To identify the contribution of anthropometric variables to predict the maturational stage in young males.
MethodsCross-sectional study that enrolled 190 male subjects aged between eight and 18 years, randomly selected from public and private schools in Natal, Northeast Brazil. Thirty-two anthropometric variables were measured following the recommendations of the International Society for the Advancement of Kineanthropometry (ISAK). The assessment of sexual maturation was based on the observation of two experienced experts, who identified the pubertal development according to Tanner guidelines (1962).
ResultsThe anthropometric variables showed a significant increase of their values during the advancement of pubertal development (p<0.05). The following variables showed the best value for prediction of maturational groups: sitting height, femoral biepicondylar diameter, forearm girth, triceps skinfold, tibiale laterale and acromiale-radiale bone lenghts. These variables were able to estimate the pubertal stages in 76.3% of the sujects.
ConclusionThe anthropometric characteristics showed significant differences between the moments of maturational stages, being found, representatively, seven variables that best predict the stages of sexual maturation.
Identificar a contribuição de variáveis antropométricas para a predição do estádio maturacional em jovens do sexo masculino.
MétodosEstudo transversal, sendo investigados 190 sujeitos do sexo masculino, com idades entre 8 e 18 anos, selecionados aleatoriamente em escolas públicas e privadas de Natal. Foram selecionadas 32 variáveis antropométricas, todas avaliadas de acordo com as recomendações da International Society for the Advancement of Kineanthropometry (ISAK). A avaliação da maturação sexual se baseou na observação de dois especialistas experientes, que identificaram o desenvolvimento da genitália, segundo as recomendações propostas por Tanner (1962).
ResultadosAs variáveis antropométricas apresentaram um aumento significativo no decorrer do avanço do desenvolvimento puberal (p<0,05). As variáveis de altura tronco-cefálica, diâmetro biepicôndilo femural, perímetro de antebraço, dobra cutânea de tríceps, alturas ósseas tibial e acrômio-radial apresentaram a melhor relação para predição dos grupos maturacionais, sendo responsáveis por estimar os estádios puberais com índice de 76,3% de chance de acerto.
ConclusãoAs características antropométricas apresentaram diferenças significativas entre os momentos dos estádios maturacionais, sendo encontradas, de forma representativa, sete variáveis que melhor predizem os estádios de maturação sexual.
Puberty is defined as the stage of development that transforms the child's body into the adult's one, with physical and hormonal changes that culminate in sexual maturation and reproductive capacity.1–3 Its onset can be used as an important analysis tool, as it occurs at an specific time and is regulated by genetic, environmental, and neuroendocrine mechanisms.4,5
The most often used method for clinical assessment of pubertal development was proposed by Tanner, based on the observation of secondary sexual characteristics, with five maturational stages. In males, this method is based on the characteristics of genital and pubic hair and genitalia development itself, with stage 1 representing the prepubertal, and stage 5, the postpubertal.6,7 Although it is widely used in the monitoring of biological maturation, the method has some disadvantages that may compromise its use in services outside the doctor's office. Most commonly, the embarrassment of the evaluated individual or lack of privacy in the chosen environment can compromise the course of the evaluation process, and thus become a limiting factor for its use.8,9
In an attempt to decrease these limitations, some studies have proposed the use of self-assessment, using illustrative photographs of the main aspects of each maturational stage, allowing for a supplementary visual identification so that the assessed individual can identify with the picture that most resembles his current maturational stage. However, national and international studies have shown, in general, a low reliability of this method, which also has the limitation of promoting situations of embarrassment to individuals.6,10–13
Given this perspective, studies have demonstrated that morphological changes are common during puberty in males, as the increased production of sex hormones have a significant association with the modification of some body measurements.14,15 Thus, the analysis of anthropometric and body composition parameters can be considered an important tool to monitor pubertal development, as changes in the external body morphology are related to advancing stages of sexual maturation.16–18
Among the methods used to verify the association between these variables, multivariate analysis can be acknowledged as the best one, as it can provide an estimate of the contribution of each anthropometric characteristic for predicting the stages of pubertal maturation, taking into account the existence of interrelations of all the variables.19 The best statistical test to attain this objective is discriminant analysis, which, similarly to multiple linear regression, verifies the level of association between the variables and creates a prediction equation for a non-metric variable based on metric variables.
In this context, this study aimed to identify the anthropometric variables that best predict the differences between the sexual maturation stages.
MethodsThis was a cross-sectional study of 190 male subjects aged 8 to 18 years, randomly selected from public and private schools in Natal, RN, Brazil. The schools were chosen by convenience, according to the four city regions (North, South, East, and West). Subsequently, the research was introduced to the students, and those who agreed to participate and whose parents or guardians signed the informed consent were enrolled in the study.
Prior to the school evaluations, a pilot study was performed at the outpatient clinic of Hospital de Pediatria of Universidade Federal do Rio Grande do Norte (HOSPED), which allowed for sample size calculation based on a confidence interval of 95%, standard deviation, and standard error estimation. The result of this estimate defined the need for a minimum sample of 181 individuals.
The criteria used for sample selection excluded subjects with genetic syndromes, cognitive impairment, those undergoing treatment with growth hormone (GH), agonists of gonadotropin-releasing hormone (GnRHa), and sex steroids or presence of conditions that could affect the interpretation of results. Initially, 196 subjects were evaluated. However, there was a sample loss of six individuals after the exclusion criteria were applied. The procedures used in this study were previously approved by the Ethics Committee in Research of Universidade Federal do Rio Grande do Norte (UFRN), process number 618/11.
A total of 32 anthropometric variables were selected and all were assessed according to the recommendations of the International Society for the Advancement of Kinanthropometry (ISAK).20 The measured variables were: weight, height, sitting height, length of lower limbs (LL), five bone diameters (biacromial, biiliocristal, transverse thoracic, humeral, and femoral biepicondylar), five bone heights (acromial-radial, radial-styloid, styloid-dactylic, tibial-trochanteric, and lateral-tibial), 11 circumferences (head, neck, relaxed arm, contracted arm, forearm, wrist, chest, waist, abdomen, hip, and calf) and seven skinfolds (triceps, subscapular, biceps, abdominal, supraspinatus, Suprailiac, and calf).
Weight and height were assessed on a Welmy electronic scale (Electronic of Welmy Indústria e Comércio, São Paulo – Brazil), with a capacity of 300 kg and precision of 50 g, and an attached anthropometric ruler with a scale between 1.00 m and 2.00 m and an accuracy of 0.1 cm. Measures of circumferences and sitting height were performed using a 2-meter Sanny anthropometric tape (Sanny, São Paulo – Brazil) with 0.1 cm precision. Diameters and bone lengths were measured using two instruments: a 2-meter Sanny segmometer (Sanny, São Paulo – Brazil) with a precision of 0.1 cm, and a Cescorf metal caliper (Cescorf, Rio Grande do Sul – Brazil) with a precision of 0.1 cm. Skinfold measurements were performed using a Harpenden caliper (John Bull British Indicators Ltd, West Sussex – England) with a unit scale of 0.2 mm and measure interpolation of 0.1 mm.
The anthropometric measurements were performed by two experienced examiners with adequate technical error of measurement (TEM), according to the values shown in the literature, of 5% for skinfolds and 1% for the others.21 Variables in which the TEM exceeded the recommended values were excluded from the analysis. Two other examiners were trained in advance and conducted the longitudinal and girth measures, under the supervision of one of the experienced examiners.
The assessment of sexual maturation was based on the observation of two specialized and experienced physicians, with rates of interobserver agreement reported in a previous study.6 For that purpose, the pubertal status of the subjects was evaluated according to the recommendations of Tanner,22 separated by the stage of sexual maturation of the genitalia (G1-G5).
The descriptive analysis was performed for central tendency values. Data distribution was analyzed by the Shapiro-Wilk test and Levene's test, and non-parametric distribution was found only for skinfolds. These were represented by the median and interquartile range, and were submitted to neperian logarithmic transformation. The inferential analysis was performed by one-way ANOVA with post hoc Scheffé test.
Using a multivariate approach, conditions were initially observed for the assumptions of the discriminant analysis by the colinearity tests (tolerance > 0.1 and tolerance inflation factor < 10) and Box's M (0.118). Then, the variables were evaluated by simultaneous estimation in order to generate a function that could predict sexual maturity based on anthropometric variables.
The level of significance was set at p<0.05, and the analysis was performed using SPSS software, release 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, New York – USA).
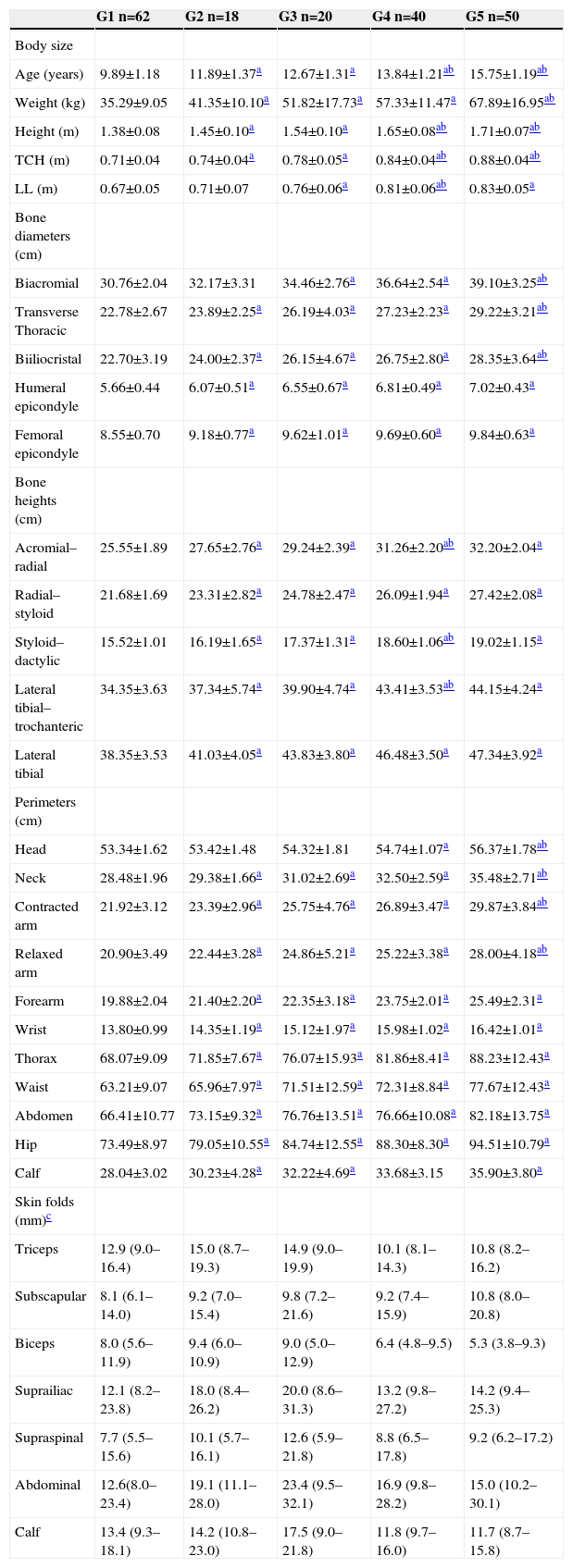
ResultsThe mean values of each anthropometric characteristic separated by pubertal stages are described in Table 1. Chronological age was higher with advancing pubertal development, with mean and standard deviation for G1 of 9.89±1.18; for G2, 11.89±1.37; for G3, 12.67±1.31; for G4, 13.84±1.21; and for G5, 15.75±1.19.
Central tendency and dispersion values of anthropometric variables, according to the stages of pubertal maturation (G1–G5)
| G1 n=62 | G2 n=18 | G3 n=20 | G4 n=40 | G5 n=50 | |
|---|---|---|---|---|---|
| Body size | |||||
| Age (years) | 9.89±1.18 | 11.89±1.37a | 12.67±1.31a | 13.84±1.21ab | 15.75±1.19ab |
| Weight (kg) | 35.29±9.05 | 41.35±10.10a | 51.82±17.73a | 57.33±11.47a | 67.89±16.95ab |
| Height (m) | 1.38±0.08 | 1.45±0.10a | 1.54±0.10a | 1.65±0.08ab | 1.71±0.07ab |
| TCH (m) | 0.71±0.04 | 0.74±0.04a | 0.78±0.05a | 0.84±0.04ab | 0.88±0.04ab |
| LL (m) | 0.67±0.05 | 0.71±0.07 | 0.76±0.06a | 0.81±0.06ab | 0.83±0.05a |
| Bone diameters (cm) | |||||
| Biacromial | 30.76±2.04 | 32.17±3.31 | 34.46±2.76a | 36.64±2.54a | 39.10±3.25ab |
| Transverse Thoracic | 22.78±2.67 | 23.89±2.25a | 26.19±4.03a | 27.23±2.23a | 29.22±3.21ab |
| Biiliocristal | 22.70±3.19 | 24.00±2.37a | 26.15±4.67a | 26.75±2.80a | 28.35±3.64ab |
| Humeral epicondyle | 5.66±0.44 | 6.07±0.51a | 6.55±0.67a | 6.81±0.49a | 7.02±0.43a |
| Femoral epicondyle | 8.55±0.70 | 9.18±0.77a | 9.62±1.01a | 9.69±0.60a | 9.84±0.63a |
| Bone heights (cm) | |||||
| Acromial–radial | 25.55±1.89 | 27.65±2.76a | 29.24±2.39a | 31.26±2.20ab | 32.20±2.04a |
| Radial–styloid | 21.68±1.69 | 23.31±2.82a | 24.78±2.47a | 26.09±1.94a | 27.42±2.08a |
| Styloid–dactylic | 15.52±1.01 | 16.19±1.65a | 17.37±1.31a | 18.60±1.06ab | 19.02±1.15a |
| Lateral tibial–trochanteric | 34.35±3.63 | 37.34±5.74a | 39.90±4.74a | 43.41±3.53ab | 44.15±4.24a |
| Lateral tibial | 38.35±3.53 | 41.03±4.05a | 43.83±3.80a | 46.48±3.50a | 47.34±3.92a |
| Perimeters (cm) | |||||
| Head | 53.34±1.62 | 53.42±1.48 | 54.32±1.81 | 54.74±1.07a | 56.37±1.78ab |
| Neck | 28.48±1.96 | 29.38±1.66a | 31.02±2.69a | 32.50±2.59a | 35.48±2.71ab |
| Contracted arm | 21.92±3.12 | 23.39±2.96a | 25.75±4.76a | 26.89±3.47a | 29.87±3.84ab |
| Relaxed arm | 20.90±3.49 | 22.44±3.28a | 24.86±5.21a | 25.22±3.38a | 28.00±4.18ab |
| Forearm | 19.88±2.04 | 21.40±2.20a | 22.35±3.18a | 23.75±2.01a | 25.49±2.31a |
| Wrist | 13.80±0.99 | 14.35±1.19a | 15.12±1.97a | 15.98±1.02a | 16.42±1.01a |
| Thorax | 68.07±9.09 | 71.85±7.67a | 76.07±15.93a | 81.86±8.41a | 88.23±12.43a |
| Waist | 63.21±9.07 | 65.96±7.97a | 71.51±12.59a | 72.31±8.84a | 77.67±12.43a |
| Abdomen | 66.41±10.77 | 73.15±9.32a | 76.76±13.51a | 76.66±10.08a | 82.18±13.75a |
| Hip | 73.49±8.97 | 79.05±10.55a | 84.74±12.55a | 88.30±8.30a | 94.51±10.79a |
| Calf | 28.04±3.02 | 30.23±4.28a | 32.22±4.69a | 33.68±3.15 | 35.90±3.80a |
| Skin folds (mm)c | |||||
| Triceps | 12.9 (9.0–16.4) | 15.0 (8.7–19.3) | 14.9 (9.0–19.9) | 10.1 (8.1–14.3) | 10.8 (8.2–16.2) |
| Subscapular | 8.1 (6.1–14.0) | 9.2 (7.0–15.4) | 9.8 (7.2–21.6) | 9.2 (7.4–15.9) | 10.8 (8.0–20.8) |
| Biceps | 8.0 (5.6–11.9) | 9.4 (6.0–10.9) | 9.0 (5.0–12.9) | 6.4 (4.8–9.5) | 5.3 (3.8–9.3) |
| Suprailiac | 12.1 (8.2–23.8) | 18.0 (8.4–26.2) | 20.0 (8.6–31.3) | 13.2 (9.8–27.2) | 14.2 (9.4–25.3) |
| Supraspinal | 7.7 (5.5–15.6) | 10.1 (5.7–16.1) | 12.6 (5.9–21.8) | 8.8 (6.5–17.8) | 9.2 (6.2–17.2) |
| Abdominal | 12.6(8.0–23.4) | 19.1 (11.1–28.0) | 23.4 (9.5–32.1) | 16.9 (9.8–28.2) | 15.0 (10.2–30.1) |
| Calf | 13.4 (9.3–18.1) | 14.2 (10.8–23.0) | 17.5 (9.0–21.8) | 11.8 (9.7–16.0) | 11.7 (8.7–15.8) |
TCH, trunk-cephalic height; LL, length of lower limbs
Regarding the other variables, there was also an increase in their values with advancing pubertal stage. It can also be observed that all variables, except head circumference, biacromial diameter, and LL showed a significant difference between G1 and G2 (p<0.05), albeit not significant between G2 and G3. Moreover, the body size variables showed significant changes from G1 to G2 (p<0.01), which stabilized in G3 and again showing differentiation in G4 and G5 (p<0.05).
As for skinfold thickness, no significant difference was found between the pubertal stages. This demonstrates that, for absolute values, body adiposity represented by subcutaneous fat does not change in boys, even with the onset of puberty.
Of the 32 anthropometric variables that were submitted to discriminant analysis, six (sitting height, femoral biepicondylar diameter, forearm girth, triceps skinfold, and tibial and radial-acromion bone heights), plus age, were selected as the best predictors for the stages of pubertal maturation.
After that, four discriminant functions were created, aiming at algebraically describing the separation between the five groups analyzed. The impact of each function for pubertal maturation prediction and their contribution to explain the association between all variables were obtained by canonical correlation, which showed acceptable values only for the first three functions (0.927, 0.440, 0.352), suggesting little contribution of function number four (0.120) for the subsequent statistics.
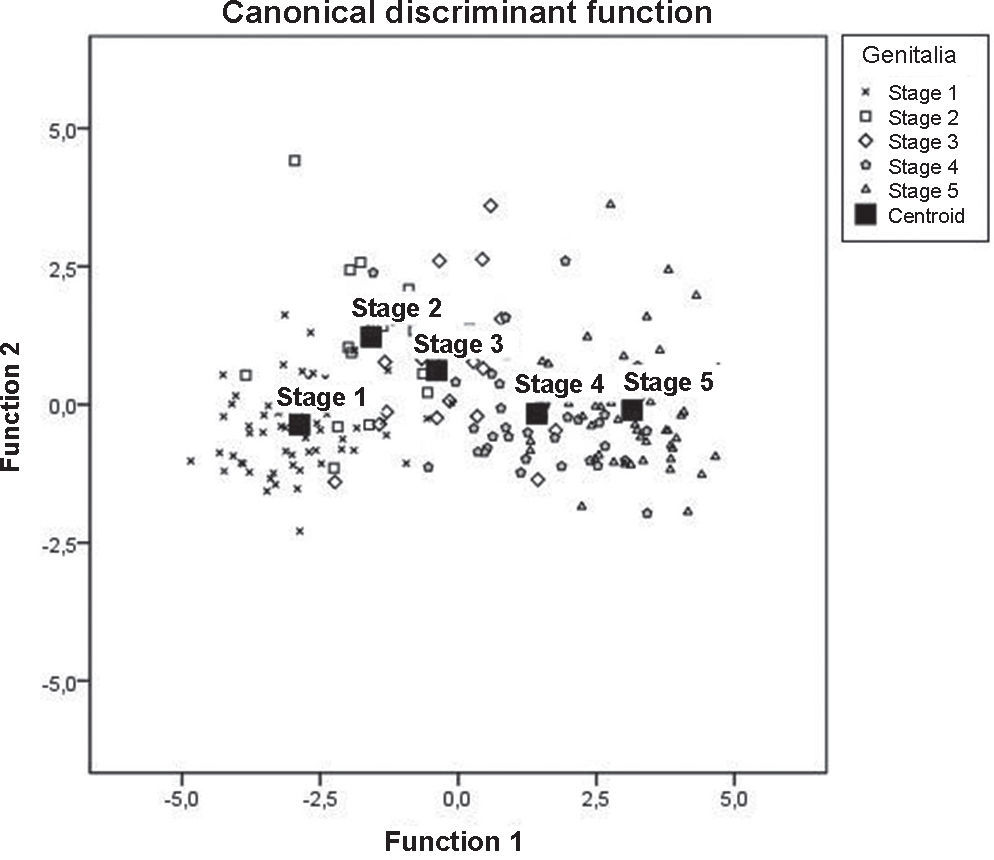
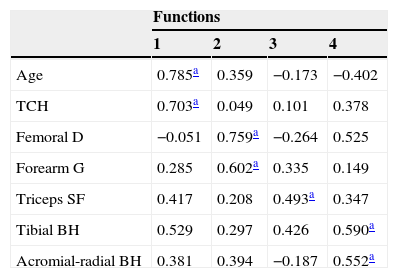
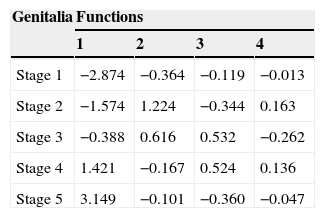
Tables 2 and 3 show, respectively, the values of the discriminant loads of each variable and the midpoint (centroid) values of each function.
Level of contribution of anthropometric variables for the pubertal maturation prediction model, based on the creation of discriminant functions
| Functions | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age | 0.785a | 0.359 | −0.173 | −0.402 |
| TCH | 0.703a | 0.049 | 0.101 | 0.378 |
| Femoral D | −0.051 | 0.759a | −0.264 | 0.525 |
| Forearm G | 0.285 | 0.602a | 0.335 | 0.149 |
| Triceps SF | 0.417 | 0.208 | 0.493a | 0.347 |
| Tibial BH | 0.529 | 0.297 | 0.426 | 0.590a |
| Acromial-radial BH | 0.381 | 0.394 | −0.187 | 0.552a |
TCH, trunk-cephalic height; D, diameter; G, girth; SF, skin fold; BH, bone height;
Mean central values (centroids) of functions for prediction of pubertal maturation based on anthropometric variables
| Genitalia | Functions | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Stage 1 | −2.874 | −0.364 | −0.119 | −0.013 |
| Stage 2 | −1.574 | 1.224 | −0.344 | 0.163 |
| Stage 3 | −0.388 | 0.616 | 0.532 | −0.262 |
| Stage 4 | 1.421 | −0.167 | 0.524 | 0.136 |
| Stage 5 | 3.149 | −0.101 | −0.360 | −0.047 |
Age and sitting height showed to be the best predictors for function 1, which is related to the differential analysis between stages 4 and 5 vs. the other stages. For function 2, which is related to the difference between stages 2 and 3 vs. the other stages, there was a large contribution from the femoral biepicondylar diameter and the forearm girth.
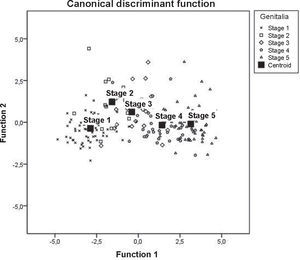
The data provided by Fig. 1 also indicate an analysis of the centroids of each pubertal stage, but emphasize the dispersion of each subject in relation to it. Thus, the difference between the mean values for the discriminant z-scores of the five stages can be observed, and the overall discriminant analysis can be comparatively verified.
The probability of pubertal stage prediction based on anthropometric variables was 76.3%, as shown by the classification values for the discriminant analysis. Specifically regarding the groups, stage 2 attained the minimum percentage value (61.1%), stage 1 had the highest value (87.1%), and stages 3, 4 and 5 had, respectively, 65%, 62.5%, and 84%.
DiscussionAnthropometric changes that occur with advancing maturational stages can be recognized as an alternative analysis to identify the variables that best predict these stages. As previously observed, of the 32 anthropometric variables that were assessed, six, in addition to age, were able to reclassify a high percentage of maturational groups, demonstrating the viability of this perspective.
In the study by Perez et al,9 the maturational stages were also successfully predicted based on eight anthropometric variables, indicating the significant effect of maturation on anthropometric characteristics. This association is ultimately responsible for the body changes in males, as observed in Table 1.3,7,23
All anthropometric variables, except head circumference, biacromial diameter, and LL showed a significant difference between G1 and G2, which was not confirmed for the subsequent period, between G2 and G3. This may have occurred for two reasons. First, the minimum age used, which, although similar to other studies, may have included males whose development is delayed in relation to puberty onset, thus underestimating the values shown in G1. Conversely, this difference may be explained by the description found in the study by Wright et al,24 wherein the stage 1 represents a period prior to puberty, with a small difference when compared to stage 2. Subsequently, peak growth velocity will be achieved, typically between stages 3 and 4.3,25
In relation to skin folds, it was observed that the variation was very high, indicating a high variability between subjects. Therefore, it was necessary to apply statistical techniques to attenuate the distribution of variables, and, after analysis of variance, no significant differences were found for any comparison between the stages, corroborating the study by Veldre and Jürimäe.8 It is known that, in boys, the increase in weight during this phase is accompanied mainly by gains in muscle mass (80% to 90%) and by stability in the levels of fat mass, resulting in few changes in absolute levels of subcutaneous fat. 26,27
The seven variables used in the discriminant analysis were responsible for the creation of four functions. Based on the Wilks-Lambda test, only the first three functions were able to discriminate the stages of maturation (p<0.05), respectively accounting for 85.9%, 19.4%, and 12.4% of the amount of variance of the discriminant analysis. These rates are considered adequate to proceed with the other interpretations of this method, as they can predict the differences between each stage of sexual maturation with high accuracy.19
Tables 2 and 3 show the specific characteristics of the functions for each selected variable and pubertal stages. Based on these, age (0.785) and sitting height (0.703) can be identified as the most predictive characteristics of the first function. The femoral diameter (0.759) and forearm girth (0.602) are better predictors of the second function. The centroid values show that the first function may contribute to the separation between the G4 and G5 groups vs. the other stages, due to the differences shown in the discriminant variables. As for function 2, such separation is represented by G2 and G3 vs. the other stages, i.e., the best trends for this discriminant analysis is based on three groups: G1; G2+G3; G4+G5.
The results are similar to those obtained by Wright et al24 on the effectiveness of growth characteristics at puberty, which proposes its analysis at three specific phases: prepubertal (Tanner stage 1), “at puberty” (stages 2 and 3 of Tanner), and “completing puberty” (stages 4 and 5 of Tanner).
Literature shows that the sequence of maturational changes during puberty is well-defined. Stages 4 and 5, when referred in relation to the characterization of the genitalia, represent an advanced maturation stage, in which the individual is close to the adult stage. It is during this period that the main body changes occur, especially in relation to peak growth velocity, contributing to a greater differentiation from early maturational stages.3,28,29
For the percentage of viability of correctly classified predictions, the discriminant analysis accounted for a total of 76.3% of correct answers, considered a good predictive level.19 This means that the seven variables used in the functions accounted for a rate of 76.3% of prediction of maturation stages, which is acceptable for this type of method.
The remaining 23.7% are related to the error rate originated by biases that accompany this study, especially in relation to the anthropometric assessment, which is characterized as a method that requires appropriate prior training and adequate capacity, so that the error intrinsic to the examiner is not as accentuated. However, the calculation of inter- and intra-rater errors was performed to minimize this problem.
In more specific analyses, it appears that the intermediate stages G2, G3, and G4 had the lowest indices, indicating greater difficulty in identifying anthropometric changes during these stages of puberty. However, the moments related to G1 and G5 had a high percentage of correctness, demonstrating high prediction accuracy, justified as extreme moments in the maturational process in which alterations that are characteristic of puberty have yet to initiate (prepubertal stage) or have already been completed (post-pubertal stage).
Body changes that occurred with advancing pubertal maturation were significant and demonstrated usefulness in the clinical setting for young males. Although this was characterized as a cross-sectional study that does not substitute for the direct method used in clinical practice, these results constitute an innovative proposal, as this study found significant differences in anthropometric characteristics between the stages of sexual maturation, identifying seven variables that best discriminate and predict these stages, represented by a classification index that is considered to be good (76.3%).
Therefore, these findings confirm that the evaluation of anthropometric characteristics has a significant association with pubertal stage in young male individuals and represents a new perspective for the development of novel methods for prediction of biological maturation, which are not limited by invasiveness and high cost.
Conflicts of interestThe authors declare no conflicts of interest.
FundingCoordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for the Master's Degree Scholarship.