Oro-nasal fistulae are amongst the most common sequels after surgical repair procedures of cleft palate patients. The aim of the present study was to present the experience of using tongue flaps for closure of wide (over 1cm) anterior palatal fistulae, or in those cases when surgery had previously failed. Closure with tongue flaps of anterior palatal fistulae larger than 1cm, or when previous treatments have failed is one of the most successful treatment options reported in scientific literature. In the present article we introduce the case of a 23 year old male with bilateral cleft palate and lip surgery sequels. The patient attended the Maxillofacial and Oral Clinic of the Graduate and Research School. Intra-oral exploration revealed a 2.5 diameter anterior palatal fistula. The patient informed of a history of several failed surgical attempts; it was therefore decided to close the anterior palatal fistula with an anterior based tongue flap.
Las fístulas oronasales son una de las secuelas más comunes consecutivas a la reparación quirúrgica del paladar hendido. El propósito de este reporte es presentar la experiencia con el uso de colgajos de lengua para el cierre de fístulas palatinas anteriores amplias (mayores de 1cm) o con intentos quirúrgicos previos fallidos. El cierre de las fístulas palatinas anteriores mayores de 1cm o con tratamientos previos sin éxito. Mediante colgajos de lengua es una de las opciones de tratamiento reportados en la literatura con un alto porcentaje de éxito. En este artículo presentamos un caso clínico de un paciente masculino de 23 años de edad con diagnóstico de secuelas labio y paladar hendido bilateral, que se presenta a la clínica de cirugía oral y maxilofacial de la división de estudios de postgrado e investigación, a la exploración intraoral presentaba una fístula palatina anterior de 2.5cm de diámetro, con el antecedente de varios intentos quirúrgicos sin éxito, por lo que se decide realizar el cierre de la fístula palatina anterior con un colgajo de lengua de base anterior.
For over 100 years, the tongue has been used to reconstruct the oral cavity. In 1901, Eisenberg was the first to use pedunculated tongue flaps to repair intra-oral defects. In 1909, Lexer reported the use a posterior based tongue flap to repair a defect in the retro-molar and tonsillar area. In 1956 Klopp and Schurter described the use of a tongue flap to repair soft palate.1–4
In 1957 Conley proposed tongue flap variations to temporarily cover wounds or for the final reconstruction of intra-oral defects. In 1963, Guerrero Santos reported the use of tongue flaps to repair lip defects, in 1966 to close palatal fistulae and in 1973 to cover bone grafts.3,4
In 1972 Jackson reported modifications to the anterior based tongue flap for closure of children's palatine fistulae. The author combined said flaps with mucosa flaps and with iliac crest bone grafts to close anterior naso-alveolar and palatal fistulae. Hockstein in 1977 as well as Carreirao and Lessa in 1980 reported the use of full-thickness tongue flaps with favorable results. In 1987 Postnik and Getz suggested the use of wide thickness tongue flaps so as to ensure its viability. Busic in 1989 and Assunçao in 1992 used thin flaps and showed their versatility and safety to close palatal fistulae.3–5
Defect reconstruction in the mouth is always a challenge. Defects can be reconstructed with local or distanced flaps, or with soft tissue free grafts. Defect anatomy, location and size are important factors in order to decide upon treatment and to determine the type of flap required for each reconstruction type.1
For the surgeon, palatal fistulae which are a result of cleft palate repair procedures can represent a simple procedure or a great challenge. When it is not possible to close the fistula using adjacent tissues in a direct closure procedure, tissue will need to be displaced from a neighboring anatomical area. For example, muco-periosteal local flaps, Vomer flaps, total palate re-surgery, tongue flaps, naso-labial flaps, flaps of the cheeks or neck, combination of pharyngeal flaps, of tongue flaps of temporal muscle flaps.4,6
Intra-oral flaps can be islands of palatal tissue, buccinator's myo-mucosal flaps, oral adipose body flaps and tongue flaps. All the aforementioned have been commonly used to reconstruct small to mediumsized intra-oral defects. Due to its abundant vascularity and its low morbility, the tongue is an excellent donor site for the reconstruction of oral cavity soft tissues. These types of flaps have also been described in the handling of palatal defects resulting from infections, trauma and neoplasia.1,3–5
PALATAL FISTULAPrimary treatment of cleft palate must result in a separation between nasal and oral cavities. Nevertheless, multiple causes such as size of the deformation, healing failure, technique defects, flap tension, necroses, hematoma, trauma at closing site etc, might cause dehiscence and palatoplasty, and leave palatal fistulae in the hard or soft palate.3,7
It must be taken into account that communication in the alveolar process at the anterior region, in cases when it has not intentionally been repaired during the surgical phase, must not be considered as a fistula, but rather as a residual cleft.4,7
Symptoms of palatal fistulae depend on their size and position; these symptoms are regurgitation of liquids from oral to nasal cavity, speech defects and halitosis.3
CLASSIFICATIONBased on defect size, Cohen and Posnick propose the following classifications:
• According to Posnik et al
- -
Simple cleft. Lesser than 1.5cm in diameter. It is commonly located at the midline. Usually caused by a small dehiscence on the hard palate.
- -
Small fistulae. Less than 1.5cm diameter. Commonly located at the midline. Commonly caused by a small dehiscence at the union between soft and hard palate, or by a small necrosis at the flap borders.
- -
Large fistulae. Larger than 1.5cm diameter. Commonly caused by necrosis of the flap's anterior third, probably due to a lesion or the palatine artery, it can be communicated to the alveolar cleft.3
• According to Cohen et al
- -
Small fistulae; 1 to 2mm.
- -
Midsize fistulae: 3 to 5mm.
- -
Large fistulae: Larger than 5mm.7
There are two techniques too lift dorsal tongue flaps: anterior based and posterior based flaps. Anterior based flaps are indicated for treatment of hard palate defects as well as defects of the anterior oral mucosa, anterior floor of the mouth and lips. Posterior based flaps are recommended for soft palate defects as well as defects of the retromolar area and posterior oral mucosa.4,8
INDICATIONSTongue flaps have been used to close intra-oral defects which occur after tumor resection procedures, severe infections, trauma and fistulae in cleft lip and palate patients. They have also been used for closure of defects occurring after radiotherapy.
Posterior based flaps are indicated for the soft palate, retro-molar area and posterior oral mucosa. Anterior based flaps are useful for closure of hard palate, anterior oral mucosa, lips and floor of the mouth defects. Due to their vascularity, posterior flaps are safer, nevertheless, anterior based flaps are more versatile with respect to their mobility and elasticity.
Closure of intra-oral defects begins with conservative treatments such as vestibular flaps, palatal flaps or combined flaps bone grafts. Tongue flaps are indicated in cases of fistula recurrence, in palates with excess scarring, as well as in palates where the quality and quantity of residual palatal tissue does not allow suitable closure, as well as in defects which are larger than 1cm diameter. Success has been reported in local flaps in fistulae measuring less than 1.5cm diameter.9
The lingual artery passes in depth in front of the hyoglossus muscle, it branches out becoming then the sublingual artery and the deep lingual artery or ranina. The sublingual artery irrigates the sublingual gland, the mylohyoid muscle as well as adjacent muscles. Ranina arteries traverse deeply in front of the ventral mucosa, producing branches which ascend to the dorsum of the tongue. Both ranina branches are separated by a medial fibrous septum except at the base, where a transversal branch joins them, and at the tip where they anatomose. These ranina branches irrigate anterior based tongue flaps.1,8–10
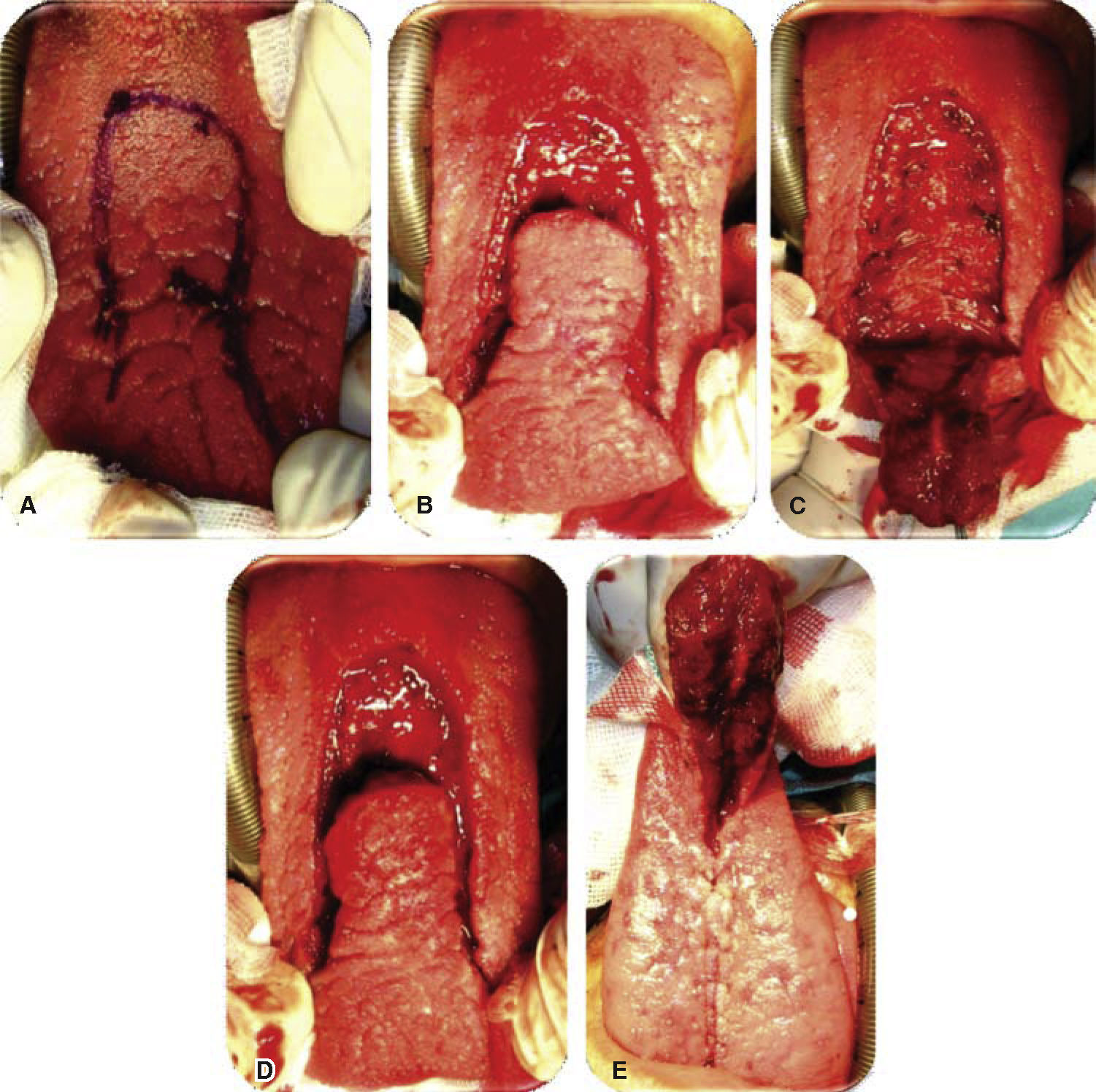
SURGICAL TECHNIQUEUnder general anesthesia and naso-tracheal intubation (although there are also reports of success with oro-tracheal intubation) local anesthetic with vasoconstrictor is infiltrated into the palate and the dorsum of the tongue. The base of the flap must be placed underneath the posterior edge (border) of the defect when the tongue is in neutral position; it must measure approximately 2.5 to 3cm wide, or of the same width or up to 20% greater width than the defect itself. Length should be sufficient to avoid tension in the pedicle during healing. The flap can extend up to 5-6cm without endangering tissue viability allowing thus tongue mobility. In general, these flaps should be 5 to 7mm thick and include mucosa and adjacent muscular tissue, thus protecting the sub-mucosal vascular plexus. Circumvallate papillae (major taste buds) must be circumvented during flap design and dissection. These papillae are very evident, so it should not be difficult to identify them. Suture at the donor site must be accomplished in one or two planes, taking great care of hemostasis and to close the empty space left by the flap, avoiding thus edema or hematoma formation which might compromise flap viability.
Prior to suturing the tongue flap over the tongue, a nasal mucosa plane must be achieved. To execute this plane, a peri-fistular incision must be undertaken, with palatal mucosa dissection, the mucosa borders are everted, and then sutured, achieving thus defect closure at a nasal mucosa plane. Suturing the aforementioned plane is often difficult, and sometimes 100% closure of the nasal mucosa plane is not achieved. Defect margins must be de-epithelialized in order to receive the tongue flap.
Once the nasal plane is secured, the tongue flap is raised, rotated, and carefully sutured on the defect.
Some authors recommend the use of intermaxillary fixation so as to prevent uncontrolled tongue movements and thus avoid tension in the flap.
Under local anesthesia, the pedicle must be separated at approximately three weeks after treatment; most of the pedicle might be repositioned into the donor site, avoiding thus esthetic sequels.1,3,5,8,9
In this procedure, complications might arise immediately, such as bleeding, hematoma, epistaxis, temporary loss of sensitivity and taste, In the short term other complications might arise such as infection, dehiscence or necrosis. No alterations in tongue mobility or in word articulation and enunciation have been reported; the only reported sequel was a thinner tongue.1,4,9,10
Patients subjected to tongue dorsum pedicled flaps must remain on a clear liquid diet during the first hours after surgery, and blended food diet until the pedicle is separated.1
CASE REPORT23 year old male patient with a diagnosis of sequels to bilateral cleft lip and palate and oro-nasal fistulae. The patient informed of a non contributory familial and pathological history, unrelated to the present condition. Surgical history included lip repair surgery at 4 months of age, palate repair surgery at 18 months of age. Oronasal fistula closure procedure at 11 years of age, the rest of patient history was non-contributory. The patient did not use obturators and informed of passage of liquids from the oral to the nasal cavities, repeated infections in upper respiratory airways, and rhinolalia. Due to all the aforementioned symptoms, the patient attended the Maxillofacial and Oral Surgery Service of the Graduate and Research School, National School of Dentistry, National University of Mexico (UNAM) to be assessed and diagnosed.
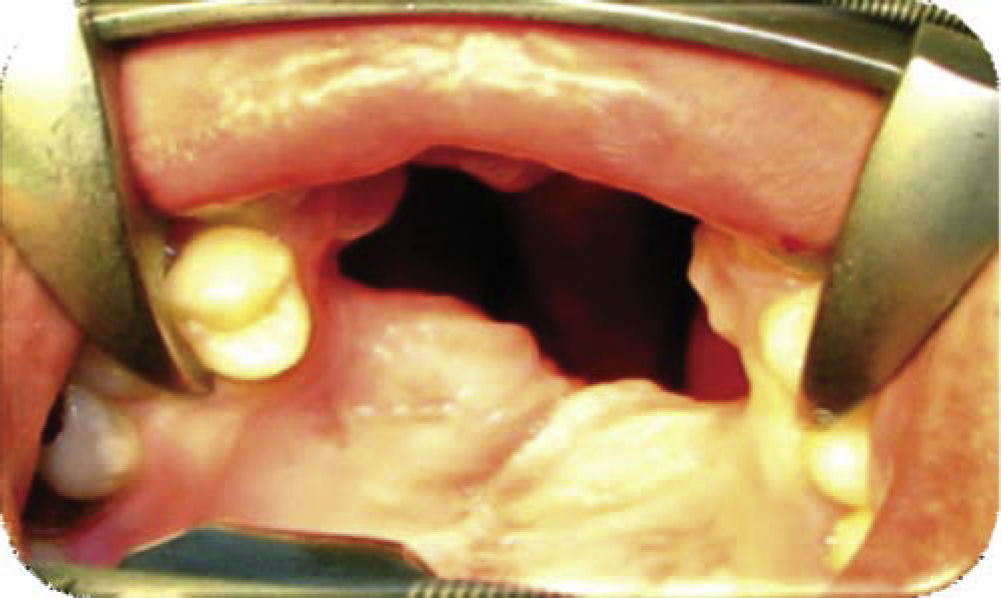
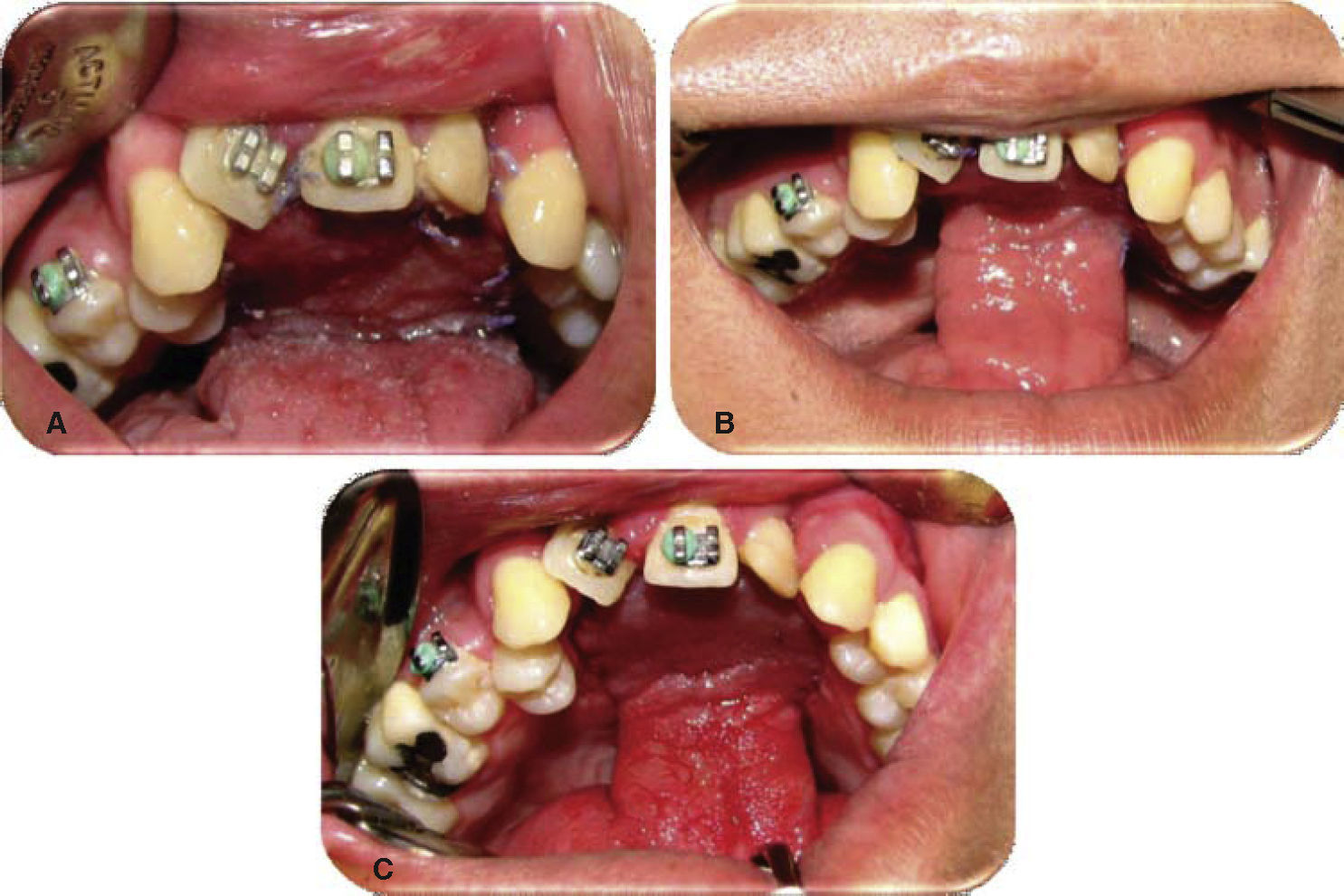
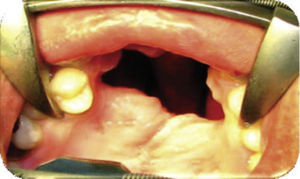
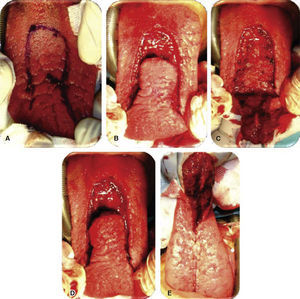
Physical exploration. 23 year old male patient. Intra-oral examination revealed cleft palate sequels, oro-nasal fistula measuring 2.5 × 2.5cm at the anterior palatal region with communication to the nasal cavity, with healthy surrounding tissue (Figure 1).
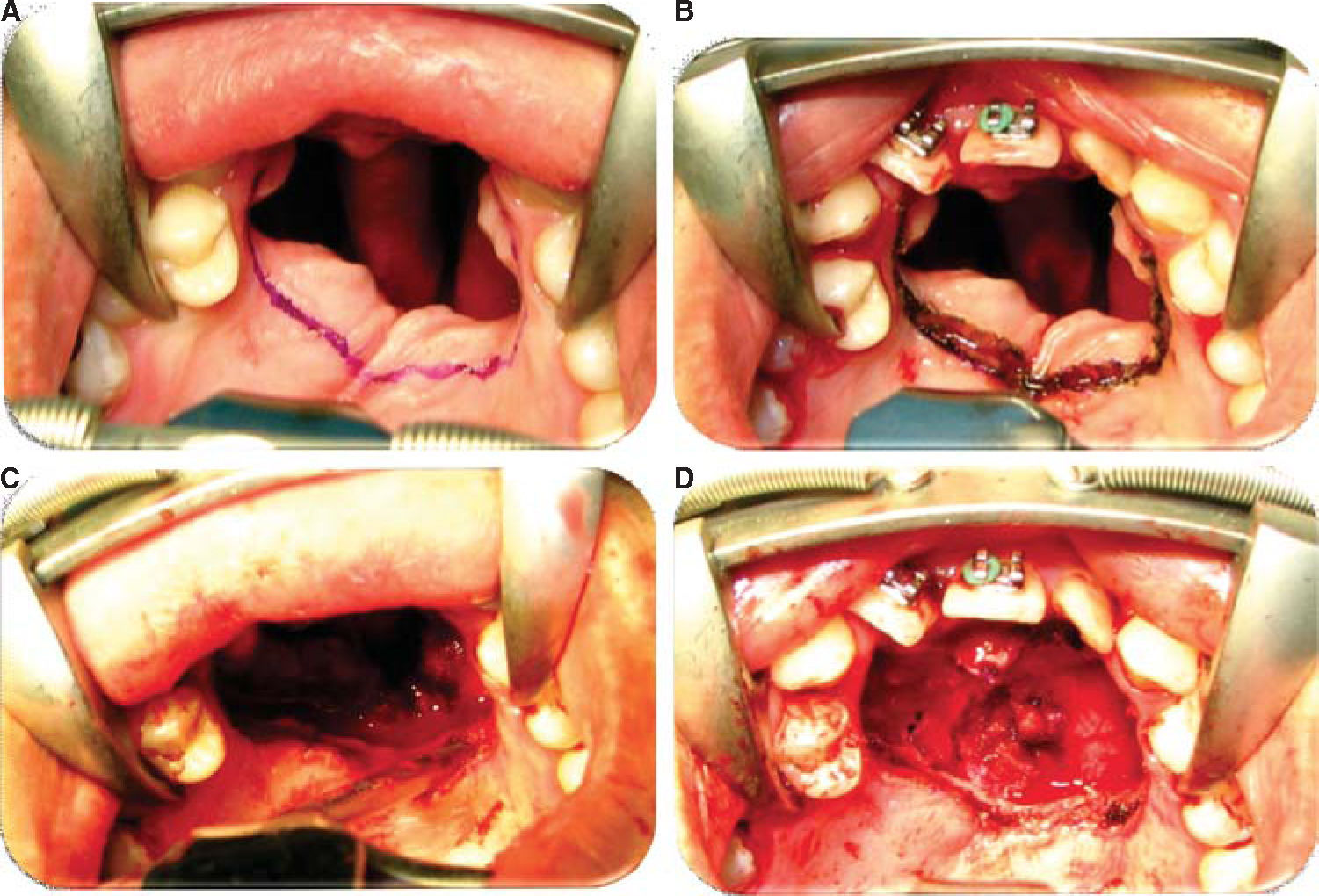
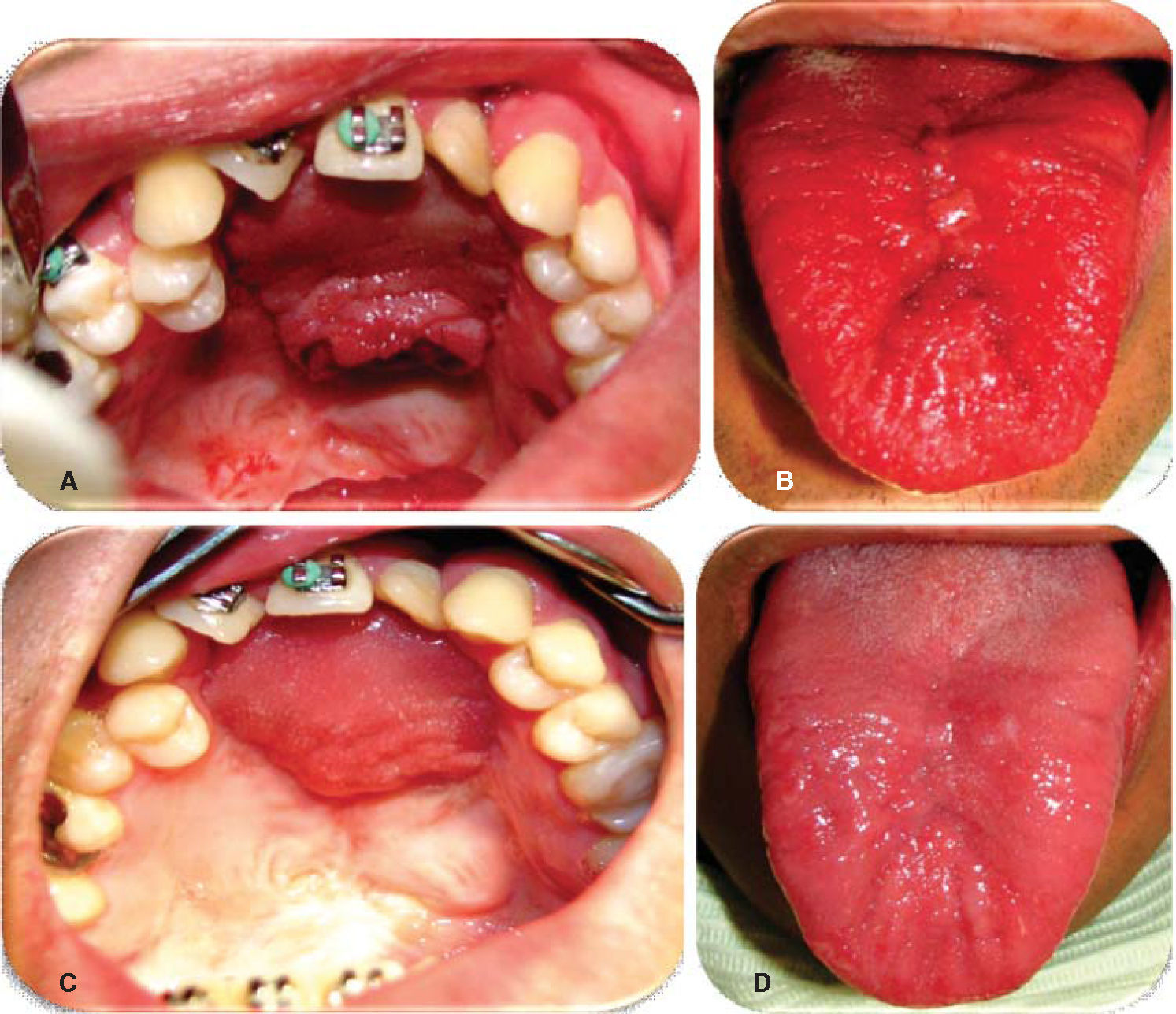
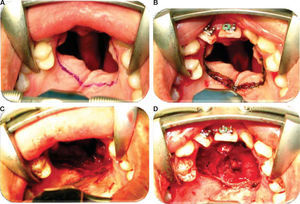
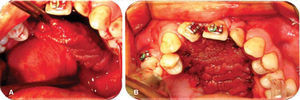
Oronasal fistula surgical closure was programmed with anterior based tongue distanced flap. Under general anesthesia with naso-tracheal intubation, palate and donor site were infiltrated with lidocaine and epinephrine at 2% and 1:100,000 to achieve hemostasis. A Digman type mouth prop was placed. A peri-fistular incision was executed with number 15 surgical blade and electric scalpel. The defect's borders were dissected and everted and then sutured with 910 4-0 polyglactine, 100% closure was achieved in the nasal mucosa plane. Nasal irrigation was undertaken with physiological solution, no egress of liquid was observed from the sutured defect. The Digman type mouth prop was removed and a Mackinson mouth opener was placed. The tongue was pulled with one 2-0 silk suture point at the tongue's tip. The anterior-based tongue flap was designed so as to be of a size 20% larger than the defect and with an approximate length of 5cm, taking great care to cover the whole defect and avoid tension. Hemostasis was achieved with electrocautery and with polyglactin 910 sutures. Suturing of donor site was executed in two planes with 4-0 polyglactin 910; no hematomas were observed. The tongue flap was rotated over the defect and sutured over the defect borders with polyglatin 910 simple stitches. Surgical event was completed without complications or accidents (Figures 2 to 4). No inter-maxillary fixation was applied since, due to his age, the patient was considered to be cooperative.
The patient was dismissed from hospital 24hours after surgical procedure Prescribed treatment was chlorhexidine rinses, amoxicillin, by mouth, 500mg every eight hours for seven days, and ibuprofen, by mouth, 400mg every eight hours for five days. A control appointment was scheduled for seven days after surgery.
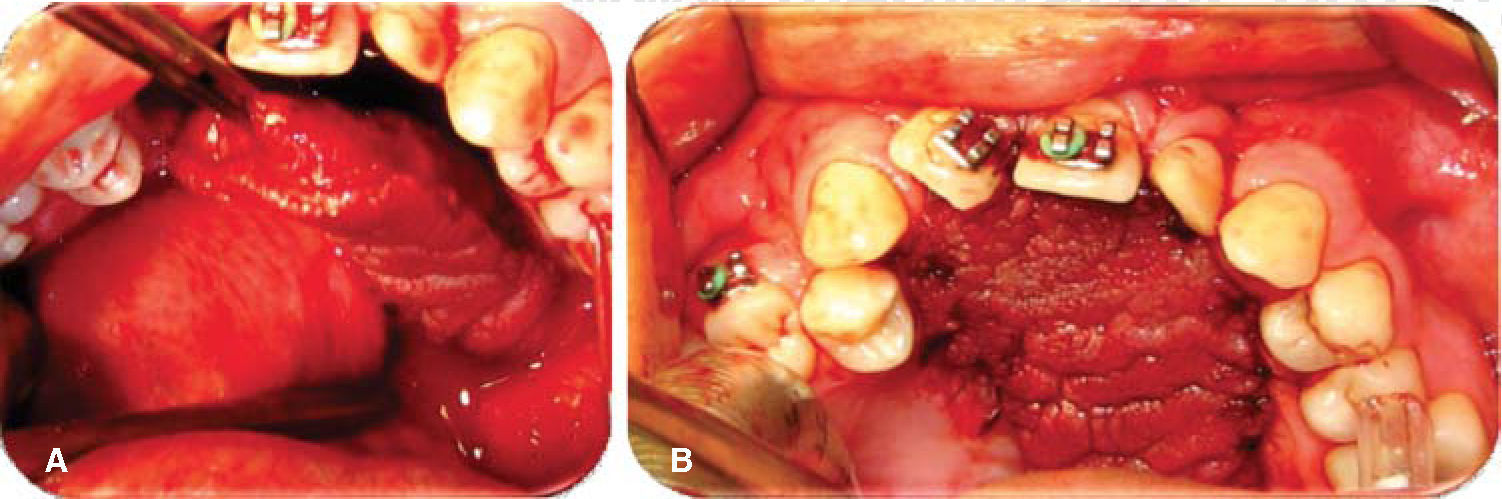
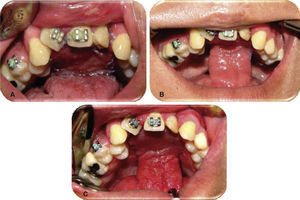
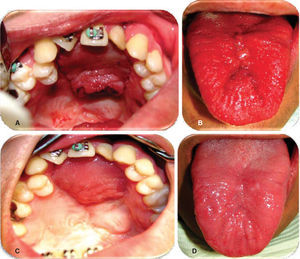
Evolution. Weekly controls were conducted at 7, 14, 21 and 28 days after surgery. At controls executed at 7, 14 and 21 days, excellent evolution was observed: with well-hydrated mucosa of suitable color, surgical wounds free of dehiscence, infection or compromised vascularity (Figures 5 and 6). Suitable tongue mobility was observed without phonation alterations. On day 21, under local anesthesia the tongue flap was separated with clamps, no accidents or complications were encountered. The patient was programmed for another visit seven days later, and was prescribed with amoxicillin, by mouth, 500mg every eight hours and ibuprofen by mouth, every eight hours for five days. At the 28th day control, clean surgical wounds were observed without signs of dehiscence or infection, as well as 100% closed oro-nasal fistula, dorsum of the tongue without functional or esthetic alterations. The patient was discharged and programmed for a one month later control appointment.
Prognosis. Favorable for life and function.
DISCUSSIONSeveral authors have reported incidence of palatal fistulae after palatoplasties, as well as the difficulty of their treatment. Said incidence varies in different publications, ranging from 0 to 36%. They are normally related to varying factors such as type of cleft, surgeon's experience, patient's age and palatoplasty technique.7
Use of tongue flaps to close palatal fistulae resulting from palatoplasty procedures is a safe, low morbidity alternative; Assunçao AG in a study published in 1993, reported 100% confirmed success rate. Series reported by Guerrero Santos and Altamirano reported 70% success rate, whereas Piggot reported 85% success in closure of palatal fistulae by means of anterior based tongue flaps.5,7 One report in literature suggests avoidance of electrocautery use.
If necessary, oral opening restriction can be achieved with a Barton type dressing, inter-maxillary fixation, suturing the flap to the upper incisors or even to the upper lip. All these measures greatly depend on the age and patient disposition to follow post-surgical care.1,4,7
CONCLUSIONSTongue flaps are an excellent alternative to close wide or recurrent palatal fistulae, since they are versatile and can be designed for each fistula type.
They are indicated when other techniques have failed and in fistulae measuring over 1cm, the excellent vascularity present in this anatomical area gives the surgeon great confidence in the treatment's success.1,11
Flap mobility restriction can be achieved with a Barton type dressing, inter-maxillary fixation of flap, suture to upper incisors or even the upper lip in cases when the patient were to be un-cooperative of that surgery be performed on a very young patient.1,4,7
The second phase of this treatment is conducted approximately 21 days after surgery, the pedicle must be removed under local anesthesia, with or without intravenous sedation; the pedicle must be placed at the donor site, thus limiting possible esthetic sequels in the tongue.1,3,5,8,9
In this procedure, complications might be immediate. Among them we can count bleeding, hematoma, epistaxis, temporary loss of taste and sensitivity. Among mid-term complications we can find infection, dehiscence, and necrosis. No reports have been made on tongue mobility alterations or word enunciation and articulation, thinning of the tongue was the only reported sequel.1,4,9
Patients subjected to pedicled flaps of the tongue's dorsum must be kept in a clear liquid diet for the first hours after surgery, and blended diet until flap separation from the pedicle.1
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam