In Mexico the NOM-013-SSA-2006 for the Prevention and Control of Oral and Dental Diseases3 recommends monthly BI's assessment in sterilization devices. This procedure has become compulsory since its publication in the Diario Oficial de la Federación (Federation's Official Gazette). In the United States, ADA (American Dental Association), OSAP (Organization for Safety, Asepsis and Prevention) and CDC (Centers for Disease Control and Prevention) recommend their weekly application.4
Biological indicators are vials containing endospores. They are considered a manner in which to routinely monitor and validate sterilization cycles.5
Each unit consists of a tube containing bacteriological culture medium, combined with a pH indicator and a disc of spores Geobacillus stearothermophilus ATCC7953 (for pressure steam sterilization cycles) or Bacillus subtilis of the ATCC9372 niger variety (for dry heat or ethylene oxide sterilization cycles).6 Spores of Bacillus pumilus ATCC 27142 strains are used to verify ionizing radiation sterilization.
Sterilization cycle verification is not a new procedure. Appropriate BI's use is described in the Mexican States Pharmacopea (MSP).
The acronym ATCC stands for «American Type Culture Collection», the number corresponds to its location in the catalogue of this international reference culture collection.7
Selected microorganisms are much more resistant to sterilization methods, when compared to pathogenic micro-organisms present due to natural contamination.
Spores are a resistance behavior adopted by some bacteriae; in the presence of untoward environmental circumstances (nutrient scarcity, desiccation, etc.) they are able to forego their vital state (vegetative) in order to adopt a latent life state (spore). They can return to vegetative phase when the environment presents once again suitable circumstances for their germination.8
The operating mechanism of these indicators is based on fluorescent detection of the presence of α-D glucosidase, an enzyme present in the Geobacillus stearothermophilus spores. This enzyme is destroyed at a 132°C temperature.9–11
The enzyme α-D glucosidase breaks a fluorescent substrate present in the culture medium. A negative result (green light or -) is synonym for sterilization; a positive result (red light or +) indicates failure in sterilization process.
Sterilization process effectiveness is based on verification of biological annihilation produced in these indicators.
The aim of the present study conducted at the UNITEC School of Dentistry, was the assessment of sterilization processes in order to determine suitable sterilization procedures.
MATERIAL AND METHODSAn observational, cross-sectioned, analytic and prospective study was conducted on the three autoclaves present at the UNITEC's School of Dentistry. A total of 96 surgical and non-surgical packs were included. These packs had been sterilized during the period comprised from May 2012 to April 2015. Monthly determinations were conducted, according to NOM-013-SSA-1994-2006 modification stipulations. To this effect, rapid-lecture biological indicators (1292 3M Attest™) with Geobacillus stearothermophilus endospores were used (Figure 1).
Sterilization procedures consisted in instrument washing, drying and packaging, conducted by undergraduate and graduate students.
All three autoclaves were pre-heated for 20minutes at a 121°C temperature, with 1 atmosphere pressure (15 pounds per square inch). In order to achieve success, time-temperature-pressure variables were essential.
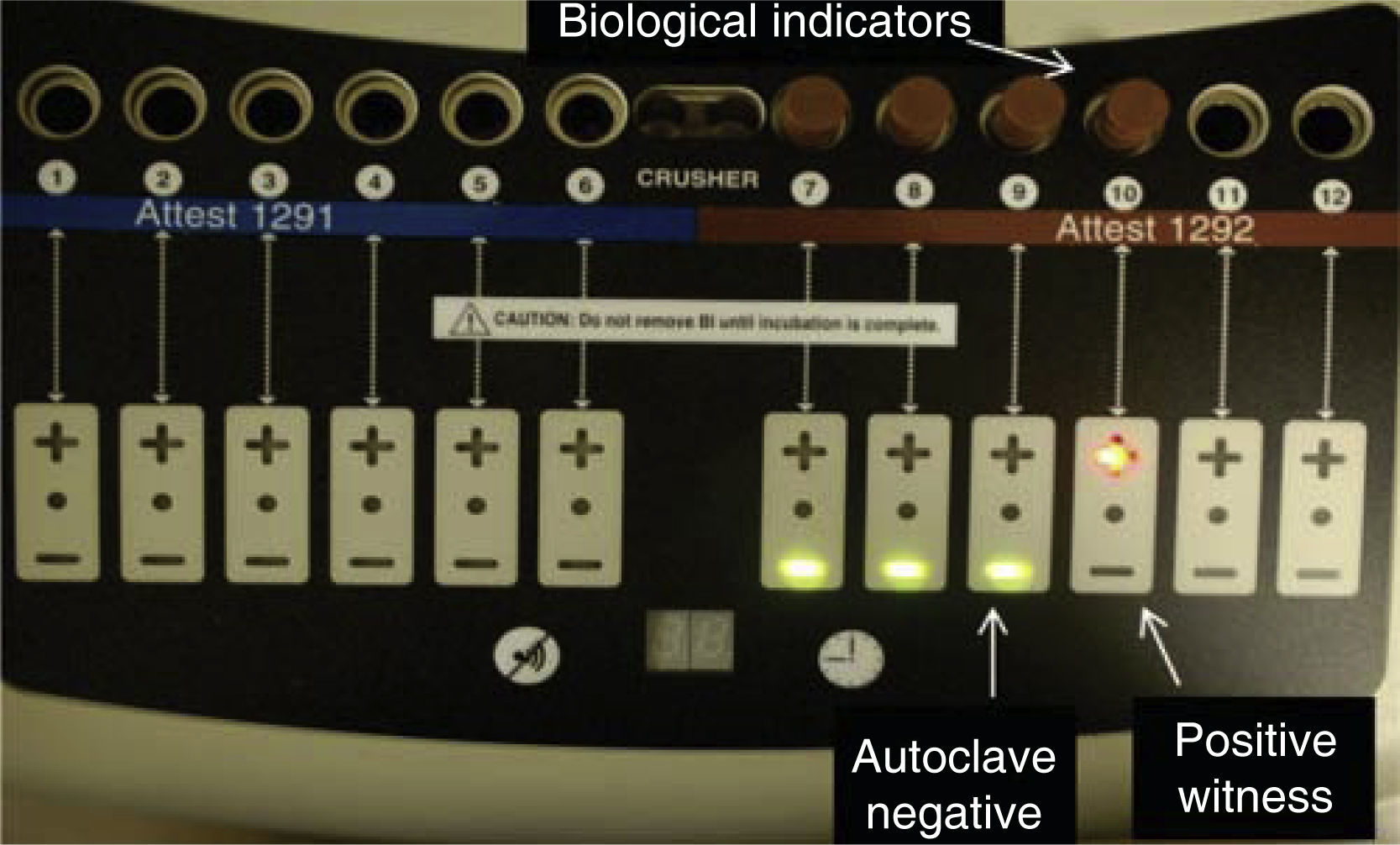
A biological indicator was placed in each autoclave close to the drain, next to the instruments; sterilization process was then undertaken (Figures 2 and 3). After this, biological indicators were placed in the optic reader in order to achieve reading. A biological indicator was used as control; this indicator was not placed within the autoclaves; in this manner, indicators’ accurate readings were ensured.
RESULTSAfter completing sterilization cycles in each autoclave, biological indicators’ reading was achieved with an optical reader; this procedure lasted for 3hours. Red light indicated positivity, that is to say, presence of the α-D-glucosidase enzyme of Geobacillus stearothermophilus spores, which was present at the biological indicator used as control.
Green light indicated absence of the aforementioned enzyme, which would indicate absence of Geobacillus stearothermophilus spores, thus, effective sterilization was achieved. These results were obtained in all three autoclaves (Figure 4).
During the period in which this monthly monitoring was conducted (May 2012-April 2015), 96 packs were examined. All packs exhibited negative results at the time of reading (Table I).
Results of biological indicators’ readings for the period comprised between May 2012-April 2015.
| Autoclave | ||||
|---|---|---|---|---|
| Month | A | B | C | Control |
| 2012 | ||||
| May | - | - | - | + |
| June | - | - | - | + |
| July | - | - | - | + |
| September | - | - | - | + |
| October | - | - | - | + |
| November | - | - | - | + |
| December | - | - | - | + |
| 2013 | ||||
| February | - | - | - | + |
| March | - | - | - | + |
| April | - | - | - | + |
| May | - | - | - | + |
| June | - | - | - | + |
| July | - | - | - | + |
| August | - | - | - | + |
| September | - | - | - | + |
| October | - | - | - | + |
| November | - | - | - | + |
| December | - | - | - | + |
| 2014 | ||||
| February | - | - | - | + |
| March | - | - | - | + |
| April | - | - | - | + |
| May | - | - | - | + |
| June | - | - | - | + |
| July | - | - | - | + |
| September | - | - | - | + |
| October | - | - | - | + |
| November | - | - | - | + |
| December | - | - | - | + |
| 2015 | ||||
| January | - | - | - | + |
| February | - | - | - | + |
| March | - | - | - | + |
| April | - | - | - | + |
Aforementioned results stressed the fact that sterilization at the school's three autoclaves was satisfactory.
DISCUSSIONIn the dental environment there is little scientific literature published on studies targeted to assess effectiveness of sterilization with biological indicators.12–14
In Mexico City, in 2001, Acosta conducted monitoring of 2,930 sterilization cycles in autoclaves with BI's. He reported flaws in 7.6%.15
In San Luis Potosi, Patiño assessed 30 autoclaves with biological indicators strips. Results revealed a 13.3% failure rate. Upon analyzing sterilization procedure, it was found that auxiliary personnel undertaking this task executed the process with inadequate pre-heating, time and temperature procedures. This study reported that only 16.1% of all dentists used biological indicators. Therefore, it is important to inform dentists about BI's, since they represent a practical, reliable and market-available method.16
In the present study, monthly BI readings in the three autoclaves were negative during the period comprised between May 2012-April 2015. This would indicate that sterilization procedures have been satisfactorily conducted.
Even though BI application is a resource allowing identification of possible flaws in sterilization processes, and additionally contributing to patient safety, its use is not well known. This could be due to the fact that BI's are frequently confused with heat indicators commonly known as «witness strips».15
Positive reading of biological control compels to discontinue use of the autoclave, until the time when BI results are negative.
Process cost does not exempt responsibility to conduct an effective sterilization procedure.
In addition to conducting sterilization monitoring according to norm NOM-013-SSA2-2006 it is recommended to use BI s in situations17 such as autoclave or sterilization means mechanical failures, change of personnel in charge of sterilization, or changes in the process (greater load). Institutions such as ADA (American Dental Association), OSAP (Organization for Safety, Asepsis and Prevention), and CDC (Centers for Disease Control and Prevention) recommend conducting weekly monitoring in order to achieve efficient sterilization control.18
It is necessary to conduct greater number of research projects in this area and report them so as to create greater awareness on the importance of BI use.
CONCLUSIONSMechanical and human error are the main reasons for sterilization procedures failure. It is therefore essential to train personnel in charge of sterilization procedures. Additionally, sterilization devices must be suitably maintained in order to avoid equipment flaws.
It is of the utmost importance for dentists to possess accurate information on BI use, since these are practical and reliable devices, and thus allow effective sterilization procedures.
Rapid lecture biological indicators (RLBI) confer greater security to instrument handling, either in the educational or the professional environment.
Few studies have been conducted on autoclave monitoring in the dental environment, therefore, references are hard to find. This fact confers the present article with an impact on the dental environment in relation to infection control.
In the present article, effectiveness of the three SAPC (CEYE) autoclaves of the UNITEC's School of Dentistry was corroborated. Norm NOM-013-SSA2-2006 established by the Mexican Health Ministry was complied with.
The present study will be furthered by modifying monitoring frequency, which will be conducted weekly as recommended by institutions such as ADA, OSAP and CDC in order to achieve better sterilization control.
Dentist, Specialist in Periodontics of the School of Dentistry.
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam