To assess the prevalence of vitamin D deficiency and its associated factors in women and their newborns in the postpartum period.
MethodsThis cross-sectional study evaluated vitamin D deficiency/insufficiency in 226 women and their newborns in Viçosa (Minas Gerais, BR) between December 2011 and November 2012. Cord blood and venous maternal blood were collected to evaluate the following biochemical parameters: vitamin D, alkaline phosphatase, calcium, phosphorus and parathyroid hormone. Poisson regression analysis, with a confidence interval of 95%, was applied to assess vitamin D deficiency and its associated factors. Multiple linear regression analysis was performed to identify factors associated with 25(OH)D deficiency in the newborns and women from the study. The criteria for variable inclusion in the multiple linear regression model was the association with the dependent variable in the simple linear regression analysis, considering p<0.20. Significance level was α<5%.
ResultsFrom 226 women included, 200 (88.5%) were 20–44 years old; the median age was 28 years. Deficient/insufficient levels of vitamin D were found in 192 (85%) women and in 182 (80.5%) neonates. The maternal 25(OH)D and alkaline phosphatase levels were independently associated with vitamin D deficiency in infants.
ConclusionsThis study identified a high prevalence of vitamin D deficiency and insufficiency in women and newborns and the association between maternal nutritional status of vitamin D and their infants’ vitamin D status.
Avaliar a prevalência de deficiência de vitamina D e os fatores associados em mulheres e recém-nascidos no período pós-parto.
MétodosEstudo de delineamento transversal; avaliou-se a deficiência/insuficiência de vitamina D em 226 mulheres e seus recém-nascidos no município de Viçosa (MG), entre dezembro de 2011 e novembro de 2012. Coletaram-se 5mL de sangue do cordão umbilical e sangue venoso materno a fim de avaliar os parâmetros bioquímicos: vitamina D, fosfatase alcalina, cálcio, fósforo e paratormônio. Usou-se regressão de Poisson e adotou-se a Razão de Prevalência (95% IC), a fim de se avaliar a deficiência de vitamina D e fatores associados. Fez-se a análise de regressão linear múltipla para identificar os fatores associados à deficiência de 25(OH)D dos recém-nascidos e das mulheres do estudo. O critério para inclusão das variáveis na regressão linear múltipla foi a relação com a variável dependente na análise de regressão linear simples, considerando p<0,20. O nível de significância adotado foi α<5%.
ResultadosDas 226 mulheres, 200 (88,5%) tinham entre 20 e 44 anos; a mediana foi de 28. Encontrou-se prevalência de níveis deficientes/insuficientes de vitamina D em 192 (85%) mulheres e 182 (80,5%) recém-nascidos. A 25(OH)D materna e a fosfatase alcalina materna se comportaram como preditores independentes da deficiência de vitamina D dos recém-nascidos.
ConclusõesFoi possível identificar a alta prevalência de deficiência e insuficiência de vitamina D nas mulheres e recém-nascidos em nosso meio e a relação entre o estado nutricional materno de vitamina D e o do recém-nascido.
Maternal–fetal vitamin D deficiency (VDD) is currently a frequent morbidity. Lifestyles, environmental factors (inadequate sunlight exposure), lack of vitamin D (VD) supplementation for children and pregnant women and insufficient intake of that vitamin and/or calcium are responsible for the high prevalence of VDD in developed and developing countries.1–3
VDD in a pregnant woman and her newborn (NB) is closely associated.4 There is a greater transfer of 25(OH)D to the fetus via the placenta during the last months of pregnancy, which is the main source of this vitamin for infants during the first months of life.5,6 Additionally, the placenta contains VD receptors and produces the enzyme that converts 25(OH)D into its active form, thereby increasing VD levels for the fetus.5
In the first six to eight weeks of postnatal life, the NB's VD status depends on the VD acquired by placental transfer in the uterus, as demonstrated by its direct association with the levels found in maternal blood.6,7 In most NBs, VD stock acquired from the mother run out up to the eighth week of life.7
Studies carried in India, United States, Bangladesh, Korea and other parts of the world have shown that many children worldwide are born with low VD reserves as a result of high maternal VDD, with a high prevalence of deficiency/insufficiency of 25(OH)D, ranging from 22.3% to 73.6% and, therefore, at risk of rickets.1,8–11 Normal plasma levels of VD promote the absorption of 30% of dietary calcium and more than 60–80% during periods of growth, due to the high calcium demand. Therefore, during childhood, VDD can cause growth retardation and bone abnormalities, increasing the risk of fractures later in life.12 Additionally, low levels of 25(OH)D in cord blood were associated with increased risk of acute respiratory infections and wheezing in childhood.13
In most individuals, skin synthesis is the major source of VD and the remainder is obtained through diet and supplements.1 Risk factors for nutritional rickets are: latitude, use of clothing covering most of the body, time of sunlight exposure, increased skin pigmentation, diets high in vegetables and low in calcium, exclusive breastfeeding, use of sunscreens and lifestyle.14,15 VDD during pregnancy is a worldwide public health problem. Studies have reported VDD prevalence ranging from 18% to 84%, depending on the country and local clothing customs.1,4,8,9,13 However, to date, there has been no study on the prevalence of VDD in Brazil in newborns and women in the immediate postpartum period. Given the magnitude of this problem in the population, the aim of this study was to evaluate the prevalence of vitamin D deficiency and associated factors in women and their newborns in the postpartum period.
MethodThis was a cross-sectional study, approved by the International Review Board of Universidade Federal de Viçosa (n. 211/2011). A total of 226 women and their newborns were evaluated in the postpartum period, between December 2011 and November 2012.
Sample calculation was performed using EpiInfo software 7.0. For the calculation, we obtained the number of newborns living in the city in 2010, adding up 806 children. We used a prevalence of VDD estimated at 20%10 and 95% confidence level. To the number of 188 obtained at the sample size calculation, we added 20% for possible losses, resulting in 226 pairs of mothers and newborns.
Children born at the study hospital in Viçosa, with gestational age ≥37 weeks and whose mothers signed the informed consent form, were included in the study. Infants that were admitted to the Neonatal Intensive Care Unit, born with congenital malformations, syndromes and the result of twin pregnancies were excluded.
Obstetric and NB data (weight, length, head circumference, ethnicity) were obtained from the Obstetrics Book and Birth certificates (BC). Socioeconomic and prenatal care data (including sunlight exposure, use of sunscreens and multivitamin supplements) were obtained through a questionnaire applied to women after 30 days postpartum at the city's Polyclinic. The Brazilian Criteria for Economic Classification (ABEP) was used for socioeconomic classification, which takes into account the ownership of consumer goods and the family head's level of schooling.16
The skin color of the mother and their newborns was self-reported and confirmed in the BC by researchers when the questionnaire was applied, and it was categorized as Caucasian or non-Caucasian. The sample was divided into two groups according to the time of sunlight exposure during pregnancy: adequate (>60min/week) or inadequate (≤60min/week).17
Plasma levels of 25(OH)D, alkaline phosphatase (AP), parathyroid hormone (PTH), calcium (Ca) and phosphorus (P) were evaluated in the women and their newborns in the postpartum period, by collecting 5mL of cord blood and 5mL of maternal venous blood for biochemical in Vacuette® gel tubes. Serum was separated in a refrigerated centrifuge, divided into aliquots and stored at −20°C until the analyses were performed. Ca was measured by colorimetric endpoint Calcium Arsenazo III,18 and AP by Kinetic–IFCC method (Bioclin).18 25(OH)D levels were measured by Liaison® competitive chemiluminescence immunoassay (CLIA-DiaSorin),19 and PTH in a Beckman immunochemiluminometric assay by Coulter®.18p was obtained by UV endpoint.18
The sample was divided into two groups according to the VD levels of newborns and the women: VD sufficiency and non-sufficiency (VD insufficiency and deficiency). VDD for women and children was defined as 25(OH)D<20ng/mL; the insufficiency of 25(OH)D between ≥20 and <30ng/mL, and sufficiency as 25(OH)D levels ≥30ng/mL.10 The cutoff for high PTH was 46pg/mL; for hypocalcemia, plasma calcium levels <9mg/dL for children and <8.8mg/dL for the women; AP was considered high when ≥375U/L for infants and 100U/L for the women.9
The analysis was performed using the IBM® SPSS® software, release 20.0 for Windows (SPSS, Chicago, IL, USA) and STATA version 9.1 (Stata Corp., College Station, TX, USA). The Kolmogorov–Smirnov test was used to assess the normality of the quantitative variables. Categorical variables were analyzed using Poisson regression and as magnitude measure, we used the prevalence ratio (PR) and their respective 95% confidence intervals (95% CI) to evaluate non-sufficiency of VD. Spearman's correlation was calculated between 25(OH)D and the variables AP, PTH, Ca and P of the women and newborns. Mann–Whitney test was used to compare the levels of Ca, PTH, 25(OH)D, P and AP between the women and infants with VD sufficiency and non-sufficiency.
Multiple linear regression analysis was performed to identify factors associated with 25(OH)D deficiency in the newborns and the women participating in the study. The criterion defined for inclusion of variables in the multiple linear regression was the association with the dependent variable at the simple linear regression analysis, considering a value of p<0.20. Statistical significance level was set at α<5%.
ResultsOf the 226 mothers and their newborns assessed in the study, 200 women (88.5%) were aged between 20 and 44 years (mean 28 years), and 26 (11.5%) were adolescents. Regarding ethnicity, 118 (52.2%) women reported being Caucasian. It is noteworthy that 62 (43.5%) participants had 8 years or less of schooling, 192 (85%) lived in the urban area and 176 (77.9%) lived with a partner. It was observed that 152 (67.3%) had more than one child. Regarding the type of delivery, 179 (79.2%) underwent a cesarean section. During pregnancy, 142 (97.3%) women reported taking a multivitamin supplement, 79 (54.1%) did not meet the sun exposure recommendations and 91 (40.3%) reported not using sunscreen. There was no association (p>0.05) between the levels of 25(OH)D identified in the women and age, ethnicity, schooling, marital status, parity, place of residence, type of delivery, vitamin supplement intake, sunlight exposure or use of sunscreens.
Of the newborns, 117 (51.8%) were females and 119 (52.7%) were Caucasian. The infants’ mean gestational age and weight were 38.7±1.0 weeks and 3248±446g, respectively; the infants’ length was 48.8±2.1cm, and head circumference was 33.9±1.5cm. There was no association between the infants’ levels of 25(OH)D and gender, ethnicity, season of the year, weight, length and head circumference or gestational age.
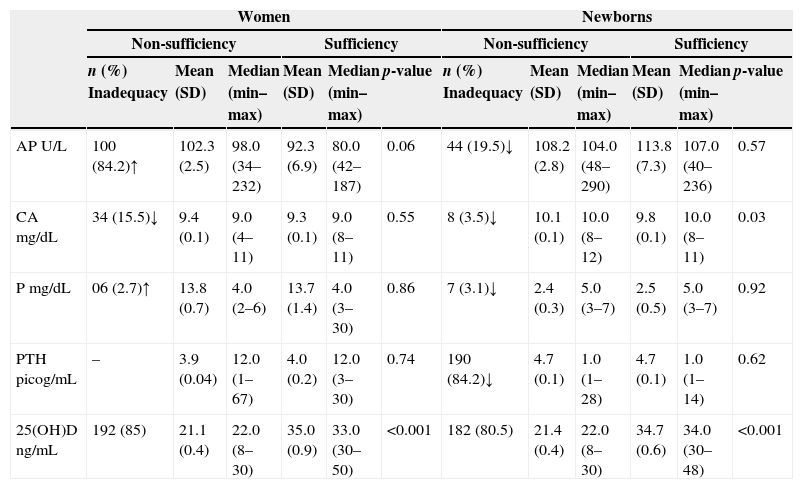
There was no difference in the levels of Ca, AP, P and PTH between the women with VD sufficiency and non-sufficiency (Table 1). However, there were differences in plasma levels between newborns with VD sufficiency and non-sufficiency (p<0.001) and calcium levels (p<0.03). There was no difference regarding the levels of AP, P and PTH between infants with VD sufficiency and non-sufficiency (Table 1).
Biochemical parameters of women and their newborns classified according to the sufficiency or not of 25(OH)D in the postpartum period.
| Women | Newborns | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-sufficiency | Sufficiency | Non-sufficiency | Sufficiency | |||||||||
| n (%) Inadequacy | Mean (SD) | Median (min–max) | Mean (SD) | Median (min–max) | p-value | n (%) Inadequacy | Mean (SD) | Median (min–max) | Mean (SD) | Median (min–max) | p-value | |
| AP U/L | 100 (84.2)↑ | 102.3 (2.5) | 98.0 (34–232) | 92.3 (6.9) | 80.0 (42–187) | 0.06 | 44 (19.5)↓ | 108.2 (2.8) | 104.0 (48–290) | 113.8 (7.3) | 107.0 (40–236) | 0.57 |
| CA mg/dL | 34 (15.5)↓ | 9.4 (0.1) | 9.0 (4–11) | 9.3 (0.1) | 9.0 (8–11) | 0.55 | 8 (3.5)↓ | 10.1 (0.1) | 10.0 (8–12) | 9.8 (0.1) | 10.0 (8–11) | 0.03 |
| P mg/dL | 06 (2.7)↑ | 13.8 (0.7) | 4.0 (2–6) | 13.7 (1.4) | 4.0 (3–30) | 0.86 | 7 (3.1)↓ | 2.4 (0.3) | 5.0 (3–7) | 2.5 (0.5) | 5.0 (3–7) | 0.92 |
| PTH picog/mL | – | 3.9 (0.04) | 12.0 (1–67) | 4.0 (0.2) | 12.0 (3–30) | 0.74 | 190 (84.2)↓ | 4.7 (0.1) | 1.0 (1–28) | 4.7 (0.1) | 1.0 (1–14) | 0.62 |
| 25(OH)D ng/mL | 192 (85) | 21.1 (0.4) | 22.0 (8–30) | 35.0 (0.9) | 33.0 (30–50) | <0.001 | 182 (80.5) | 21.4 (0.4) | 22.0 (8–30) | 34.7 (0.6) | 34.0 (30–48) | <0.001 |
AP, alkaline phosphatase; CA, calcium; P, phosphorus; PTH, parathyroid hormone; ↑, plasma levels above the reference values; ↓, plasma levels below the reference values.
VDD occurred in 61 women (27%) and 66 newborns (29.2%); 131 women (58%) and 116 infants (51.3%) had VD insufficiency, and only 34 women (15%) and 44 (19.5%) newborns showed VD sufficiency. Severe VDD (VD levels≤0ng/mL) was observed in 15 women (11.9%) and 4 newborns (1.8%).
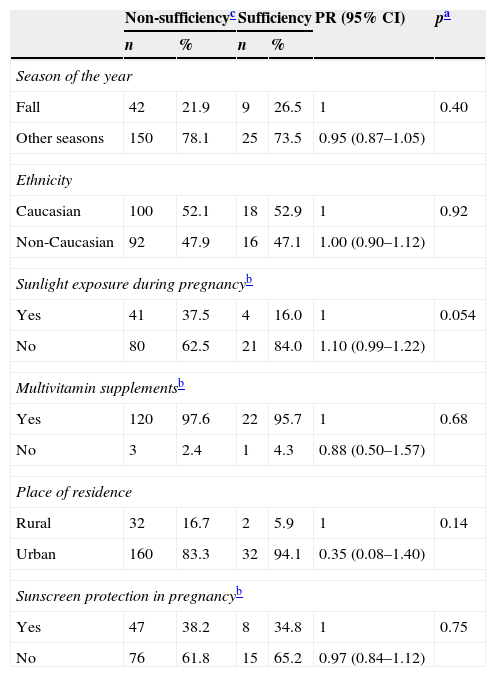
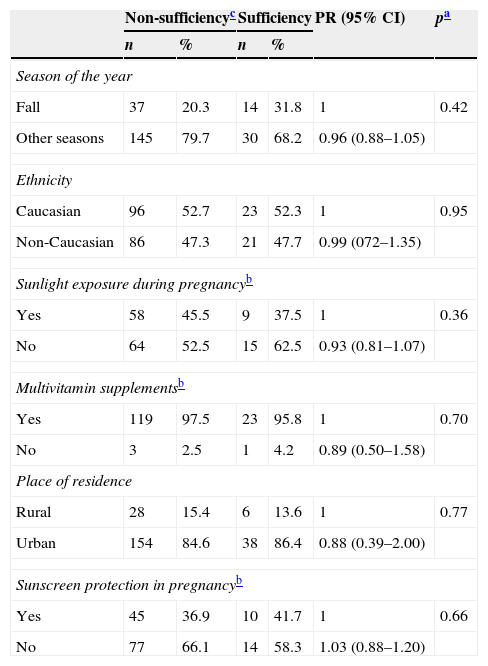
There was no association between VD non-sufficiency in women or the newborns and season of the year, ethnicity, place of residence, sunlight exposure, use of multivitamin supplement and sunscreen use (Tables 2 and 3).
Possible factors associated with non-sufficiency of vitamin D in women in the postpartum period.
| Non-sufficiencyc | Sufficiency | PR (95% CI) | pa | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Season of the year | ||||||
| Fall | 42 | 21.9 | 9 | 26.5 | 1 | 0.40 |
| Other seasons | 150 | 78.1 | 25 | 73.5 | 0.95 (0.87–1.05) | |
| Ethnicity | ||||||
| Caucasian | 100 | 52.1 | 18 | 52.9 | 1 | 0.92 |
| Non-Caucasian | 92 | 47.9 | 16 | 47.1 | 1.00 (0.90–1.12) | |
| Sunlight exposure during pregnancyb | ||||||
| Yes | 41 | 37.5 | 4 | 16.0 | 1 | 0.054 |
| No | 80 | 62.5 | 21 | 84.0 | 1.10 (0.99–1.22) | |
| Multivitamin supplementsb | ||||||
| Yes | 120 | 97.6 | 22 | 95.7 | 1 | 0.68 |
| No | 3 | 2.4 | 1 | 4.3 | 0.88 (0.50–1.57) | |
| Place of residence | ||||||
| Rural | 32 | 16.7 | 2 | 5.9 | 1 | 0.14 |
| Urban | 160 | 83.3 | 32 | 94.1 | 0.35 (0.08–1.40) | |
| Sunscreen protection in pregnancyb | ||||||
| Yes | 47 | 38.2 | 8 | 34.8 | 1 | 0.75 |
| No | 76 | 61.8 | 15 | 65.2 | 0.97 (0.84–1.12) | |
PR prevalence ratio; 95% CI, confidence interval.
Possible factors associated with non-sufficiency of vitamin D in the newborns.
| Non-sufficiencyc | Sufficiency | PR (95% CI) | pa | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Season of the year | ||||||
| Fall | 37 | 20.3 | 14 | 31.8 | 1 | 0.42 |
| Other seasons | 145 | 79.7 | 30 | 68.2 | 0.96 (0.88–1.05) | |
| Ethnicity | ||||||
| Caucasian | 96 | 52.7 | 23 | 52.3 | 1 | 0.95 |
| Non-Caucasian | 86 | 47.3 | 21 | 47.7 | 0.99 (072–1.35) | |
| Sunlight exposure during pregnancyb | ||||||
| Yes | 58 | 45.5 | 9 | 37.5 | 1 | 0.36 |
| No | 64 | 52.5 | 15 | 62.5 | 0.93 (0.81–1.07) | |
| Multivitamin supplementsb | ||||||
| Yes | 119 | 97.5 | 23 | 95.8 | 1 | 0.70 |
| No | 3 | 2.5 | 1 | 4.2 | 0.89 (0.50–1.58) | |
| Place of residence | ||||||
| Rural | 28 | 15.4 | 6 | 13.6 | 1 | 0.77 |
| Urban | 154 | 84.6 | 38 | 86.4 | 0.88 (0.39–2.00) | |
| Sunscreen protection in pregnancyb | ||||||
| Yes | 45 | 36.9 | 10 | 41.7 | 1 | 0.66 |
| No | 77 | 66.1 | 14 | 58.3 | 1.03 (0.88–1.20) | |
PR prevalence ratio; 95% CI, confidence interval.
When performing the multiple regression analysis to estimate the influence of obstetric, biological and socioeconomic variables in relation to the mothers’ VDD, variables with p≤0.20 were included in the model: maternal schooling (p=0.02), place of residence (p=0.11), sunlight exposure (p=0.16) and ABEP classification (p=0.04). In the final model, there was no statistically significant association of these variables with maternal VDD.
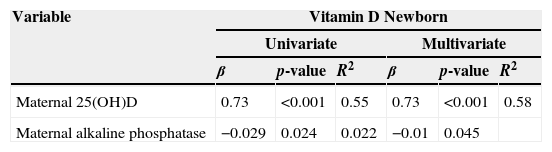
When performing the multivariate regression to estimate the influence of these variables in relation to the newborns’ VDD, variables with p≤0.20 were included in the model: season of birth (p=0.06), NB ethnicity (p=0.19), maternal 25(OH)D (p<0.001) NB Ca (p=0.15) and maternal alkaline phosphatase (p=0.0). Maternal levels of 25(OH)D and alkaline phosphatase behaved as independent predictors of VDD in the newborns (Table 4). This model accounted for 58% of the variation in VD levels of the newborns (Table 4).
Multiple regression analysis for predictors of deficiency of 25(OH)D (ng/mL) in the newborns.
| Variable | Vitamin D Newborn | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| β | p-value | R2 | β | p-value | R2 | |
| Maternal 25(OH)D | 0.73 | <0.001 | 0.55 | 0.73 | <0.001 | 0.58 |
| Maternal alkaline phosphatase | −0.029 | 0.024 | 0.022 | −0.01 | 0.045 | |
β, coefficient of regression; R2, univariate and multivariate linear regression; p<0.05.
When using Spearman's correlation, we observed an association between PTH and 25(OH)D of the newborns (r=−0.142; p=0.033; R2=0.006), with a strong association between maternal and newborn levels of 25(OH)D (r=0.73; p<0.0001; R2=0.558).
DiscussionVitamin D deficiency is a worldwide public health problem and Brazil is part of this scenario, with a high prevalence of VDD in the population, as demonstrated by studies at different age groups.20 However, no studies have evaluated maternal and NB VDD as the present study. We found a high prevalence of VD insufficiency, of 80.5% and 85% in newborns and women, respectively, although 97.3% of the women reported the use of multivitamin supplements during pregnancy. In India, 66.7% of VDD was found in the children and 81.1% in the mothers. In Korea, 18% of the infants had VDD and 73.6% had VD insufficiency at birth. In the USA, rates of 22.3% of VDD and 73.6% of VD insufficiency were identified in 290 babies, and VD insufficiency was also identified in several countries.8–11,13,21 Reports in the literature have shown that the lack of or inadequate exposure to sunlight without adequate corrective VD intake or vitamin supplements can explain the high VDD prevalence in women.1,8
Exposure to sunlight is the main source of VD and, at best, only 10% of the body's reserves are provided by dietary intake.1 There was no association between VDD in women and sunlight exposure recommendations. The exposure recommendations for obtaining VD take into account factors such as UVB radiation, exposure duration and exposed body area, skin pigmentation, sunscreen use, time of the day of exposure, season, latitude, altitude and air pollution.2
An increase in the sun's zenith angle during the winter and in the early morning and late afternoon results in a longer path for UVB rays to go through the ozone layer and be absorbed. This explains why, above and below 33° of latitude, little or no VD is produced in the skin during winter. This also explains why the synthesis and greater absorption of VD occur only between 10am and 3pm at the equator or in places closer to it. Thus, sunlight exposure of arms and legs for 5–30min, twice a week, from 10am to 3pm, is adequate.17
The Brazilian Society of Pediatrics advises that children younger than 6 months should be exposed to direct sunlight from the 2nd week of life and that 30min/week wearing only diapers (6–8min a day, 3 times a week) or 2h/week with partial exposure (17min a day), exposing the face and hands, are enough.22 The Brazilian Recommendations of Endocrinology and Metabolism advise VD supplementation of 600IU/day for pregnant women and 400IU/day for children, from birth to the first year of life, as they belong to the risk group for VDD.20,23,24
Dark-skinned individuals have difficulty to produce VD.25 There is a greater affinity of melanin, as the melanocytes are more active and easily absorb UVB rays, which hinders the conversion of 7-dehydrocholesterol molecule into VD. A dark-skinned individual requires 10–50 times more sunlight exposure to produce the same amount of VD in comparison to light-skinned individuals.25 However, in this study, skin color showed no association with VD non-sufficiency; similar results were found in studies carried out in Greece and the USA.10,21 Regarding the use of sunscreen, a protection factor (SPF) of 15 reduces VD production capacity by more than 98%,26 although most of the women in the study with VD non-sufficiency reported they had not used any type of sunscreen during pregnancy.
There was an association between 25(OH)D levels of the women and the newborns. There is a strong association of circulating VD levels between the mother and the fetus, so that maternal VDD reflects in neonatal VDD,8,13 exposing children to the risk of rickets.1 In pregnancy, the low status or low intake of VD is detrimental to both the mother and the fetus and predisposes to VD deficiency in infancy.1 VDD in childhood has been associated with increased risk of lower respiratory tract infections, while low VD plasma levels in cord blood were associated with increased risk of acute respiratory infections and wheezing in childhood.13
It is noteworthy that 44.2% (100) of the women in this study had high AP levels, which suggests osteoblastic activity or bone mobilization of Ca and P to meet the high requirements of the fetus. In the presence of mineral deficiency, there is increased AP synthesis.27
There were differences in the levels of 25(OH)D of newborns with non-sufficiency and sufficiency of VD and calcium. In the diagnosis of rickets, some of the following tests are altered: serum CA, P, PTH, 25(OH)D or AP.28 This is characterized as an important result, as 66 infants had VDD, four had severe 25(OH)D deficiency, with VD levels ≤10ng/mL and eight had hypocalcemia. Severe hypocalcemia with or without seizures is a common complication of VDD in the neonatal period or early childhood, due to the maternal VDD, along with inadequate intake of VD from breast milk or supplements. This condition can be prevented through adequate levels of maternal VD during pregnancy and supplementation in infancy.1 In this study, PTH levels in cord blood of 84.2% of the infants were below the normal level. It is suggested that this fact is a consequence of high Ca concentrations that exist during this period (supplied by maternal Ca),5,29 without the need to stimulate PTH function of the newborns as a secondary mechanism of Ca and P mobilization from bone, which are, therefore, physiological mechanisms.
This study has limitations due to its cross-sectional design and also because some risk factors, such as sunscreen use, use of multivitamin supplements and amount of time of sun exposure during pregnancy can generate recall bias, as they are self-reported information. However, the results obtained in this study can be used in other studies and in other populations, especially in Latin America, where they are scarce, as the assessed sample consisted of randomly selected individuals from the target population, so that the sample was representative of the population. Moreover, VDD is a worldwide problem throughout the life cycle. It is suggested that further studies be carried out to better understand the determinants of VD levels in other population groups.
Although Brazil is a tropical country, where VDD is less likely to occur than in countries located far from the equator, a high prevalence of VD non-sufficiency was identified in this population of women and newborns, which can be prevented by providing guidelines for women during prenatal care and the postpartum period in relation to sun exposure and VD supplementation. It was found that the risk factors that contributed to the low levels of VD in newborns were maternal 25(OH)D and AP levels. Other risk factors, namely season, ethnicity, place of residence, sun exposure, use of multivitamin supplements and use of sunscreen, were not associated with VDD in women and newborns. Therefore, the importance of assessing the nutritional status of VD during mother and child routine care is noteworthy.
FundingFAPEMIG – Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Process n. APQ 00846-11-Edital 01/2011 – Demanda Universal.
Conflicts of interestThe authors declare no conflicts of interest.