To alert the pediatrician who is following up HIV-infected patients about the possibility of non-cirrhotic portal hypertension (NCPH) in this period of life, in order to avoid the catastrophic consequences of this disease as bleeding esophageal varices.
Case descriptionA 13 years old HIV-infected patient by vertical route was receiving didanosine (ddI) for 12 years. Although the HIV viral load had been undetectable for 12 years, this patient showed gradual decrease of CD4+ T cells, prolonged thrombocytopenia and high alkaline phosphatase. Physical examination detected splenomegaly, which triggered the investigation that led to the diagnosis of severe liver fibrosis by transient elastography, probably due to hepatic toxicity by prolonged use of ddI.
CommentsThis is the first case of NCPH in HIV-infected adolescent described in Brazil. Although, the NCPH is a rare disease entity in seropositive patients in the pediatric age group, it should be investigated in patients on long-term ddI or presenting clinical and laboratories indicators of portal hypertension, as splenomegaly, thrombocytopenia and increased alkaline phosphatase.
Alertar o pediatra sobre a ocorrência de hipertensão portal não cirrótica (HPNC) na faixa etária pediátrica, no sentido de evitar as consequências catastróficas dessa doença, como o sangramento de varizes de esôfago.
Descrição do casoPaciente de 13 anos, infectado pelo HIV por via vertical, recebia esquema antirretroviral com didanosina (ddI) havia 12 anos. Apesar do controle adequado da replicação viral, com carga viral do HIV indetectável havia 12 anos, passou a apresentar diminuição gradativa dos linfócitos TCD4+, trombocitopenia prolongada e fosfatase alcalina elevada. O exame físico detectou esplenomegalia, que desencadeou o processo de investigação e culminou no diagnóstico de fibrose hepática acentuada pela elastografia, por provável toxicidade hepática devido ao uso prolongado de ddI.
ComentáriosEste é o primeiro caso de HPNC em adolescente infectado pelo HIV descrito no Brasil. Embora seja entidade mórbida rara em pacientes soropositivos para o HIV na faixa etária pediátrica, deve ser investigada nos pacientes em uso prolongado de ddI ou que apresentem indicadores clínicos e/ou laboratoriais de hipertensão portal, como esplenomegalia, trombocitopenia e aumento de fosfatase alcalina.
It is estimated that approximately 718,000 individuals live with HIV/AIDS in Brazil, representing a prevalence rate of 0.4% in the general population. AIDS detection rate in Brazil has increased about 2% in the last 10 years, especially among young individuals aged 15-24 years and adults aged 50 years or older. However, AIDS detection rate in children younger than five years old, an indicator used in Brazil to monitor HIV vertical transmission, decreased by 35.8% compared to 2003.1 The combined antiretroviral therapy (cART) resulted in a sharp decrease in mortality of children and adolescents infected with HIV.2 Nucleoside analogue reverse transcriptase inhibitors (NRTIs) were the first available antiretroviral drugs and it is not rare to find HIV-infected patients that have been using these drugs (alone or combined with other antiretroviral classes) for over 10 years.
Infectious diseases (including opportunistic infections) progressively decreased with treatment, but the non-infectious complications, including liver disease, have become significant causes of morbidity and mortality in the long-term among survivors. A retrospective study in South America (Brazil, Mexico, Argentina and Peru), including 6,000 HIV-positive adult patients, showed that terminal liver failure or cirrhosis was the leading cause of death, with 54/130 (42%) confirmed or probable cases based on clinical, laboratory or histological findings.3 Rubio et al., in 2009, used non-invasive procedures to assess hepatic involvement in 26 children chronically infected with HIV through vertical route and found that more than 60% of the population had signs of liver disorder in at least one of the tests.4
Non-cirrhotic Portal Hypertension (NCPH) is one of the clinical entities that affect HIV-infected patients. It is a rare disease, occurring in up to 0.5% of HIV-positive adult patients.5 The direct action of HIV itself, endothelial and mitochondrial damage, hypercoagulability and microbial translocation are factors possibly implicated in its pathogenesis.6–8 The aim of this case report is to alert pediatricians about the occurrence of non-cirrhotic portal hypertension (NCPH) in pediatric patients, in order to avoid the catastrophic consequences of this disease such as bleeding of esophageal varices.
Case descriptionThis case report was approved by the Institutional Review Board of UNIFESP (N. CAAE: 31701414.8.0000.5505) and informed consent forms were signed by patients and their legal guardians.
A preterm male infant, born by cesarean section, with 1,250 g birth weight, 36 cm height and Apgar scores at 1 and 5 minutes of six and eight, was infected by the human immunodeficiency virus (HIV) through vertical transmission. The mother was a user of illicit drugs with irregular use of zidovudine (AZT) during pregnancy and received intrapartum prophylaxis. The child was adopted at 6 months of life. In December 2001 (7 months old) he started follow-up of HIV infection at the Outpatient Clinic of the Discipline of Pediatric Infectious Diseases, UNIFESP/EPM (CEADIPE), with HIV viral load of 1,100,000 copies/mL and CD4+ lymphocytes of 819 cells/mm3 (24.1%). He was classified as B2, and was treated with cART (Zidovudine-AZT + didanosine-ddI + Nelfinavir-NFV).
There was good adherence to antiretroviral therapy leading to control of viral replication (undetectable HIV viral load) and normalization of CD4+ T lymphocyte values. The patient had a history of two hospitalizations at the beginning of follow-up (acute otitis media and pneumonia at 8 months of age and diarrhea two months later), and some uncomplicated clinical conditions, such as chicken pox and scarlet fever during the clinical follow-up. He received the full vaccination schedule recommended for HIV-infected children. cART was maintained until June 2007, when NFV was no longer available, being replaced by Nevirapine (AZT+ddI+nevirapine-NVP). There was control of viral replication and absence of immunosuppression (normal CD4+ levels), with appropriate physical and neurological development, without disease complications or clinical complaints until April 2009, when he started to present with thrombocytopenia (<150,000 platelets/microliter), without bleeding episodes. In March 2013 he started to show immunosuppression (decrease in CD4+ T lymphocyte levels <500 cells/mm3).
In August 2013, aged 13 years, he was clinically stable, weighed 35.6kg, with a body mass index of 14.8 (between the 3rd and the15th percentiles); 155.5cm tall; H/A=111% (>97th percentile); blood pressure 99×75mmHg; rare posterior inguinal and cervical ganglia (<0.5cm diameter), palpable liver at 3.5cm from the right costal margin and spleen at 6.0cm from the left costal margin (smooth surface, painless). Tanner pubertal stage was G3P3 and neurological examination was normal.
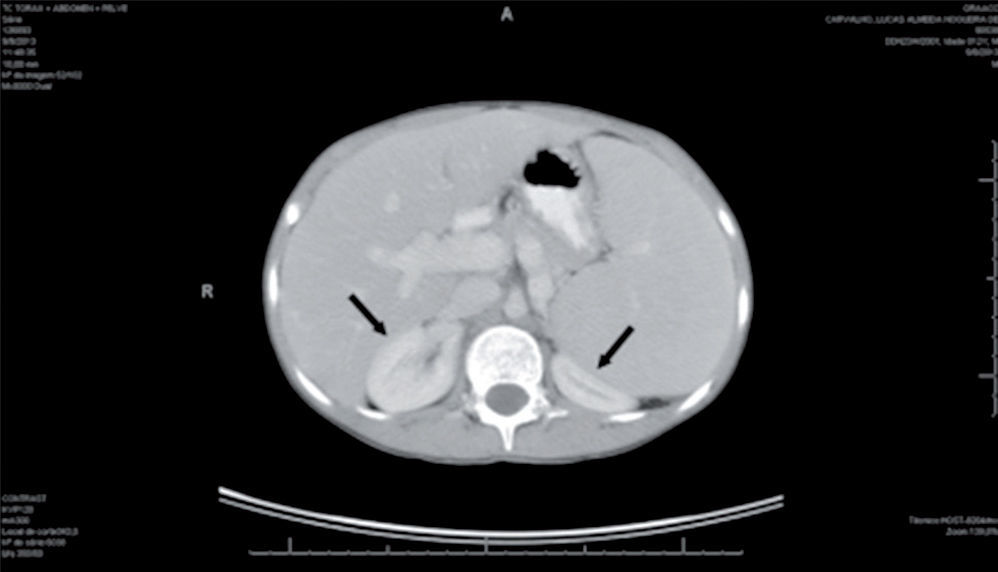
Infectious and oncological causes were investigated and ruled out for the immunosuppression and hepatosplenomegaly: immunity for CMV, hepatitis A and hepatitis B, negative serology for hepatitis C, toxoplasmosis and mononucleosis. The abdominal ultrasound confirmed splenomegaly; CT of the chest and abdomen showed normal chest, homogeneous hepatosplenomegaly, compressing the kidneys posteriorly, and increased portal vein dimensions (1.8 cm) (Fig. 1).
The myelogram without anomalous cells in the bone marrow disclosed a G/E ratio of 0.9/1; marked erythrocyte hyperplasia with preserved maturation; mild signs of dyserythropoiesis; granulocytic normoplasia with preserved maturation, as well as the presence of mild megaloblastosis; lymphocyte normoplasia and presence of plasmocytes; monocyte normoplasia, increased number of macrophages and megakaryocytic normoplasia.
The CBC showed hemoglobin (Hb) of 13.1g/dL; Hematocrit (Htc) of 40.4%, platelets of 63,000/uL, leukocytes 3,700/uL (7% band cells, 64% neutrophils, 1% eosinophils, 23% lymphocytes, 5% monocytes). Other tests: C-reactive protein (CRP) 0.45mg/L, lactate dehydrogenase (LDH) 163 U/L, aspartate aminotransferase (AST) 40U/L, alanine aminotransferase (ALT) 36U/L, gamma-glutamyl transferase (gammaGT) 68U/L, alkaline phosphatase (AP) 382U/L, total protein 7.4g/dL, albumin 4.26g/dL. APRI index of 1.11 (aspartate aminotransferase-platelets ratio index).
Considering these laboratory tests, a hypothesis of portal hypertension due to liver toxicity caused by didanosine (ddI) was suggested. The cART was modified, with ddI withdrawal and substitution of the previous regimen (Abacavir-ABC+ Lamivudine-3TC+ Lopinavir and Ritonavir-LPV/r); liver elastography and upper digestive endoscopy were requested. The liver elastography showed no steatosis, E index of 10.0kPa with marked hepatic fibrosis (F2-F3 of Metavir). The endoscopy disclosed small-caliber esophageal varices, intense portal hypertensive gastropathy and gastric polyps.
DiscussionOnly two other cases of non-cirrhotic portal hypertension (NCPH) have been previously described in children and adolescents infected with HIV: a 10-year-old in Italy9 and a 15-year-old in the United States,10 both with prolonged exposure to ddI and history of esophageal variceal bleeding. No other cases were identified in Brazil. The investigation of this case was triggered because the patient had control of HIV viral replication (undetectable HIV viral load for 12 years), had been using cART with ddI for 12 years without clinical symptoms, had thrombocytopenia, progressive decrease in CD4+ T cell levels and hepatosplenomegaly.
In addition to co-infections by hepatitis B and C virus, the infection of liver cells by HIV may contribute to the progression of liver disease by direct and indirect mechanisms.11 The HIV can directly infect hepatocytes, hepatic stellate cells (HSCs) and Kupffer cells. The glycoprotein 120 (gp120) of the HIV bound to the CXCR4 correceptor can induce apoptosis of hepatocytes and activation of HSCs, both contributing to the formation of liver fibrosis.11 Indirectly, the nucleoside analog reverse transcriptase inhibitors and the HIV itself (effect via peroxisome proliferator-activated receptor - PPAR) can contribute to liver disease by inducing metabolic syndrome. Gastrointestinal tract infection by HIV leads to lipopolysaccharide (LPS) increase, which can stimulate the three types of liver cells to produce proinflammatory cytokines and chemokines that attract activated lymphocytes and monocytes to the liver, further increasing fibrosis.11
One of the clinical presentations of liver disease in HIV mono-infected patient is the non-cirrhotic portal hypertension (NCPH), first described in adults by Maida et al.12 Parikh et al.,13 in 2014, studying HIV-infected adults with NCPH found that most of them had splenomegaly, thrombocytopenia and elevated alkaline phosphatase. Maida et al., in 2006, assessed a group of 17 HIV-infected adults with cryptogenic liver disease, and comparing them to a control group (HIV-infected patients without liver disease), found prolonged use of ddI as the only independent factor associated with the development of NCPH.12 The same was observed by Schouten et al.14 in 2012, demonstrating that the risk factors for the development of NCPH were: long-term exposure (11 years) to ddI alone and/or short-term exposure (4 years) to ddI+ Stavudine-D4T or ddI+Tenofovir-TDF combination.
The antiretroviral agents didanosine (ddI), stavudine (d4T) and zalcitabine are stronger inhibitors of mitochondrial DNA polymerase than Zidovudine (AZT), Lamivudine (3TC) and Abacavir (ABC), and cause increased mitochondrial toxicity. Drug-induced depletion of mitochondrial DNA polymerase is an assumed underlying mechanism of lactic acidosis associated with steatosis, steatohepatitis and liver failure in HIV-infected individuals. The association between mitochondrial hepatotoxicity and NCPH was also suggested by Schouten et al. in 2012.14
More recently, Parikh et al., in 2014,13 based on the prevalence of significant factors in patients with NCPH when compared with the control group, proposed a flow chart to assess this diagnosis: exposure to ddI or splenomegaly in an HIV-positive patient without known liver disease should trigger an evaluation for NCPH (excluding patients with a history of alcohol consumption and viral hepatitis detected by serological screening). In the presence of thrombocytopenia, AST>40U/L or AP>115U/L, the patient should be referred to a hepatologist and start the investigation.13
Considering that liver involvement can occur for a long period of time before the clinical manifestations of liver disease become evident, and that children infected with HIV by vertical transmission are surviving for longer periods with continuous use of antiretroviral drugs, it is necessary to seek markers capable of identifying liver damage as early as possible.
Siberry et al., in 201415 evaluated the APRI (aspartate aminotransferase/platelet ratio) index as a predictor of liver fibrosis in a cohort of HIV-infected children by vertical transmission in Latin America. The cutoff value for this index in children has not been established, but an index >1.5 seem to be quite specific, although it lacks sensitivity.15 The studied patient had an APRI of 1.11 at the start of the investigation of hepatosplenomegaly, but this index increased to 2.0 after one month.
The NCPH results in bleeding of esophageal varices. In the study by Parikh et al.,13 of 34 cases with NCPH, 55.9% had bleeding, demonstrating that NCPH is underdiagnosed, perhaps due to lack of an algorithm for the investigation. Esophageal varices were identified in almost 90% of the group with NCPH in this study, a higher prevalence than that found by other researchers: 15% in the study by Mallet et al.,5 in 2007, and 69% in the study by Maida et al.,6 in 2008. The patient we described had small-caliber esophageal varices, without bleeding. The substitution of ddI by another antiretroviral agent in this case aimed to stop the progression of liver fibrosis, decreasing the risk of disease complications.
The purpose of this clinical case report is to alert the professionals that treat individuals infected with HIV/AIDS, particularly pediatricians, about the possibility of NCPH in children and adolescents infected with HIV through vertical transmission submitted to long-term use of cART. One should consider as warning signs the presence of thrombocytopenia and hepatosplenomegaly, regardless of elevated liver enzymes. Liver fibrosis should be investigated in these patients and early identification of the disease can prevent its severe consequences, such as bleeding of esophageal varices.
FundingThis study did not receive funding.
Conflicts of interestThe authors declare no conflicts of interest.