Hialuronic acid (HA) is an essential glucosaminoglycan of the extracellular matrix of all tissues; it is found at an average concentration of 0.02%. A subject weighing 60kg contains approximately 12g of HA. The greater concentrations of this acid are found in connective tissue such as the skin, which exhibits up to 56% percent of said acid. HA plays an important role in cell migration, since it is involved in processes of growth, inflammation and reparation as well as stimulation of different connective tissue cells.
ObjectiveTo present a clinical case involving interdental papilla reconstruction with HA infiltration.
Clinical case24 year old female who reported being systemically healthy. The patient exhibited loss of interdental papilla in the area of tooth number 11 and 21 caused by presence of gingivitis and poor brushing techniques. The patient was assessed according to Nordland and Tarnow classification in order to ascertain the procedure's degree of predictability; she was additionally examined according to Cardaropoli classification in order to be able to establish pre- and post-treatment comparisons. The patient exhibited 5mm of contact point to the bone crest, therefore, HA infiltration was undertaken into the papilla, every seven days for four weeks.
ConclusionThere are very few non-surgical techniques to regenerate interdental papillae, one of them is HA use. Research conducted on this technique is not new, nevertheless, it would be suitable to further it taking into consideration different factors; conducting them in greater-sized populations with subjects of different ethnicities and gender, and using different infiltration intervals.
El ácido hialurónico (AH) es un glucosaminoglicano esencial de la matriz extracelular de todos los tejidos, estando en una concentración media del 0.02%. Una persona de 60kg de peso contiene aproximadamente 12g de AH; las mayores concentraciones de este ácido se encuentran en los tejidos conectivos como la piel, la cual presenta hasta un 56% de éste. El AH tiene un papel importante en la migración celular, ya que está involucrado en procesos de crecimiento, inflamación y reparación, así como estimulación de diferentes células del tejido conectivo.
ObjetivoPresentar un caso clínico de reconstrucción de la papila interdental infiltrando AH.
Caso clínicoPaciente femenino de 24 años de edad que al interrogatorio refiere ser sistémicamente sana. Presenta pérdida de la papila interdental de la zona OD 11 y 21 a causa de una gingivitis y mala técnica de cepillado. Se valora de acuerdo con la clasificación de Nordland y Tarnow para saber el grado de predictibilidad del procedimiento y de acuerdo con la clasificación de Cardaropoli, con el fin de poder realizar comparaciones antes y después del tratamiento. La paciente presentaba 5mm del punto de contacto a la cresta ósea, por lo que se realizó un infiltrado de AH en la papila durante cuatro semanas cada siete días.
ConclusiónExisten muy pocas técnicas no quirúrgicas para la regeneración de la papila interdental, dentro de ellas encontramos el uso de AH. Las investigaciones sobre esta técnica no son nuevas, sin embargo, se deben continuar y ampliar los estudios considerando diversos factores: realizarlos en poblaciones mayores con personas de distintas razas, sexo y utilizando diferentes intervalos de infiltración.
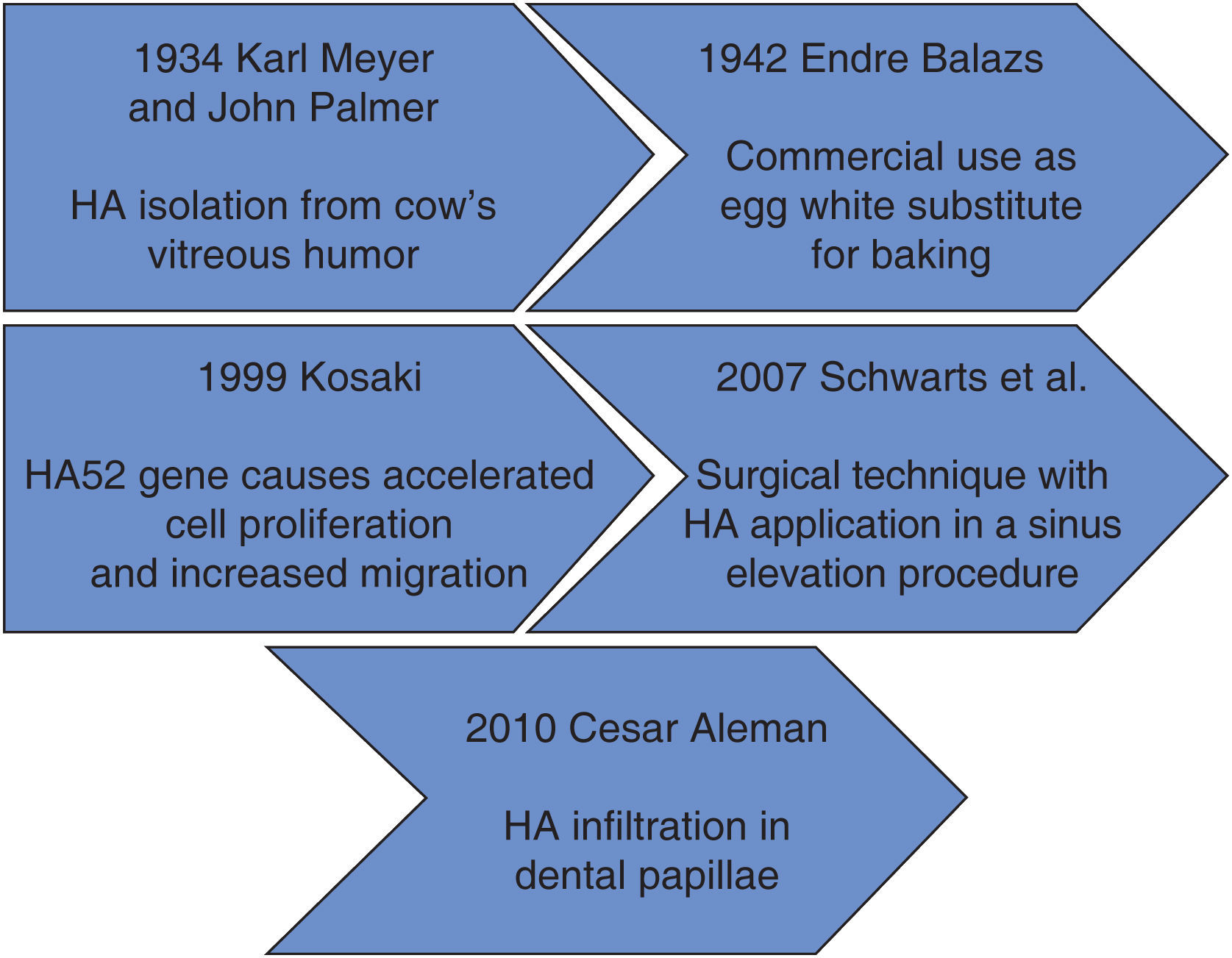
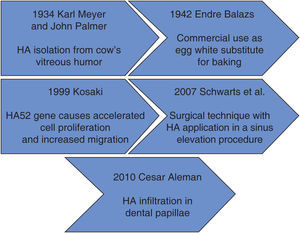
Within the context of historical background we find that in 1934, Karl Meyer and his assistant John Palmer isolated hyaluronic acid (HA) from cow's eyes vitreous humor, for them, it was an unknown chemical substance. These researches stated that it was a universal component of the extra-cellular space, and that its multiple properties allowed constitution of a matrix, which would provide support to normal functioning of cells and tissues. Moreover, they declared that this substance contained two sugar remnants, one of them being uronic acid.1
Endre Balazs used for the first time HA in 1942, he used it to substitute egg white for baking purposes. Balazs undertook the most part of HA discoveries in the last 50 years.1
Figure 1 shows HA timeline process. It can be observed that at the beginning, HA was used as a substitute for egg white, later it was used as a promoter of cell migration in different fields of medicine.
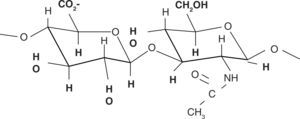
DEFINITIONHA presently is defined as a linear glucosaminoglycan formed by di-saccharide units (GAGs) constituted by glucuronic acid and N-acetylglucosamine (NacGlu). Although other sulfated GAGs such as proteoglycans are synthesized in the Golgi apparatus, this is not the case with HA, which is assembled by enzymes of the plasmatic membrane.2–4
General aspects of glucosaminoglycansGlucosaminoglycans are long polymers composed of certain repeated di-saccharides, where one or both of them contain a sulfate residue.5 They are molecules of great volume. Due to its vast hydration, the extracellular matrix behaves like a gel, this allows tissues possessing high proportions of glucosaminoglycans to withstand strong mechanical pressures, favoring moreover high rate of substance diffusion among cells.6
Within the glucosaminoglycans group, HA is the only non-sulfated; it constitutes a special case since it does not form covalent links with other molecules of the extracellular matrix, it is extracellulary synthesized by enzymes located on the cell surface, called hyaluronic acid-synthetases (HAS). Out of these there are three isoenzymes: HAS1, HAS2 and HAS3.6,7
Hyaluronic acid is frequently associated to collagen molecules or proteoglycans, providing the extracellular matrix elasticity, resistance and lubrication. Its function is very important during development, or in locations of the body where strong cell proliferation takes place, since it facilitates cell displacement. Since it is a large and rather inflexible molecule, it fills considerable volume with many free spaces.6
HA can be considered a union bridge for central proteins, and example of this would be the cartilage union protein, aggrecan and versican. Hyaluronic acid plays the role of axis or structure for large proteoglycan complexes; it adheres to surface receptors which regulate cell migration and proliferation, such as CD44.8 CD44 is an HA receptor acting as adhesion molecule and expressed in leukocytes, epithelial cells, fibroblasts and muscle cells.9
The physiological role of this receptor is to preserve organic and tissue structure through cell-cell and cellmatrix adhesion. CD44 isoforms are implicated in the initial bond of leukocytes to endothelial cells activated by inflammatory processes. It has been shown that it corresponds to a model with multiple steps, where HA bonds to CD44 receptor and mediates initial adhesion of inflammatory cells to the vessel which allows extravasation instead of inflammation.10
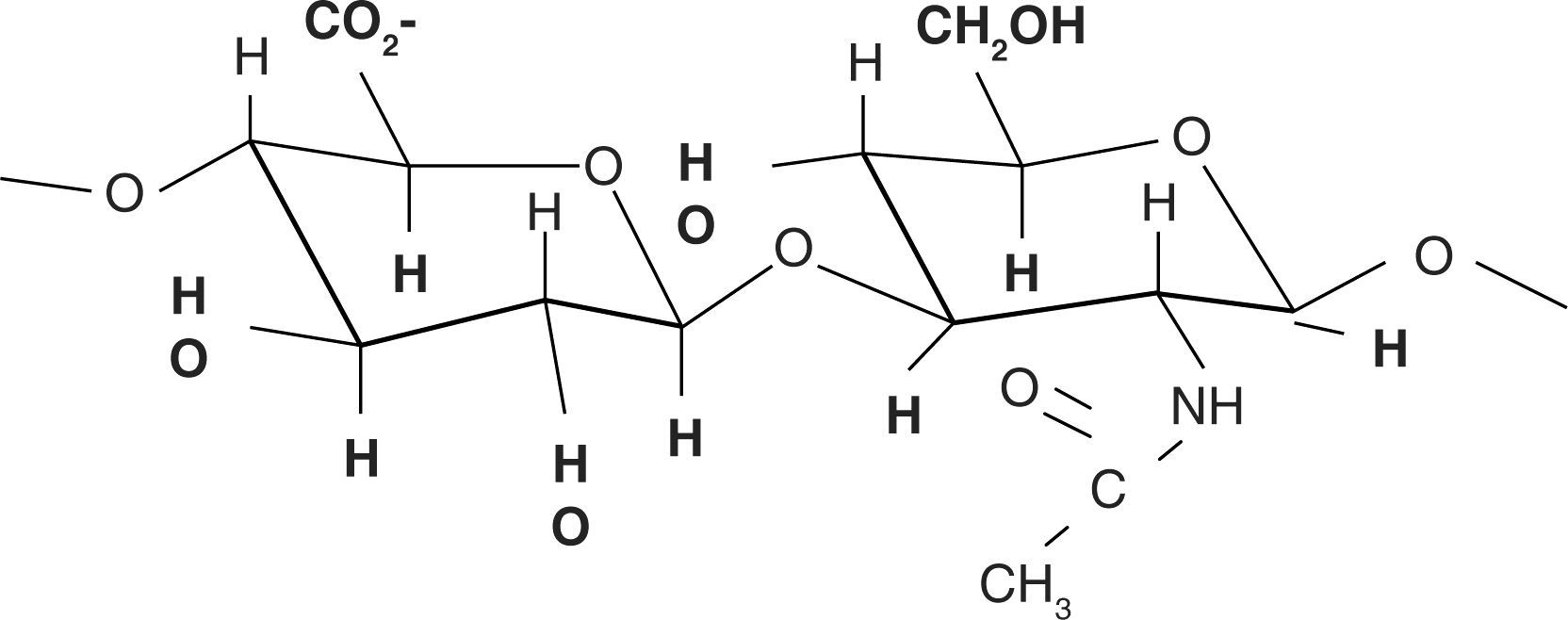
Chemical structureHA chemical structure is composed of a disaccharide unit containing glucuronic acid and N-acetylglucosamine which bond to form a uniform and linear polysaccharide molecule. These sugar units are hydrophilic, their most relevant physical property is the ability to store water, increasing in over 50 times their dry weight, which confers them a high degree of elasticity and allows them to act as a barrier to macro-molecule and foreign bodies passage. These properties are achieved by way of the OH-groups and the negative loads it possesses. Therefore, it is highly water-soluble (Figure 2).11,12
Biological activityHA plays definitive roles in the genesis, maintenance and resolution of underlying inflammation.13,14 It decreases prostaglandin types, which are causes of inflammation, and decreases inflammatory processes, moreover, it improves collagen disposition, causing better tissue healing and reparation.15,16 It is a component of synovial fluid, of vitreous humor and it is essential in fertilization processes, since many fluids of the female genital tract are HA rich.17
Thus, HA is involved in hydration-providing processes of growth/reparation and provides plastic properties to the mucosae. It participates in the process of tissue repair and healing. A de-polarization takes place in inflammatory processes which alter tissue architecture, hindering metabolic exchange. Here is where HA intervenes.18,19 Information has recently been provided confirming HA's anti-inflammatory and extracellular matrix stabilizer role, achieved by means of a protein complex called inter-alpha-inhibitor.17
Proteins which recognize HA are inter-related and are called hyalurocadherinas. Some of them are classified as type TSG6 type soluble proteins (tumor necrosis factor stimulated gene 6), as CTRL1 (cartilage tissue bonding protein), and others function as cellular adhesion molecules such as RHAMM, also called in specialized nomenclature CD168. RHAMM is a HA receptor whose activity induces chemotaxis.
Many hyalurocadherinas are soluble proteoglycans, such is the case of versican or aggrecan, and others are coupled to the membrane, such is the case of CD44. It is considered that CD44 can be a recovery route for lysosomal degradation. Within enzymes which specifically degrade HA, we find hyaluronidases or hyaluroglycosaminidases (HYAL) philogenetically preserved from bacteria.12,20 HO increases bone formation, stimulating osteoblasts in vitro through mesenchymal cell increase and their differentiation21
HA pharmacokineticsHA presents mean life span of 2 to 3 days, and its metabolization takes place in the liver.22 Its action mechanism consists in organizing disposition of collagen favoring cell differentiation and thus allowing healing. In cases when unsuitable HA concentrations were to be found, healing would be abnormal with presence of stenosis and retraction.23
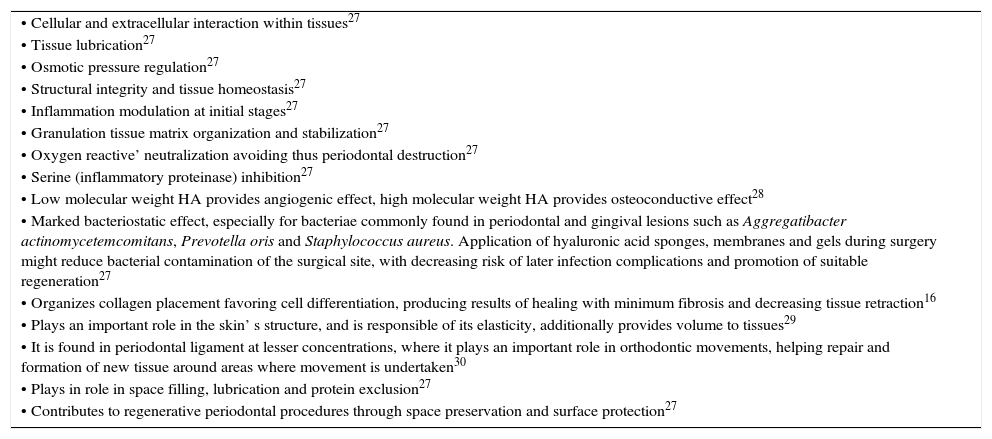
Among specific activities performed by HA we find fibroblast migration and fibrogenesis, regulation of proliferation level and epidermis thickness, as well as keratinocyte proliferation.20,24Table I shows HA's general functions.
Hyaluronic acid functions.
| • Cellular and extracellular interaction within tissues27 |
| • Tissue lubrication27 |
| • Osmotic pressure regulation27 |
| • Structural integrity and tissue homeostasis27 |
| • Inflammation modulation at initial stages27 |
| • Granulation tissue matrix organization and stabilization27 |
| • Oxygen reactive’ neutralization avoiding thus periodontal destruction27 |
| • Serine (inflammatory proteinase) inhibition27 |
| • Low molecular weight HA provides angiogenic effect, high molecular weight HA provides osteoconductive effect28 |
| • Marked bacteriostatic effect, especially for bacteriae commonly found in periodontal and gingival lesions such as Aggregatibacter actinomycetemcomitans, Prevotella oris and Staphylococcus aureus. Application of hyaluronic acid sponges, membranes and gels during surgery might reduce bacterial contamination of the surgical site, with decreasing risk of later infection complications and promotion of suitable regeneration27 |
| • Organizes collagen placement favoring cell differentiation, producing results of healing with minimum fibrosis and decreasing tissue retraction16 |
| • Plays an important role in the skin’ s structure, and is responsible of its elasticity, additionally provides volume to tissues29 |
| • It is found in periodontal ligament at lesser concentrations, where it plays an important role in orthodontic movements, helping repair and formation of new tissue around areas where movement is undertaken30 |
| • Plays in role in space filling, lubrication and protein exclusion27 |
| • Contributes to regenerative periodontal procedures through space preservation and surface protection27 |
Hyaluronic acid is used in dentistry as a biomaterial, since it is the only one with same chemical structure in all species and tissues.25 It is additionally used as an adjuvant in tissue reparation and traumatic processes.25 It is worth mentioning that it is generally used as an antiseptic and is beneficial to decrease bleeding. It is additionally used in conditions of traumatic, degenerative or inflammatory temporomandibular joint, since it improves function and decreases pain, due to its mechanical properties (lubrication decreasing articular wear) and metabolic properties (favoring nutrition to avascular areas of the condylar cartilage and disk).25,26
Other recorded uses of HA are procedures of maxillofacial surgery, orthopedics and orthognathic surgery.31 It is commonly used in aesthetic treatments due to its ability to hydrate soft tissues.28,32,33 In periodontal therapy, literature reports that this acid has been employed in gingivitis, recessions, periodontal pockets, grafts and implants.28,32,34,35
HA contraindicationsHA should not be applied in the following cases:
- •
Patient has a tendency to develop hypertrophic scars.
- •
There is history of auto-immune diseases.
- •
In children, pregnant or nursing females.
- •
In patients subjected to immunotherapy treatment.
- •
In patients with active herpes.
- •
In patients allergic to chondroitin sulfate and heparine.
- •
In cancer patients, since HA causes cell proliferation, and if this is applied to cancer patients it would generate malignant cells.36,37
Secondary reactions generated by HA use are: reddening of application area, small edema and sensitivity sensation, these reactions are very mild and tend to disappear after 24-48hours.24
Figure 3 summarizes the characteristics of hyaluronic acid.
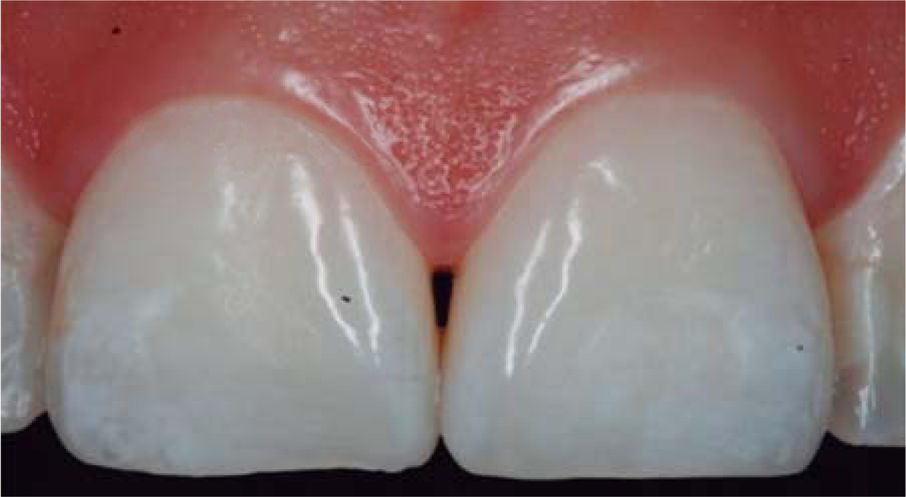
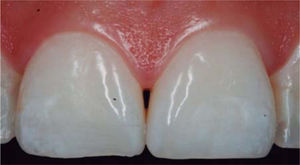
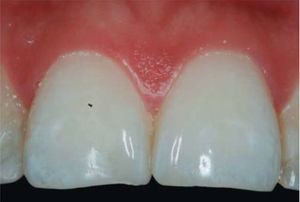
CLINICAL CASE PRESENTATIONA 24 year old female patient, who upon history taking, informed of systemic good health. Clinical exploration revealed loss of interdental papilla between upper centrals caused by gingivitis and poor brushing technique (Figure 4).
A diagnosis of interdental papilla loss was emitted according to parameters described by Nordland and Tarnow. Vertical distance between bone crest and apical point was evaluated measuring contact area between crowns and soft tissue height in the interproximal area. When distance between bone crest and contact point is 5 mm or less, and the height of the papilla does not exceed 4 mm, an interdental papilla reconstruction surgical intervention can be accomplished and thus predictably solve the problem of a black triangle.
In cases when the contact point is located at a distance surpassing 5mm from the bone crest, means should be used to apically lengthen the contact area found between teeth, and not operate, in order to improve papilla topography.38
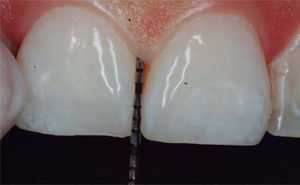
In the present clinical case, a 5mm measure was recorded from bone crest to contact point, thus the patient was considered a candidate for treatment of interdental papilla reconstruction (Figure 5).
Cardaropoli classification revealed IPP2 diagnosis since the papilla did not cover all the space underneath the interdental contact point; cement-enamel junction was not visible and was not found at the same height as adjacent papillae. Due to the aforementioned, said classification allowed to conduct an easy papilla height measurement and to observe comparisons between treatment's initial and later levels.39
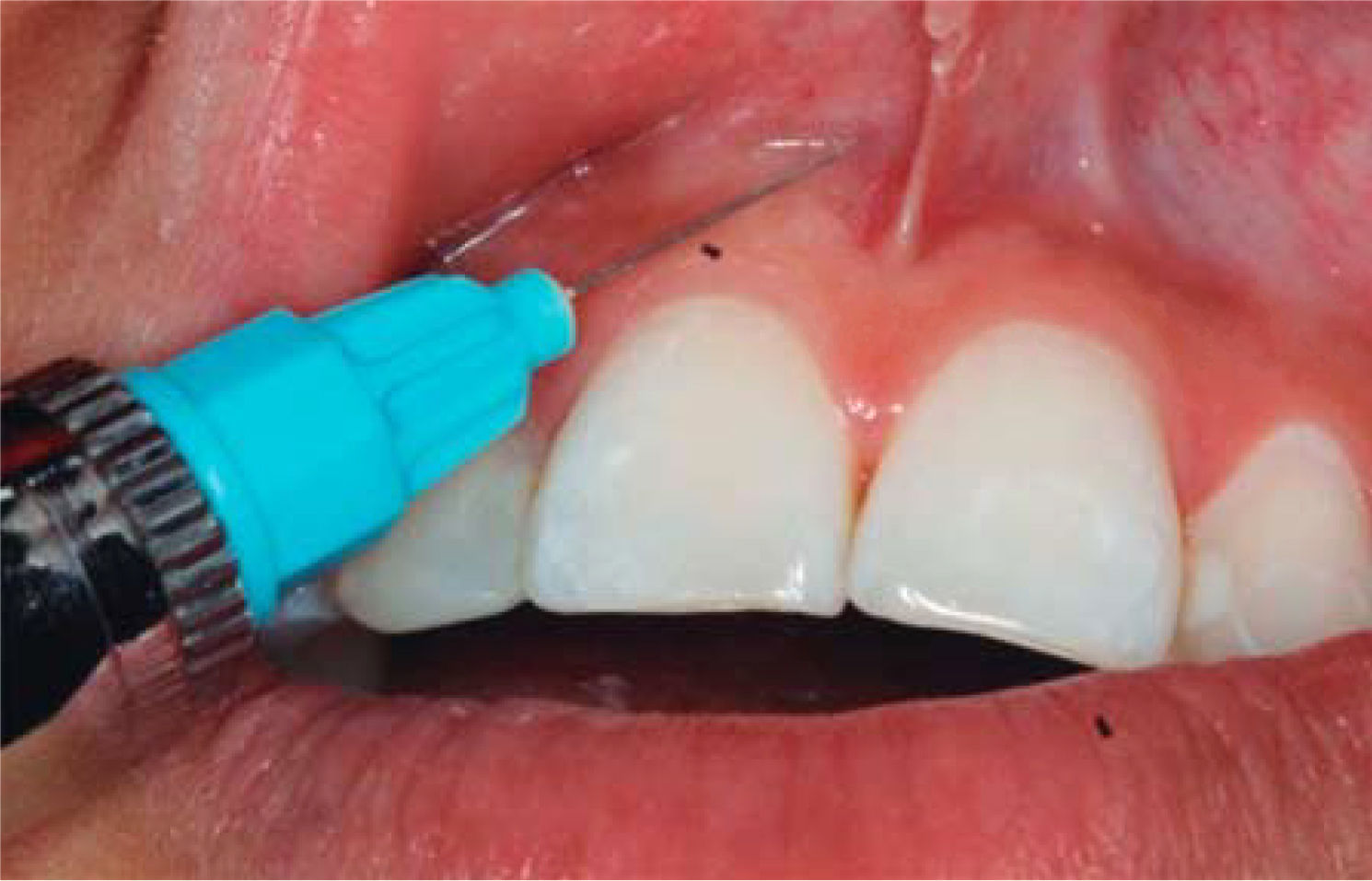
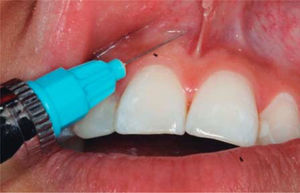
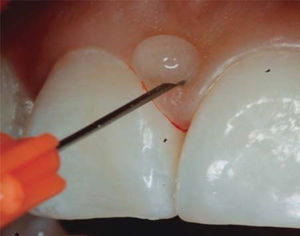
TREATMENTAfter anesthetizing the area (Figure 6), the interdental papilla was infiltrated with 1ml of HA placed in an insulin syringe.
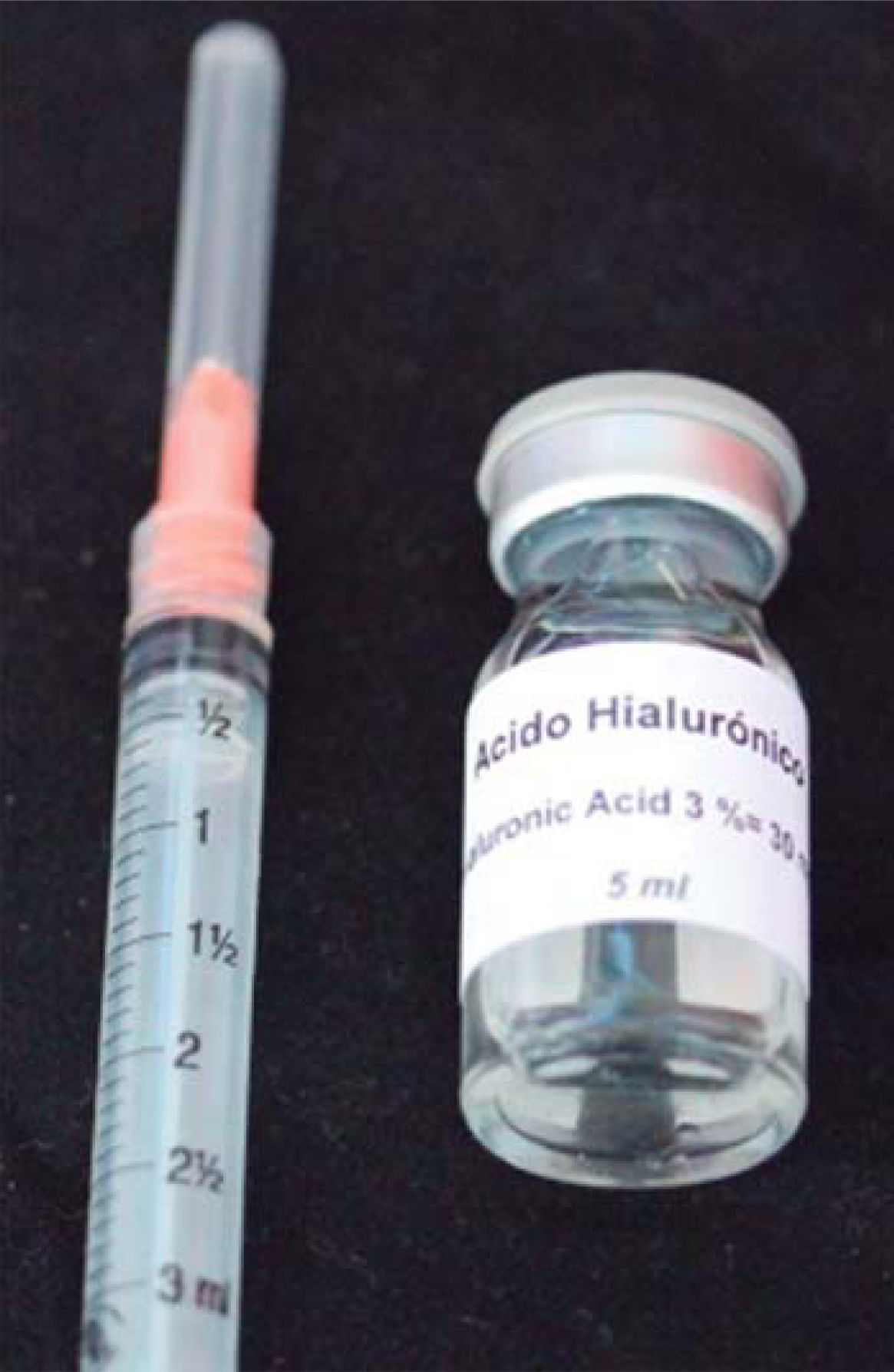
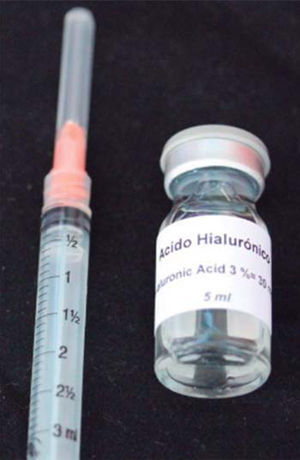
HA used in this clinical case was Vbiotek Mexico 3%. Vbiotek Mexico is prepared in Mexico and comes from Phentapharm Switzerland (Phentapharm Ltd, Engelgasse 109, PO Box CH-4002 Basel/Switzerland) where it is produced from bacterial genetic engineering (Streptococci). The product is received at the National Autonomous University of Mexico (UNAM) in powder form. In the laboratories of the Chemistry Faculty at this university, the powder is prepared into gel and is inserted in syringes or jars at different concentrations, under strict quality control and handling measures, to avoid product contamination.40
Characteristics of Vbiotek Mexico HA are:
- •
High molecular weight.
- •
6-7 pH.
- •
Hydration with distilled water.40
PH is stabilized by means of a Baker phosphate buffer, in laminar flow bell. It is later packaged and sterilized in autoclave, according to FDA standards, and thus remains sterile for one year (Figure 7).40
HA infiltration technique:
- •
Insulin syringe is used.
- •
The needle is inserted in perpendicular direction with respect to the tooth's long axis at the base of the papilla.
- •
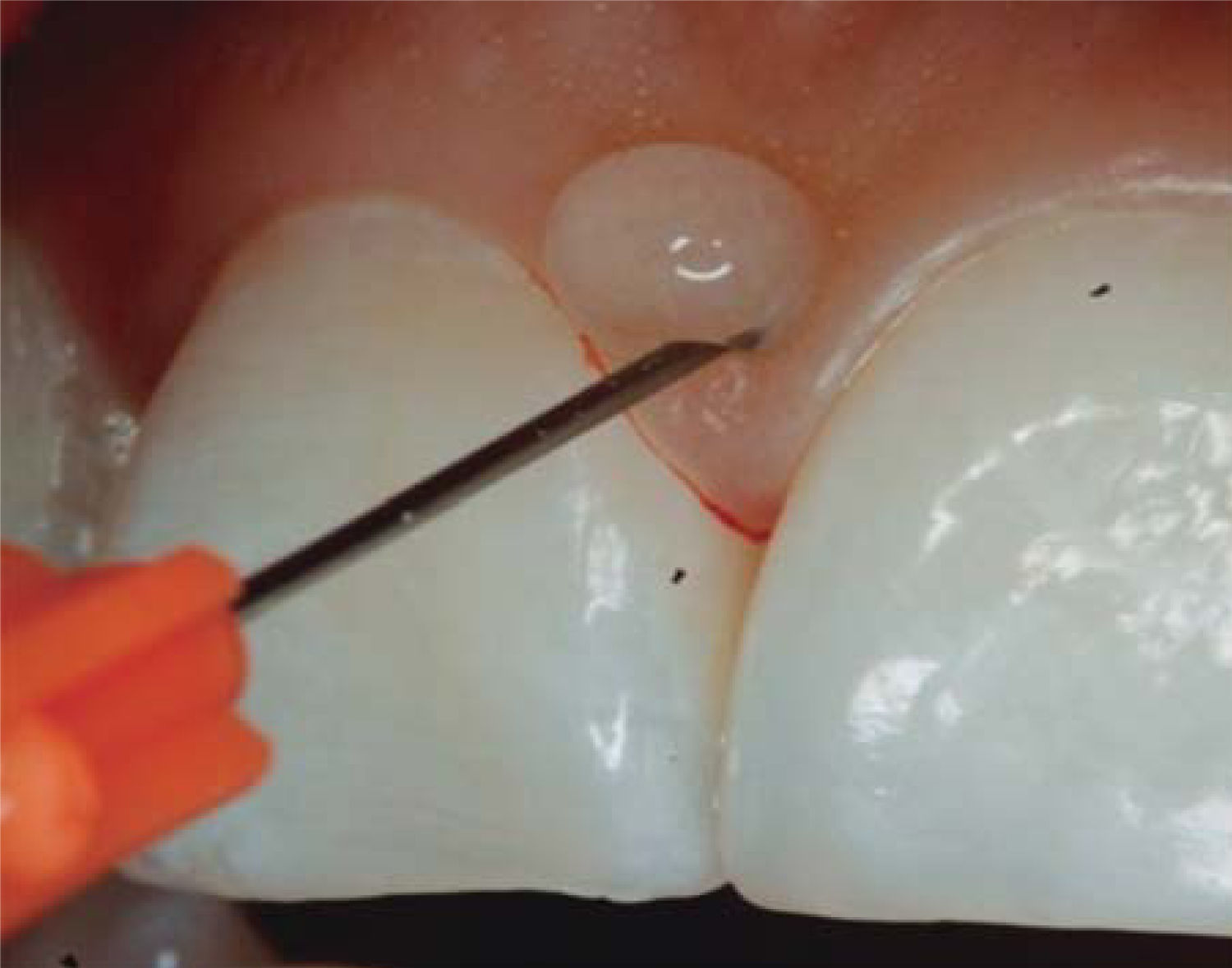
1mL HA is injected in the interdental papilla until ischemia is observed (Figure 8).
- •
The needle is placed at the point of the papilla and the same procedure is repeated.
- •
Seven days later, a second HA application is undertaken, and this procedure is repeated at same intervals until reaching four applications. In the present case, infiltrations were accomplished 7, 14 and 21 days after the initial infiltration. This is undertaken for two reasons: the wound inflicted into the gingival epithelium heals after seven days, according to histology and physiology of epithelial lable cells of the human body. Moreover, collagen synthesis is accomplished at 28 to 30 days.
- •
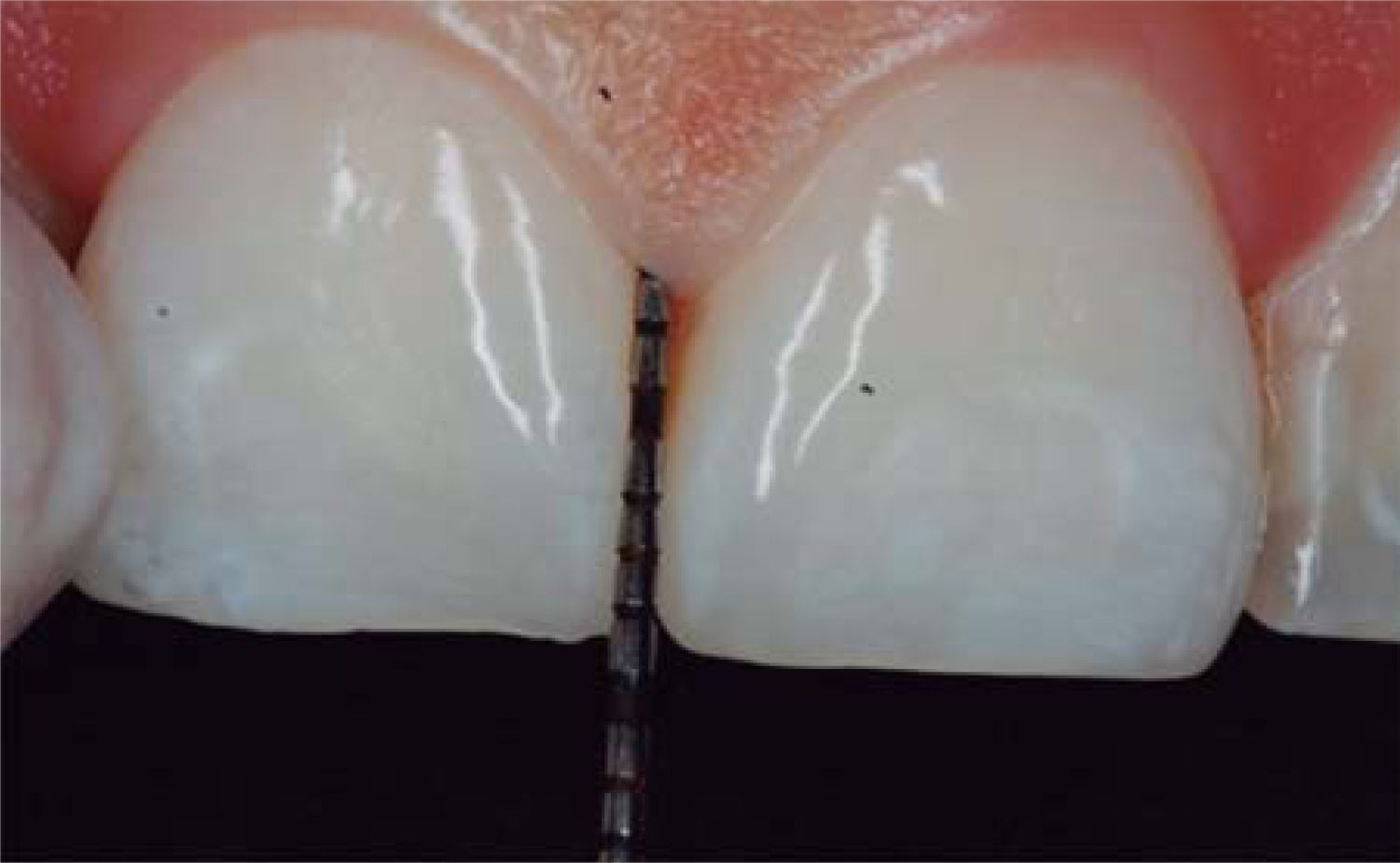
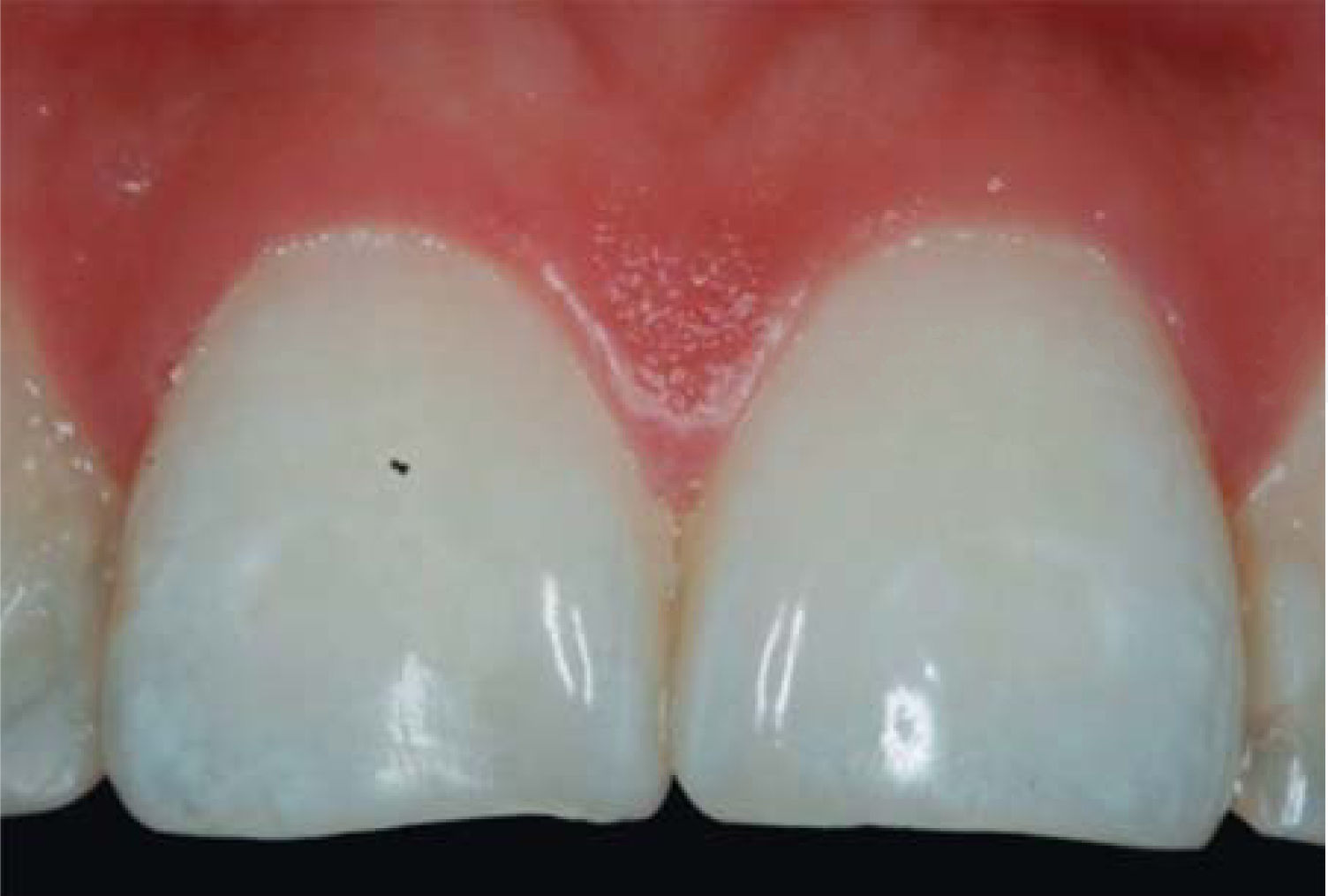
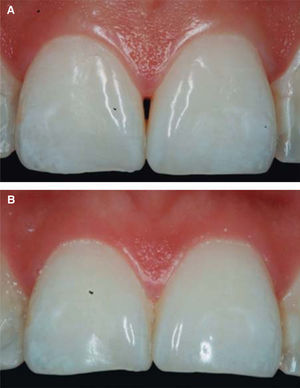
After achieving all four HA infiltrations, a clinical comparison was established taking Cardaropoli classification as a base. Figure 9 shows papilla evolution, covering all space underneath the interdental contact point without presence of black triangle.
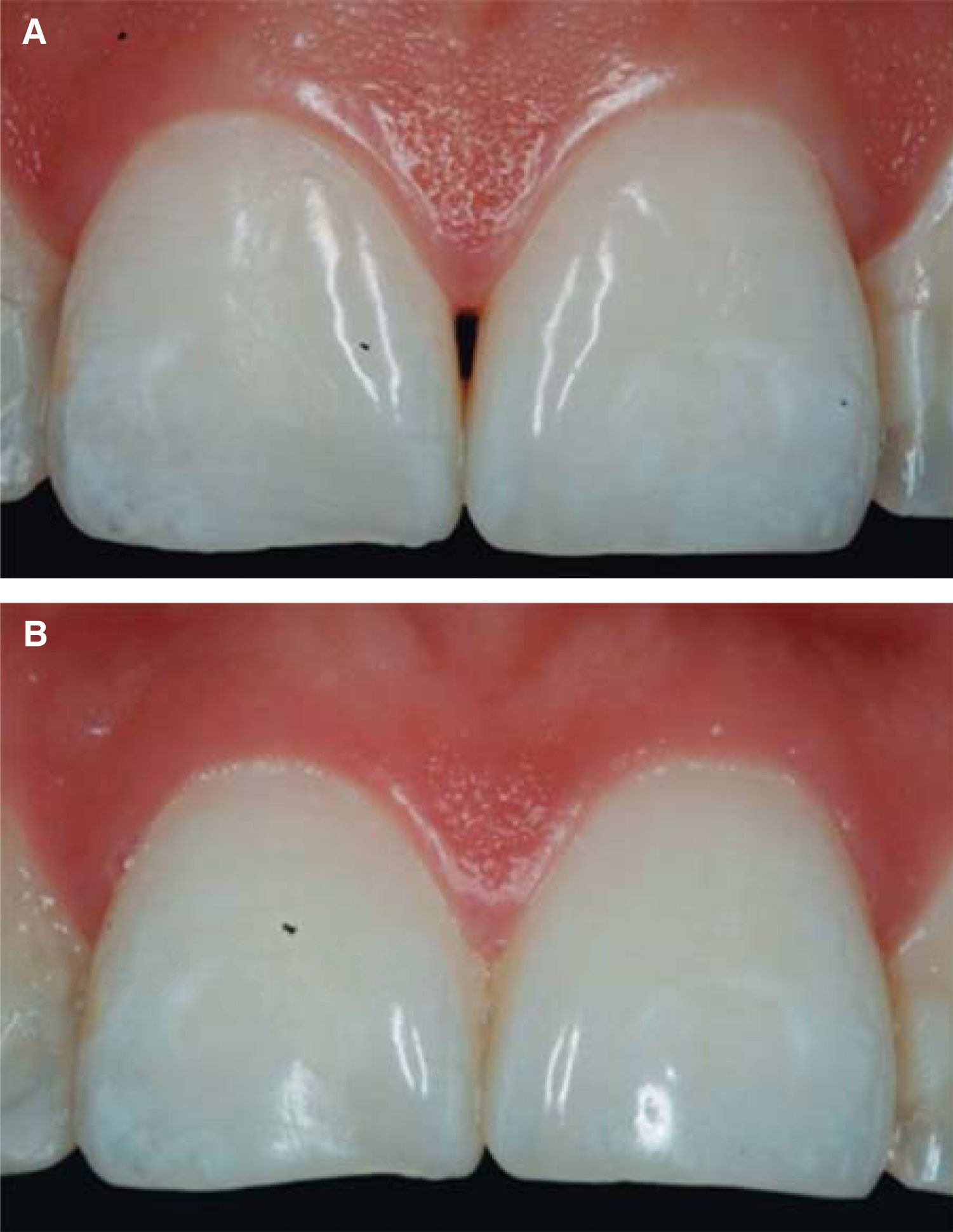
Upon clinical comparison, it was observed that interdental papilla (taking Cardaropoli classification as reference point) at the beginning of treatment did not fully cover interproximal space, that is to say, underneath the interdental contact point (black triangle). Moreover, it was not at the same height as adjacent papillae, but the cement-enamel junction still was not visible. Therefore, a diagnosis of IPP2 was emitted. Upon treatment completion it was observed that papilla covered all the space found underneath the interproximal contact point, and was found at the same height as adjacent papillae, so it was considered to be IPP1 (Figures 10A and 10B).
Results obtained in this clinical case using HA to regenerate papilla were favorable. From the clinical point of view it was observed that the papillae moved towards crown direction and covered all existing space underneath the interdental contact point, where the black triangle was no longer visible.
Results were predictable and successful due to diagnosis based on Nordland and Tarnow parameters. Using Cardaropoli classification, a clinical comparison could be observed in pre- and postreatment circumstances. It could be observed how interdental papilla increased, and the black triangle was no longer visible. Interdental papilla perfectly covered all available space underneath the contact point of upper central teeth.
DISCUSSIONDevelopment of further research is required on HA use to regenerate interdental papilla, since available studies have been conducted in small-sized populations.
Research should be furthered in larger populations, at different infiltration intervals: with subjects of different ethnicity, age, gender etc. Likewise, follow-up of cases should be undertaken along several years, in order to assess whether results are satisfactory and papilla is not again lost.
CONCLUSIONSThere is correlation between HA and interdental papilla growth as long as scientific-clinical bases are taken into account such as the Nordland and Tarnow studies. In them, it is explained that before undertaking interdental papilla reconstruction, vertical distance between bone crest and apical of crown contact area must be evaluated. As well as soft tissue height in interdental areas. When the distance between bone crest and contact point is 5mm or less, and papilla height does not exceed 4mm, a surgical intervention might be justified in order to increase papilla volume targeting resolution of the interdental black triangle problem. It is worth mentioning that the possibility arises of using a predictable, non-surgical technique with HA for interdental papilla regeneration, as long as all aforementioned parameters are used.
DDs, graduated from Faculty of Dentistry, National Autonomous University of Mexico UNAM.
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
Teacher at the Periodontics and Implantology Department, Graduate and Research School.
Research Seminar Professor, Implantology and Periodontics Department, Graduate and Research School, Professor, Professional Studies Division, National School of Dentistry, National University of Mexico.