To study the breastfeeding history (BF) and the anthropometric status of children with Sickle Cell Disease (SCD).
MethodsA cross-sectional study of 357 children with SCD aged between 2 and 6 years, regularly followed at a Newborn Screening Reference Service (NSRS) between November 2007 and January 2009. The outcome was anthropometric status and the exposures were: BF pattern, type of hemoglobinopathy and child's age and gender.
ResultsThe mean (SD) age was 3.7 (1.1) years, 52.9% were boys and 53.5% had SCA (hemoglobin SS). The prevalence of exclusive breastfeeding (EBR) up to six months of age was 31.5%, the median EBR times (p25-p75) was 90.0 (24.0-180.0) days and the median weaning ages (p25-p75) was 360.0 (90.0-720.0) days respectively. Normal W/H children experienced EBR for a mean duration almost four times longer than malnourished children (p=0.01), and were weaned later (p<0.05). Height deficit was found in 5.0% of children, while all the children with severe short stature had had SCA (hemoglobin SS) and were older than 4 years of age.
ConclusionsEBF time and weaning age were greater than that found in the literature, which is a possible effect of the multidisciplinary follow-up. Duration of EBF and later weaning were associated with improved anthropometric indicators.
Descrever a história de aleitamento materno (AM) e estado antropométrico de crianças com Doença Falciforme (DF).
MétodosEstudo transversal com 357 crianças com hemoglobinopatias SS e SC de 2-6 anos de idade, acompanhadas regularmente num Serviço de Referência em Triagem Neonatal (SRTN) entre novembro de 2007 e janeiro de 2009. O desfecho correspondeu ao estado antropométrico e as exposições foram: padrão do AM, tipo de hemoglobinopatia, faixa etária e sexo da criança.
ResultadosA média (DP) de idade observada foi de 3,7 (1,1) anos, sendo 52,9% meninos e 53,5% com hemoglobinopatia SS. A prevalência de aleitamento materno exclusivo (AME) até o 6º mês foi 31,5% a mediana (p25–p75) do tempo de AME foi de 90,0 (24,0-180,0) dias e a mediana (p25–p75) da idade de desmame foi de 360,0 (90,0-720,0) dias. Crianças eutróficas em relação ao P/A tiveram o tempo de AME, em média, quase quatro vezes maior que os desnutridos (p<0,01), bem como foram desmamadas mais tarde (p<0,05). O déficit de altura foi encontrado em 5,0% das crianças e todas as crianças com baixa estatura grave tinham hemoglobinopatia SS e mais de 4 anos de idade.
ConclusõesO tempo de AME e a idade de desmame foram superiores aos encontrados na literatura, possível efeito do acompanhamento multidisciplinar. A duração do AME e a idade mais tardia de desmame foram associadas a melhores indicadores antropométricos.
The protective effect of breast milk (BM) on children's health is a consensus in the literature, and it is considered a universal source of nutrition for young infants, contributing significantly to energy and micronutrient intake in the first year of life.1 Furthermore, many studies confirm the superiority of breastfeeding (BF) in conferring protection against several diseases2,3 and reducing the frequency of hospitalizations for pneumonias4 and diarrhea,5 pointing to the need for incentive and integrated support.
A study carried out in Latin America found that 13.9% of child deaths, secondary to several causes, could be prevented by the practice of exclusive breastfeeding (EBF) until three months of age and partial breastfeeding throughout the first year of life. Among infants younger than three months, 55% of deaths from diarrheal diseases and respiratory infections would be prevented.6
In chronic diseases, protection against preventable infections and nutritional status have considerable impact on the reduction of morbidity and mortality,7 and considering the benefits of breastfeeding, one may suggest its relevant importance for children with Sickle Cell Disease (SCD), which shows great phenotypic variety and is mainly characterized by vaso-occlusive phenomena, hemolytic anemia and increased risk of infections. It is known that social and environmental conditions, personal care, antibiotic prophylaxis, vaccination, access to health care services, hydration and proper nutrition have a strong influence on the clinical course of the individual with SCD.8
In Brazil, SCD is considered a public health problem, and Bahia is the state with the highest occurrence of SCD, with 1:565 live births in 2009.9 Furthermore, Salvador, the state capital, has a low percentage of EBF, around 9.4% of children younger than six months,10 a scenario that can contribute to increased morbidity and mortality. Moreover, according to data from the Second National Survey on Breastfeeding Prevalence, carried out in 2008, only 37.0% of children younger than six months from northeastern capital cities benefited from EBF, a lower prevalence than that observed in the North, Central-West, South and Southeast regions, whose prevalence rates were, respectively, 45.9%, 45.0%, 43.9% and 39.4%. Additionally, even with the increase in the median time of BF, which went from 296.0 days in 1999 to 342.0 days in 2008,11 this period is still much shorter than what is recommended: EBF up to six months (180 days) of life, and complemented BF up to two years or more.12,13
Thus, considering the nutritional aspects and the possible impact of BF on the course of SCD, this study aimed to describe the history of BF and anthropometric data of children with SCD, with early diagnosis obtained by neonatal screening, followed at a Neonatal Screening Reference Service (NSRS) in a state with high incidence of hemoglobinopathies.
MethodThis is a cross-sectional study carried out between November 2007 and January 2009 in children with Sickle Cell Anemia and Hemoglobin SC disease, regularly monitored at a NSRS in the state of Bahia, aged two to six years and of both genders. The outcome corresponded to the anthropometric status and the assessed exposures were the BF pattern (EBF duration and total time of BF), type of hemoglobinopathy (SS or SC), age and gender of the child.
All children aged between two and six years, with a diagnosis of Sickle Cell Anemia and Hemoglobin SC disease, regularly followed at the NSRS (who had at least two consultations during the year before the study period) were included. Patients whose parents or legal guardians did not sign the informed consent were excluded. Two children were excluded due to lack of anthropometric data, and another one was excluded only from the breastfeeding analyses, as this information was not available.
Weight and height were measured in all consultations and anthropometric assessment indicators recommended by the WHO were calculated:14 height for age (H/A) and weight for height (W/H), obtained using the Anthro® program (WHO Anthro, version 3.2.2, January 2011, for children aged between 2 and <5) and Anthro Plus® (WHO AnthroPlus software, version 1.0.4, 2011, for children aged five to six years). Weight was measured with the child without clothes, using an electronic scale (WELMY®, model W200/5, SP, Brazil, with an accuracy of 50 g and a maximum capacity of 200 kg), and height was measured using the anthropometer of the scale with a precision of 0.5 cm and maximum of 200 cm, according to the recommended standards. The BF history was reported by the mother/caregiver, using a standardized questionnaire administered by a previously trained interviewer after the research was explained and the informed consent was obtained.
The classification of nutritional status was based on weight for height (W/H) and height for age (H/A), expressed in Z-scores for all ages, according to WHO recommendations.15 The indicator H/A<–3 z-scores was considered severe short stature; between –3 and –2, short stature, and H/A>–2 z scores were classified as adequate height for age. As for the indicator W/H, values below –3 z-scores were classified as severe malnutrition; between –3 and –2, malnutrition; between –2 and +1, normal weight; between +1 and +2, risk for overweight; between +2 and +3, overweight, and above +3 z-scores as obesity. Subsequently, the W/H index was reclassified as malnutrition (present/absent) and overweight (present/absent), according to the previous definitions.
EBF was defined as a situation in which the child received only breast milk directly from the breast or expressed, with no other liquids or solids except for drops or syrups containing vitamins, mineral supplements or medications. Weaning was considered when there was no more offer of breast milk or when it was completely removed from the child's diet. The breastfeeding duration and age at weaning were expressed in days.
The BF pattern was described using measures of central tendency and dispersion, and the percentage of children that maintained EBF at the reported 90 and 180 days. Comparison of BF patterns between the types of hemoglobinopathies, gender, age and anthropometric status was performed using the nonparametric Mann-Whitney test, given the non-normal distribution for the variables ‘time of EBF’ and ‘age at weaning’. The frequency of anthropometric alterations was described in percentages and compared in relation to gender, age and type of hemoglobinopathy with the chi-square test, and for the time of EBF and age at weaning, with the Mann-Whitney or Kruskal-Wallis test. Data were analyzed using SPSS™ software (SPSS Statistics for Windows, Version 17.0. Chicago, USA). Statistical significance was considered when p<0.05.
The study was approved by the Ethics Committee on Human Research of Centro de Pesquisas Gonçalo Moniz, Fundação Oswaldo Cruz-Bahia (CEP-CPqGM/FIOCRUZ), protocol number 112/2006, and followed the ethical standards in research stated in resolution 196/1996 and complementary ones of the National Health Council, and the Declaration of Helsinki, 2008.
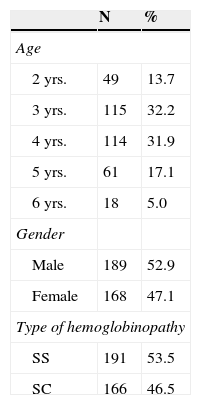
ResultsA total of 357 children between two and six years were studied, with a mean (SD) age of 3.7 (1.1) years, of which 45.9% (164/357) were younger than four years, 52.9% (189/357) were boys and with a higher frequency (191/357) of sickle cell anemia (SCA) (53.5%), as described in Table 1.
Demographic and clinical characteristics of 357 children with hemoglobinopathies SS and SC treated and monitored in the NSRS from 2007–2009.
| N | % | |
|---|---|---|
| Age | ||
| 2 yrs. | 49 | 13.7 |
| 3 yrs. | 115 | 32.2 |
| 4 yrs. | 114 | 31.9 |
| 5 yrs. | 61 | 17.1 |
| 6 yrs. | 18 | 5.0 |
| Gender | ||
| Male | 189 | 52.9 |
| Female | 168 | 47.1 |
| Type of hemoglobinopathy | ||
| SS | 191 | 53.5 |
| SC | 166 | 46.5 |
EBF until the 3rd and 6th months of life was found, respectively, in 52.3% (186/356) and 31.5% (112/356) of the children studied. The median (p25-p75) of EBF was 90.0 (24.0 to 180.0) days, and the median (p25-p75) age at weaning was 360.0 (90.0 to 720.0) days.
There was no significant variation in EBF duration according to hemoglobinopathy type, with a mean (SD) of 102.0 (91.0) days in children with hemoglobin SC disease and 93.0 (72.0) days in children with SCA. However, the mean (SD) age at weaning showed that children with hemoglobin SC disease were breastfed, on average, almost two months longer than children with SS [460.1 (392.8) versus 406.3 (371.3) days], although it was not statistically significant (p=0.18).
Regarding the W/H indicator, 2.2% (8/357) of children suffered from malnutrition, with higher prevalence among children with SCA, although non-significant [3.1% (6/191) versus 1.2% (2/166); p=0.39]. The W/H indicator deficit was more frequent, but non-significant (p=0.11), between the ages of four and six years, with a prevalence of 3.6% (7/193), when compared to children aged between two and four years, with 2.0% (1/49) of malnutrition. The opposite was observed for the high weight for height, which was observed in 16.3% (8/49) of patients aged two to three years, 3.5% (4/115) between three and four, and 5.2% (10/193) after four years of age (pfor trend=0.04). The frequency of excess weight observed was eight times higher than that of malnutrition in SC children (p=0.02), being, respectively, 9.6% (16/166) and 1.2% (2/166). In the SCA group, there was no difference for the type of inadequacy [3.1% (6/191) with high weight and 3.1% (6/191) diagnosed with malnutrition].
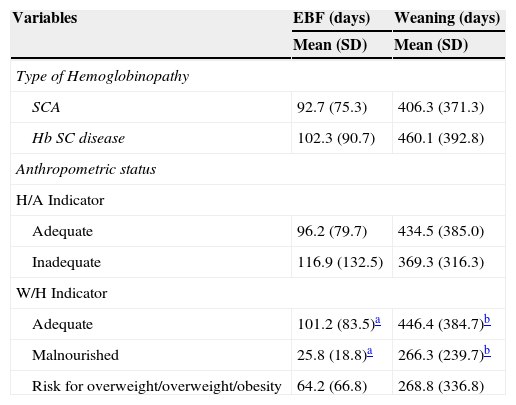
Table 2 shows that children with normal weight in relation to W/H had a mean duration of EBF (101.2 days, 95% CI: 92.1-110.3) almost four times greater than the malnourished ones (25.8 days; 95% CI: 10.0-41.5, p<0.01) and were weaned later (p<0.05). Furthermore, the mean age at weaning was well below the one recommended by the WHO, regardless of the type of hemoglobinopathy and nutritional status, although a large variation was observed in the studied population.
Time of exclusive breastfeeding and weaning age in days, according to type of hemoglobinopathy and anthropometric status (H/A and W/H).
| Variables | EBF (days) | Weaning (days) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Type of Hemoglobinopathy | ||
| SCA | 92.7 (75.3) | 406.3 (371.3) |
| Hb SC disease | 102.3 (90.7) | 460.1 (392.8) |
| Anthropometric status | ||
| H/A Indicator | ||
| Adequate | 96.2 (79.7) | 434.5 (385.0) |
| Inadequate | 116.9 (132.5) | 369.3 (316.3) |
| W/H Indicator | ||
| Adequate | 101.2 (83.5)a | 446.4 (384.7)b |
| Malnourished | 25.8 (18.8)a | 266.3 (239.7)b |
| Risk for overweight/overweight/obesity | 64.2 (66.8) | 268.8 (336.8) |
H/A, height for age; W/H, weight for height.
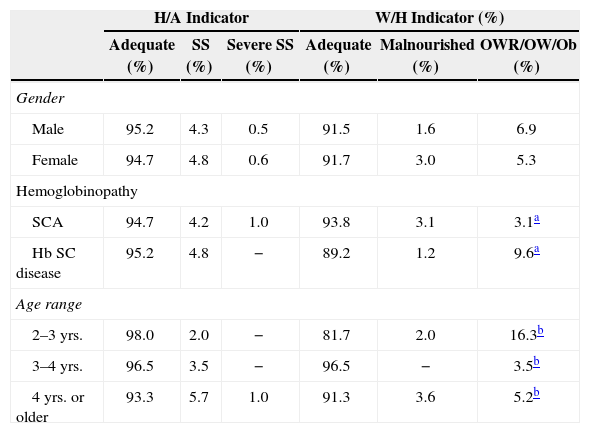
Height deficit was found in 5.0% (18/357) of children, similar in both genders [4.8% (9/189) of boys versus 5.4% (9/168) of the girls] and type of hemoglobinopathy [4.8% (8/166) among hemoglobin SC disease versus 5.2% (10/191) among SCA]. However, all children with severe short stature had sickle cell anemia. The prevalence of short stature increased with age, but without statistical significance (p=0.10). It was found in 2.0% (1/49) of children between two and three years; 3.5% (4/115) between three and four years, and 6.7% (13/193) in those older than four years, an age group that concentrated all children with severe short stature (Table 3).
Anthropometric status (H/A and W/H) according to gender, type of hemoglobinopathy and age range.
| H/A Indicator | W/H Indicator (%) | |||||
|---|---|---|---|---|---|---|
| Adequate (%) | SS (%) | Severe SS (%) | Adequate (%) | Malnourished (%) | OWR/OW/Ob (%) | |
| Gender | ||||||
| Male | 95.2 | 4.3 | 0.5 | 91.5 | 1.6 | 6.9 |
| Female | 94.7 | 4.8 | 0.6 | 91.7 | 3.0 | 5.3 |
| Hemoglobinopathy | ||||||
| SCA | 94.7 | 4.2 | 1.0 | 93.8 | 3.1 | 3.1a |
| Hb SC disease | 95.2 | 4.8 | − | 89.2 | 1.2 | 9.6a |
| Age range | ||||||
| 2–3 yrs. | 98.0 | 2.0 | − | 81.7 | 2.0 | 16.3b |
| 3–4 yrs. | 96.5 | 3.5 | − | 96.5 | − | 3.5b |
| 4 yrs. or older | 93.3 | 5.7 | 1.0 | 91.3 | 3.6 | 5.2b |
SS, short stature; OWR/OW/Ob, Overweight risk/overweight/obesity. H/A, height for age; W/H, weight for height.
For the presence of excess weight, it was observed that this was most frequent alteration in patients with hemoglobin SC disease (p=0.04), as well as younger children (p=0.02).
DiscussionIn the present study, more than half of the children with SCD benefited from EBF at three months of life, although the time of EBF and the age at weaning were lower than those recommended by the WHO, regardless of the type of hemoglobinopathy and nutritional status. Patients with normal weight had a mean BF duration superior to the ones that had nutritional deficits.
Studies on BF in the state of Bahia are scarce. In 1980, a study applied to infants from the semiarid region of Bahia found a BF median of 90 days.15 An investigation carried out in 1996 with a representative sample of healthy infants residing in Salvador, capital of the state of Bahia, evaluated the time of BF, finding a median duration of EBF of 31 days, and the total BF (weaning) of 131 days, periods much shorter than the findings of this study.16 In 2001, in Feira de Santana, Bahia, 39% of children maintained EBF at six months, also lower than the percentage found in this study.17 Despite the time elapsed between the three studies and the present one, the observed difference cannot be credited, in our view, only to governmental and non-governmental investments to encourage the practice of BF. The indicators found among children with SCD, although lower than those recommended by the WHO,12 seem to reflect better adherence to the practice of BF than those observed in children in the general population. The presence of early diagnosis of a chronic illness in the context of a system of multidisciplinary care in the first days of life can encourage the practice of BF. Additionally, a study with mothers of children with hemoglobinopathy describes the maternal option of “temporarily leaving the world of work” to devote themselves to the care of the child with SCD.18
Indeed, studies have shown that continuous incentive to BF might increase its frequency. Research carried out on the “National Day of Multivaccination” in 2001, in a city in the state of São Paulo, found 48% of children exclusively breastfed at six months and attributed the results being above those previously reported mainly to the existence of BF support and incentive groups since 1994 in the Basic Health Units, with weekly meetings between mothers/babies and health professionals to assist in the task of breastfeeding.19 A similar fact has been described in Feira de Santana, Bahia.17 This beneficial effect can be attributed to the National Neonatal Screening Program (PNTN), as patients receive specialized and regular monitoring. It is also possible that children with SCD be weaned later, in view of the psychological representation of suckling at the breast for both the infant and the mother, as a means of calming the child, especially for those who get sick more frequently.20 The image of the child's frailty, from the maternal perception, and the possibility of suffering with weaning may contribute to prolonged breastfeeding.20
Children with SCD have normal birth weight,21 with subsequent significant decline in weight and height growth and delayed sexual maturation.22 The association between malnutrition and SCD is well established in children and adolescents, being more evident with increasing age,23 in agreement with the findings of the present study. It was observed that the H/A deficit almost tripled when the group aged two to three years was compared to four to six years, although without statistical significance; one should emphasize that all children with severe short stature were older than four years and were homozygous for hemoglobin S. This fact suggests that the likely poor weight gain early in life is adjuvant to poor linear growth, culminating in short stature still in infancy. The high protein-calorie demand and low food intake observed in SCD establish a cycle of disease and malnutrition that is difficult to manage,23 with the SCA group showing higher impairment due to more severe physiopathology, resulting in greater nutritional risk.
In this study, malnutrition measured through W/H was more prevalent in children with sickle cell anemia (SCA) and in the age group of four to six years, suggesting the cumulative effect of increased crisis frequency over the binomial disease/loss of appetite. The importance of crisis frequency in the nutritional status can also be reinforced by the higher prevalence of overweight and obesity in SC children, with known clinical outcome, on average, less severe than that of children with sickle cell anemia.24
The H/A deficit was lower than that reported in the literature in studies with healthy Brazilian preschoolers. Data from the National Health and Nutrition Survey in 1989 showed short stature in 14.9% of the children younger than five years and 24.9% among children from the Northeastern region.25 In 2006, the National Demographic and Health Survey found 5.8% of short stature in Northeastern children younger than five years.25 Furthermore, another study showed that short stature in childhood is inversely proportional to the nutritional status and maternal level of education, as well as family income.26 When compared to the results of this study, these data are surprising and probably reflect the impact of PNTN on screened patients, as the follow-up, as previously mentioned, is performed early, regularly and by a multidisciplinary team,24 as a high proportion of stature/height deficit is expected in patients with sickle cell disease.8
It was observed that children with adequate W/H had longer EBF time and were weaned later. That is, the malnourished children had a four-fold shorter mean time of EBF and were breastfed, on average, six months less than the ones with normal weight. Despite the absence of temporality between exposure and the assessed outcome, it is possible that the association with higher frequency of crises, poorer affective bond and the risks involved in the early introduction of water, infusions and complementary foods must have contributed to the poor nutritional status. The association between the early introduction of liquid and semi-solid foods with consequent interruption of EBF has been well described. In addition to being considered an unnecessary practice, it reduces the consumption of human milk, being inversely associated with time of EBF.27 All disadvantages of early weaning for infants are exacerbated by the physiopathological events characteristic of SCD, a fact of concern, considering the coexistence of a disease that brings an increased risk of infections. Thus, the protective character of BF on the nutritional status was also observed in the assessed children. Other studies that compared the BF pattern in children with chronic diseases are scarce, but they emphasize the importance of breastfeeding, reporting that children with cystic fibrosis who were breastfed longer had fewer infections in the first three years of life28 and less frequent use of antibiotics.29
However, complications related to the clinical course of the disease and prognostic markers that may have interfered directly or indirectly with nutritional status were not evaluated in this study, which is a limitation to be emphasized. Other limitations should be acknowledged. Recall bias possibly occurred in the retrospective collection of BF history, which may also have influenced the more prolonged time of EBF and weaning age in our findings, when compared to the healthy population. Moreover, the fact that an association was found between children's age and a higher prevalence of malnutrition may have been overestimated, as the patients were evaluated only once, exposing the reverse causality of cross-sectional studies.
The present study found longer time of EBF and older weaning age in children with sickle cell disease followed at the NSRS than those described in the literature, although still below the current recommendations. Furthermore, the association between adequate nutritional status and more prolonged EBF and BF among children with SCD was clearly demonstrated. The results probably reflect the benefit of multidisciplinary follow-up at an early age in the NSRS.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil, 26/2006.
Conflicts of interestThe authors declare no conflicts of interest.