A Obesidade é um problema global de saúde publica que na maioria das vezes pode ser melhorado com a mudança nos hábitos alimentares, diminuindo o consumo de alimentos processados e aumentando a actividade física como forma de combater os diferentes desequilíbrios hormonais que podem surgir em muitos indivíduos. Entre os factores causadores ou concomitantemente observados na hipertensão endócrina inclui-se um aumento na actividade hormonal, como por exemplo, da aldosterona, dos mineralocorticóides, do cortisol, das catecolaminas (ou pela sua acção através do sistema nervoso simpático) e as hormonas do crescimento, da tiróide e da paratiróide. Por outro lado, a insulino-resistência, a deficiência na hormona do crescimento, a testosterona, a Vitamina D e as hormonas da tiróide podem também contribuir para a hipertensão endócrina. Neste trabalho apresenta-se uma revisão dos mais recentes avanços obtidos na já tradicional hipertensão endócrina de causa suprarrenal e são ainda indicados outros aspectos menos referidos desta hipertensão, como por exemplo a nossa hipótese de que a dependência da comida, de doces e de sal é um dos aspetos mais importantes em muitas sociedades e como tal devem ser abordados de forma holística e conforme o antigo pensamento filosófico de que uma mente sã levará a um corpo são.
Obesity is a global health problem, most of which can probably be improved by changing dietary habits, with less consumption of processed and preserved foods and by increasing physical activity to combat the various hormonal imbalances that have developed in many individuals. Among the factors responsible for, or observed in, endocrine hypertension, are excessive increases in the action of hormones, including, aldosterone, mineralocorticoids, cortisol, and catecholamines (or by their action through the sympathetic nervous system), growth hormone, thyroid hormone, and parathyroid hormone. On the other hand, insulin resistance and deficiency of growth hormone, testosterone, vitamin D, and thyroid hormone may also be seen to contribute in patients with endocrine hypertension. A review is presented of recent advances in the traditional adrenal causes of endocrine hypertension and some non-traditional aspects of endocrine hypertension are illustrated, including our hypothesis that addiction to food, sweets, and salt are the main issues in many societies, and should be approached holistically and by the ancient philosophical method that a healthy mind will lead to a healthy body.
Introduction
The 2012 report F as in Fat (Trust for America's Health) clearly underscores how obesity threatens America's future, mainly by an increase in obesity-related health care costs, including those for type 2 diabetes, coronary heart disease, stroke, and hypertension. In 2011, 69% of Mississippians were overweight or obese, leaving only 31% with normal body mass index (BMI), when defined as <27 kg/m2.1 Although the extent of obesity and type 2 diabetes is still lower in Europe, including Portugal, than in America, it is also rising (obesity in Portuguese adults: approx. 18%), especially in children (approx. 25% in Portugal).2 History and the last several decades have shown that the fundamental drivers of the obesity epidemic are changes in food supply and physical environment, both socioeconomically driven, and hard to change by an individualistic free-market approach to obesity prevention.3,4 As we now know, the BMI definition of obesity is not perfect when assessing the risk for developing type 2 diabetes, as it does not tell the body composition, i.e. lean and fat mass.5 It also does not assist in separating obese individuals with insulin resistance and an inflammatory state from those with insulin sensitivity and/or no inflammatory state. This explains why obesity-associated hypertension is a complex entity that belongs to a form of endocrine hypertension, as in some patients, testosterone deficiency and growth hormone deficiency, as well as vitamin D deficiency, may coexist with obesity.6 In women with polycystic ovarian syndrome, it remains controversial whether associated hypertension is independent of obesity. On the other hand, elevated blood pressure related to the intake of oral contraceptives and postmenopausal hormone therapy can be reversed by stopping hormonal therapy. This review aims to provide an update on the classical forms of endocrine hypertension, as well as providing insights into the non-traditional, less thought of forms of hormonal causes involved in causing or being associated with high blood pressure.7
Syndromes of mineralocorticoid excess
Among this group of disorders causing hypertension (Figure 1), is primary aldosteronism (PA), the most common form of syndromes of mineralocorticoid excess, with an estimated prevalence of up to 13% in patients with moderate to severe hypertension, and of up to 23% in patients with resistant hypertension.8 Of note; screening for PA should be limited to selected patient groups, as there presently are limited data linking such screening to improvement of quality of life, reduced morbidity or mortality.9
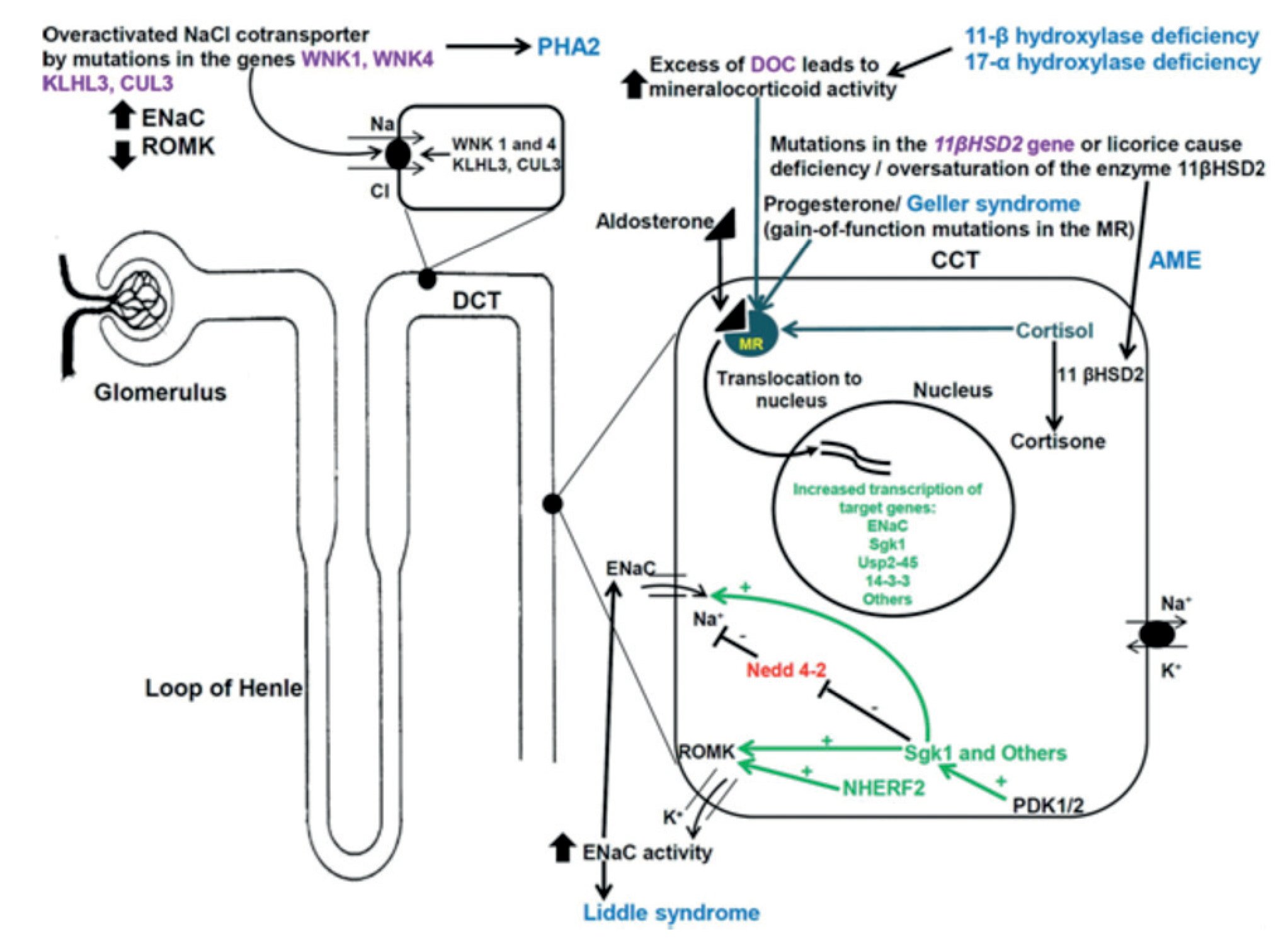
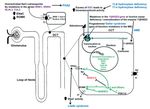
Figure 1. Inherited endocrine condition related to mineralocorticoid excess (modified after ). The picture shows the molecular pathways involved in dysregulation of NaCl homeostasis located in the distal renal tubules. +: activation; - inhibition; AME: apparent mineralocorticoid excess; CUL 3: cullin 3; ENaC: epithelial sodium channel; GRA: glucocorticoid-remediable aldosteronism; KLHL3: kelch-like 3; MR: mineralocorticoid receptor; Nedd4-2: ubiquitin-protein ligase; deubiquitylating enzyme Usp2-45; NHERF2: Na+/H+ exchange regulating factor 2; PDK1,2: 3-phosphoinositide-dependent kinases 1 and 2; PHA2: pseudohypoaldosteronism type 2; ROMK: renal outer medullary potassium channel; Sgk1: serum glucocorticoid kinase 1; WNK: with-no-lysine kinase 1,4; 14-3-3 proteins.
Conditions with low renin concentrations include:
• Mineralocorticoid Excess
• Primary aldosteronism
• Hypercortisolism (Cushing's syndrome)
• Glucocorticoid / cortisol resistance (Chrousos syndrome)
• Apparent mineralocorticoid excess syndrome
• Licorice or carbenoxolone in excess
• Congenital adrenal hyperplasia (11beta- and 17alpha-hydroxylase deficiencies)
• 11-Deoxycorticosterone (DOC), 18-hydroxy-DOC excess
• Geller Syndrome
• Familial hyperkalemic hypertension (Gordon's syndrome)
• Liddle's syndrome
After the first report of curing hypertension and hypokalemia in a Polish patient by removing an adrenocortical adenoma in 1953,12 major advances have been accomplished, namely improving our tools of measuring aldosterone, plasma renin activity (involves generating angiotensin from angiotensinogen, radioimmunoassay (not standardized among laboratories) or direct active renin (automated chemiluminescence immunoassay in Europe), of localization / imaging (adrenal vein sampling, computed or magnetic resonance tomography), and of medical or surgical therapy (with robotic assisted and/or laparoscopic adrenalectomy). Deciphering the human genome has helped identify several disease causing genes, including some being involved in primary aldosteronism, i.e. in the K-channel gene KCNJ5, encoding Kir3.4, a member of the inwardly rectifying K+ channel family. The presence of these mutations in the KCNJ5 gene alter the K+ conductance/ selectivity of this channel and consequently increase the Na+ conductivity (influx) with a further impact on voltage-gated calcium channels leading to cellular proliferation in the adrenal cortex.14 The recurrent somatic mutations (G151R, L168R) in the adrenal potassium channel KCNJ5 have been related to benign aldosterone-producing adenomas (APAs) with initial estimates reporting almost half of APAs being associated with these mutations.13-16 Subjects with the KCNJ5 G151E mutation have no features of APAs and hyperplasia, a different clinical course (not progressive) and excellent control of blood pressure with spironolactone.
We now know of familial aldosteronism type 1, type 2, and type 3, although the precise molecular pathogenesis of aldosteronomas still needs to be elucidated.17-19 Given the heterogeneity of tumors, not only between, but also within one individual, it may be more prudent to analyze family members with a known gene defect and identical tumors in a whole genome and exome wide sequencing project for targeted genes known to be involved in cell growth regulation. Some of these patients with familial aldosteronism (overall less than 5% of patients with PA) may require bilateral adrenalectomy to control their hypertension, which can occur at a young age (sometimes in childhood).
Despite all the advances in diagnosing and treating patients with primary aldosteronism over the last 60 years, it should be noted that approximately 30% of patients with primary aldosteronism have concurrent essential hypertension, which limits the surgical cure of hypertension, although biochemical cure of aldosterone excess occurs.20 Also, about 25% of patients with essential hypertension have low renin hypertension, emphasizing the need to evaluate patients with refractory hypertension, or those with an adrenal incidentaloma, by measuring the aldosterone and renin ratio, demonstrating aldosterone excess by a plasma aldosterone level that typically exceeds 15-20 ng/dl.21 The aldosterone to plasma renin activity ratio can be performed without posture stimulation and while the patient is taking antihypertensive drugs, except for mineralocorticoid receptor antagonists such as spironolactone and eplerenone, which should be discontinued at least 6 weeks prior to morning testing. Some authors recommend stopping beta-blockers 4 weeks before testing, as these drugs lower renin, and to a lesser extent, plasma aldosterone, thereby raising the aldosterone-renin ratio, with the resulting increase in false-positive tests. 18-Hydroxycorticosterone, a precursor of aldosterone has been controversially discussed as a marker for patients with aldosteronomas compared to patients with bilateral adrenal hyperplasia.21 18-hydroxycortisol and 18-oxocortisol are markedly increased in patients with familial aldosteronism type 3. The more sensitive biochemical and imaging tools have led to the detection of patients with normokalemic aldosteronism, and one should note that around 5% of all patients with hypertension have primary aldosteronism.21,22 Of note, plasma potassium is more reliable than serum potassium. A recent study showed that in patients with PA compared to those with essential hypertension, the 24 h urine sodium and potassium excretions are higher (150 mmol vs. 105 mmol; 75 mmol vs. 30 mmol), underscoring that both patient groups would benefit from a salt restricted diet.23 Salt loading and aldosterone suppression testing for patients suspected of having primary aldosteronism is usually performed by obtaining a 24 h urine sodium excretion of more than 200 mmol,24 which should be an easy task considering the recent data from the Centers for Disease Control and Prevention, showing that 9 in 10 Americans consume more than 2.3 g of sodium daily, which corresponds to 100 mmol. This important fact illustrates how difficult it is to combat sodium intake and high blood pressure, as well as obesity, in various countries, especially when considering that many people no longer prepare fresh foods, but use preserved or processed food items.
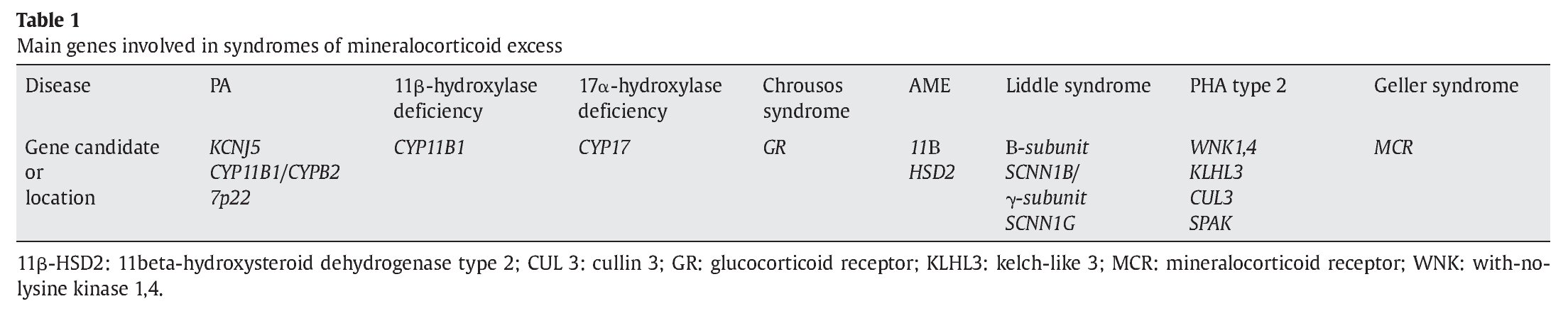
Low renin hypertension in childhood can be caused by PA, apparent mineralocorticoid excess, 11-beta hydroxylase deficiency, or 17alpha-hydroxyalse deficiency (Table 1). Certain populations have an increased risk.8,25 Signs and symptoms in patients with 11-beta hydroxylase deficiency are variable, and hypokalemia is infrequently observed. In the non-classic form, women may present with a clinical phenotype of polycystic ovarian syndrome. Patients with 17-alpha-hydroxylase deficiency are frequently Dutch Mennonites immigrated from Portugal and Spain, and as genotypic XY individuals may show genital ambiguity, sometimes with breast development during puberty, whereas no breast development is usually seen in XX individuals, often then referred to as sexual infantilism.26,27 In obese individuals, one could perhaps propose esterified and free estradiol in adipose tissue. Hypertension has now also become a problem in patients with 21-hydroxylase deficiency congenital adrenal hyperplasia, with elevated blood pressure and insulin resistance being more common in classic than in non-classic patients, and being associated with suppressed plasma renin activity in a cohort of 24 patients on chronic glucocorticoid therapy.28
In patients with Cushing's syndrome, it still is very important to increase awareness of fellow colleagues to only use the lowest possible dose of any glucocorticoid needed to achieve a desired effect, while avoiding exogenous over-replacement effects, including those that increase blood pressure. This now may be made easier in patients with adrenal insufficiency on a novel hydrocortisone dual-release formulation, which has shown a reduced blood pressure, lower body weight, and improved glucose metabolism, compared to traditional thrice-daily conventional hydrocortisone tablets.29 The morbidity and mortality of patients with Cushing's syndrome is mainly determined by the hypertension and type 2 diabetes / insulin resistance.30 This should certainly be kept in mind when discussing and exploring newer treatment options for patients with endogenous hypercortisolism. Among the contemporary adrenal suppressing agents ketoconazole, metyrapone, etomidate, mitotane, and aminogluthetimide, and newer agents such as the glucocorticoid receptor type 2 antagonist mifepristone and the somatostatin analog pasireotide have been recently approved.31-35 That hypertension is linked to subclinical Cushing's syndrome has been controversially discussed, but will become increasingly important, similar to the emerging group of patients with adrenal or pituitary incidentalomas and subclinical / silent hypercortisolism or with silent pheochromocytoma.21,36-38 In this context, it is worthwhile thinking about a given hormone measurement, the effect at its receptor, and the distribution and density of such receptors in target tissues. This is exemplified by Chrousos syndrome or primar y generalized or sporadic glucocorticoid resistance and hypersensitivity.39,40 Individuals with certain single nucleotide polymorphisms and/or mutations in the glucocorticoid receptor gene have a higher propensity for hypertension, insulin resistance, and obesity than those without such sequence variants.5
Pheochromocytoma
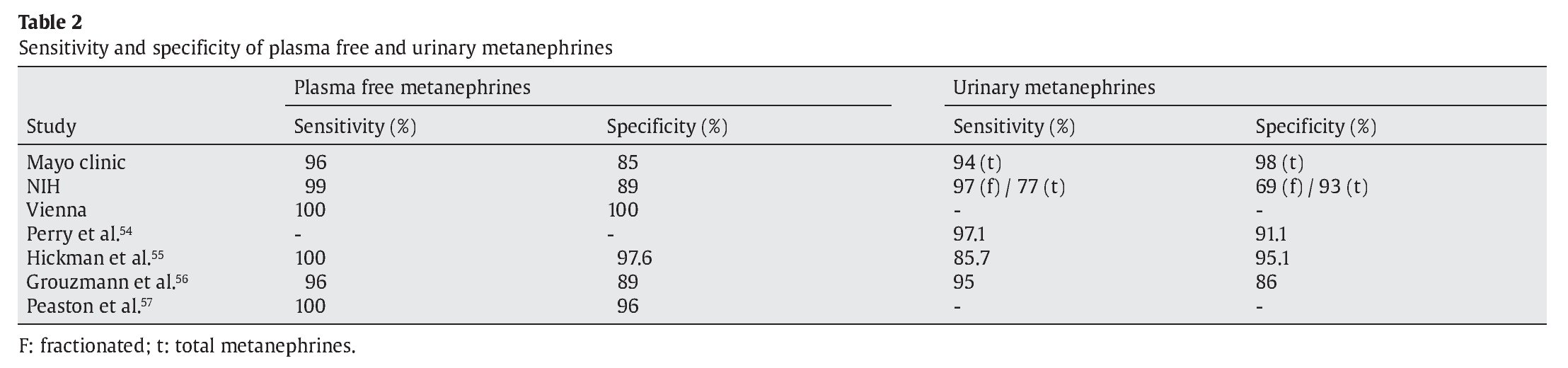
The prevalence and pretest probabilit y of patients with pheochromocytoma are, in descending order: multiple endocrine neoplasia type 2 (approx. 50% of patients), von Hippel Lindau syndrome (26%), familial paraganglioma syndrome (20%), neurofibromatosis type 1 (<5%), adrenal incidentaloma (<10%), hypertension and suggestive symptoms (0.5%!), unselected hypertensives (0.2%!). Over the last 12 years, investigators have identif ied several new genes involved in the inheritance of pheochromocytoma and extra-adrenal paraganglioma.41-47 This has led to the detection of preclinical pheochromocytoma / paragangliomas by genetic testing of affected and at risk family members, with many of these patients being asymptomatic, raising the question of when one should surgically intervene, and with which type of surgery, as there are now laparoscopic transperitoneal and retroperitoneoscopic approaches for adrenal pheochromocytomas.48 Asymptomatic patients with pheochromocytoma have previously been reported in 15 of 150 patients seen at the Mayo Clinic,49 and in 19 of 33 patients with adrenal pheochromocytomas that were incidentally discovered.50 Advances in the biochemical screening for pheochromocytoma include the addition of methoxytyramine for paragangliomas and the assessment of plasma free and urinary fractionated metanephrines by liquid chromatography, and tandem mass spectrometry.51,52 Table 2 shows test performance of plasma free and urinary fractionated metanephrines measured in various centers across the globe, where a negative test was defined as both metanephrine and normetanephrine within the reference range, and a positive test as either metanephrine or normetanephrine higher than the reference range.53
Among the newer genes and proteins is TMEM127, a negative regulator of mammalian target of rapamycin (mTOR) effector proteins.44,45 In patients with multiple endocrine neoplasia type 2, the molecular pathogenesis of pheochromocytomas includes germline mutations in the RET proto-oncogene.17,58-61
Patients with von Hippel Lindau (VHL)-associated pheochromocytoma (approx. one third) often have silent tumors, and extra-adrenal paragangliomas in up to 30%.37,62,63
These extra-adrenal pheochromocytomas or paragangliomas in patients with VHL disease can be sympathetic or parasympathetic and, if located in the head and neck, are mostly unable to produce and secrete catecholamines.62-64
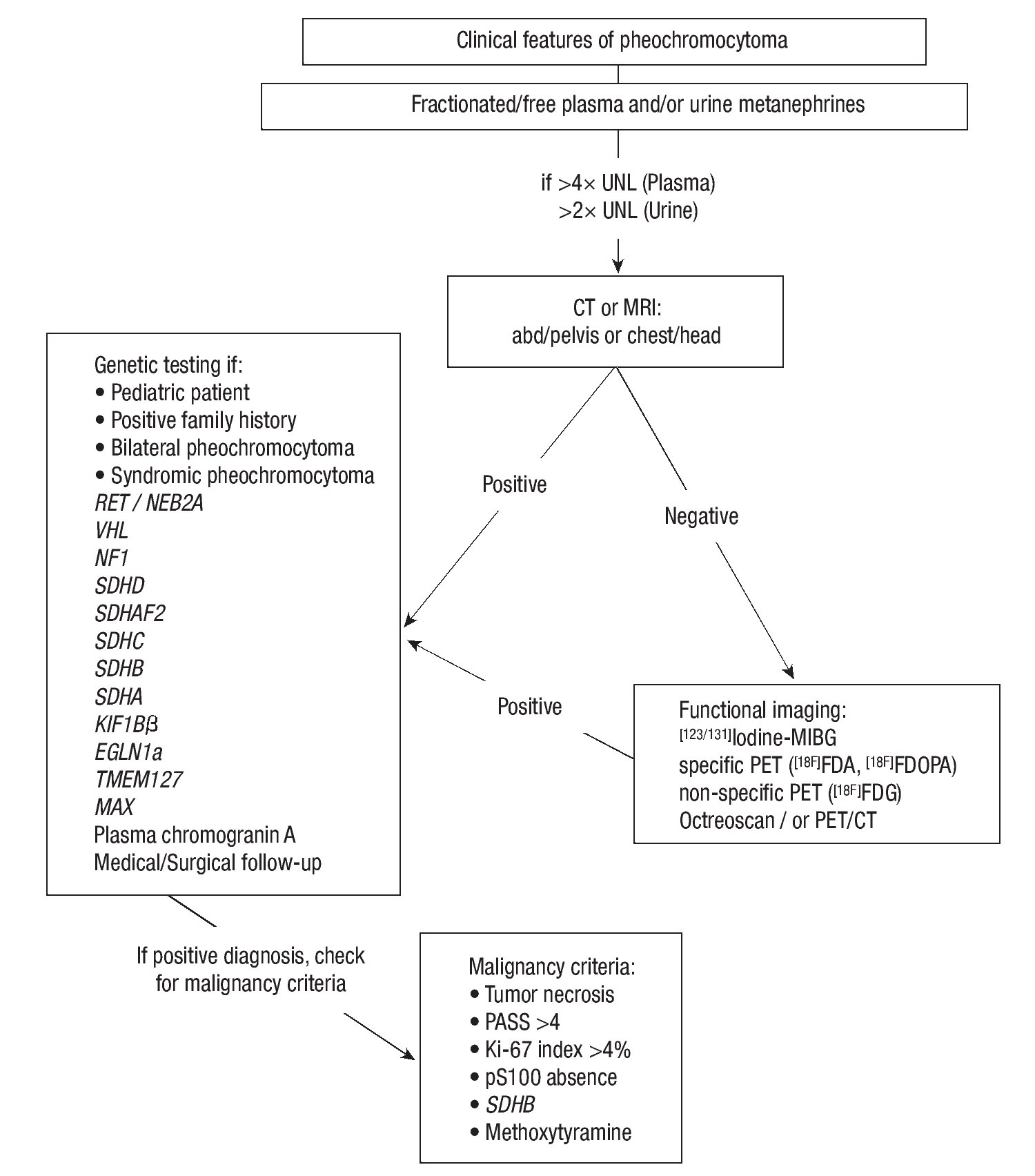
In general, extra-adrenal pheochromocytomas have a higher frequency and potential of malignancy than pheochromocytomas located in the adrenal gland.65 Malignant pheochromocytomas (defined as the presence of chromaffin tissue at locations where such tissue should not be present, i.e. lungs, liver, bone, lymph nodes) are uncommon in patients with VHL syndrome and Multiple endocrine neoplasia type 2 (MEN 2), with frequency rates less than 5%. Unfortunately, there are still no markers that can reliably distinguish a benign from a malignant pheochromocytoma, although germline mutations in the SDHB gene, PASS (pheochromocytoma of adrenal gland scaled score) >4, size and pheochromocytoma weight, Ki-67 index >4%, and lack of pS100 appear to be of prognostic value (see Figure 2 and Chapter 7 in the Endocrine Testing Protocol section, section editor: Christian A. Koch, by Eugen Melcescu & Christian A Koch 2012 at http://www.endotext.org/protocols/ protocols7/protocolsframe7.htm).
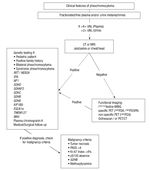
Figure 2. Flow chart for diagnostic evaluation for Pheochromocytoma. Adapted after: Waguespack SG, et al. JCEM. 2010;95:2023-37, from Chapter 7, Melcescu & Koch. Testing for endocrine hypertension. In: De Groot L, editor. Available from: www.endotext.org, section: Christian A. Koch.
Determining the screening age for pheochromocytoma in VHL germline mutation carriers should depend on the potential beneficial and negative sequelae that could occur if screening were not performed, i.e. malignancy (lower than 5% in VHL disease !), hypertension, radiation exposure from frequent imaging tests. There is marked intrafamilial variation, and the penetrance of pheochromocytoma may be as late as age 54 years. At present, experts recommend that pheochromocytoma screening should be started in childhood, at the age of 8 years. The adrenal glands can be visualized while undergoing an MRI to look for renal abnormalities in patients with VHL disease. Approximately 40% of pheochromocytomas in children have an underlying hereditary cause with germline mutations in either the SDHx genes, RET proto-oncogene, VHL gene, or neurofibromatosis type 1 gene. More than 70% of pheochromocytomas presenting in children are due to VHL disease. The diagnosis can further be established by abdominal computed tomography and/or magnetic resonance imaging. Positron emission tomography should only be performed in patients with metastatic disease.
Given the low frequency rate of extra-adrenal pheochromo cytomas (approx. 15%) and malignant pheochro mocytomas (< 5%) in patients with VHL disease, only selected patients should undergo 123I-MIBG scanning to search for extra-adrenal or metastatic lesions, before adrenalectomy is performed. Some experts report a tumor size of larger than 5 cm, others of larger than 10 cm as trigger to perform 123I-MIBG imaging.21,48 Importantly, (isolated) pheochromocytoma can be the presenting manifestation of VHL syndrome. Approximately 50% of patients with apparently isolated familial pheochromocytoma have VHL disease and approximately 10% of patients with apparently sporadic pheochromocytoma, acknowledging that a family history is not always present or reliably obtainable, and that even if VHL germline mutations were detected by certified laboratory testing in patients with non-syndromic pheochromocytoma (i.e. in 3 of 182 patients), misdiagnoses can occur.66
A cost-effective panel of genetic profiling by performing mutation analysis for the VHL, RET, SDHB, SDHD, TMEM127, MAX, SDHC, SDHA, SDHAF2 genes is debatable for all patients presenting with apparently sporadic pheochromocytoma, since up to 33% have germline mutations in the aforementioned genes.67
Whether non-functional pheochromocytomas in VHL patients (only clinically remarkable as adrenal masses on imaging with normal fractionated urinary metanephrines and/or normal plasma free metanephrines) require treatment/surgery, and if they are smaller than 5 cm, it remains unclear. Important aspects in this context are the questions such as; how fast do these tumors grow, if and when they cause symptoms, and if and when (at what size) they metastasize.
The likelihood of malignancy among hereditary pheochromocytoma syndromes when having a germline mutation seems to be in descending order in the following genes: SDHB, (MAX), NF1, (TMEM127), VHL, RET, SDHD. Therapy with sunitinib, a tyrosine kinase inhibitor of vascular endothelial growth factor, platelet derived growth factor, RET, c-KIT, and FLT-3 receptor, may be promising, when looking at a 21% tumor shrinkage (pelvic malignant pheochromocytoma) and reduction of plasma normetanephrine and chromogranin A after 6 months of therapy. Other experimental therapies include arsenic trioxide, radiolabelled somatostatin analogs, including Yttrium-90-DOTATOC (90Y-DOTA-TOC) and Lutetium-177-DOTA0-Tyr3-octreotate (177Lu-DOTA-TATE), the mTOR inhibitor everolimus (RAD001) in combination with octreotide, novel dual PI3k/mTOR inhibitor (NVP-BEZ235), and HIF1a inhibitors.46,68-71 After surgery (and 131I-MIBG therapy) for malignant pheochromocytoma, the most used and effective chemotherapy regimen still consists of a combination of cyclophosphamide, vincristine, and dacarbazine (Averbuch protocol).
Non-traditional hormone conditions linked to hypertension
Hormonal imbalances are now present in many communities and societies, with an increasing number of obese and insulin resistant individuals compared to decades ago. Obesity-associated hypertension is very complex as there are various forms of obesity, some without insulin resistance or without any inflammatory state. As the ancient Greeks, Romans, and Egyptians emphasized "mens sana in corpore sano" should be the motto for many people today. Unfortunately, only a few are still able to listen to their internal voice and body, and train both mind and body to be strong.
Inflammatory conditions including some forms of obesity and type 2 diabetes can lower testosterone and growth hormone, leading to a decline in the lean body mass, and a further increase in fat body mass, thereby contributing to more stress and increased sympathetic nervous system activity, resting tachycardia, insulin resistance, and hypertension.72-77 Chronic stress can also reduce growth hormone levels, with subsequent loss of lean body mass and an increase in fat mass, with more predisposition to insulin resistance and the development of the metabolic syndrome including hypertension. While data on the relationship between growth hormone deficiency and hypertension are emerging, the link between growth hormone excess and hypertension is strong, with good evidence showing an improvement or resolution of hypertension after successful treatment of acromegalic patients.73,78
Whether headaches in acromegalics are linked to hypertension or the GH excess alone, or bone sequelae from it, remains unclear. Many acromegalics presenting with headaches report improvement of their headaches after normalizing insulin-like growth factor 1 levels. One should remember that mortality in acromegalics (and GH levels >2.5 ng) has been shown to be related to cardiovascular issues including, among others, hypertension, cardiomyopathy, and arrhythmias.
Testosterone deficiency has now been recognized as a problem, not only for sexual function and bone health, but also for glycemic control and cardiovascular health.79 Serum total testosterone levels, even when measured with the best assay (currently with liquid chromatography and mass spectroscopy) often do not correlate well with symptoms and signs, illustrating the challenge of assessing intra- and inter-individual testosterone activity. A total serum testosterone level of greater than 200 ng/dl has been shown to be beneficial for bone health and risk of osteoporosis, whereas there is a wide range for sexual function and glycemic control, with most authorities considering a cut-off of 350 ng/dl.72
Obese individuals also frequently are found to have low 25-hydroxy vitamin D (25-OHD) levels, with data showing an improve ment in cardiovascular blood flow and function, including blood pressure, after raising 25-OHD (80). In this context, it is important to remember that biologically active vitamin D helps resorb calcium better, with some investigators having concerns about increasing cardiovascular risk due to calcium intake. However, recent studies, including those with an intake of 1 g of calcium citrate demonstrated no such risk, but rather improved coronary blood flow.81-83 Patients with longstanding severe primary hyperparathyroidism frequently show calcifications of their coronary arteries, and several studies have linked primary hyperparathyroidism to hypertension. However, the data in this context, including studies on reversing high blood pressure by surgical treatment of primary hyperparathyroidism, are conflicting.84,85
Similarly, there are data for and against the role of hypo- and hyperthyroidism in blood pressure regulation. Recently, studies showed that hypothyroidism can raise blood pressure by volume expansion with low plasma renin levels.86-88 Typically, marked reduced sensitivity to sympathetic agonists, increasing peripheral vascular resistance, and arterial stiffness are observed in hypothyroid patients, whereas hyperdynamic circulation changes are seen in patients with hyperthyroidism.89 Beta-blockers are preferred for blood pressure control in hyperthyroid patients, whereas calcium channel blockers and diuretics, as well as a low sodium diet, often improve blood pressure in patients with hypothyroidism. Women with subclinical hypothyroidism identified during pregnancy are at increased risk for severe pre-eclampsia compared to euthyroid women.90 Increased cardiac output leads to a reduction in plasma renin activity and in peripheral vascular resistance, together with isolated systolic hypertension in hyperthyroid women.91
Neuroendocrinological considerations
Mineralocorticoid receptors (MR) apparently play a role in certain areas of the brain including, the (anterior) hypothalamus, hippocampus, nucleus ventromedialis hypothalami, nucleus ambiguus, nucleus tractus solitarii, locus ceruleus, area postrema, and anterior pituitary, influencing homeostatic control of blood pressure, sodium intake, water and electrolyte balance, and sympathetic activation of heart and kidneys.92,93 One of the mechanisms of intracerebral aldosterone / MR action leading to an increase in vascular resistance and sympathetic tone appears to be a reduction of baroreceptor discharge.94
Excessive MR activation can promote inflammation, fibrosis, and heart disease, as well as psychiatric illness including anxiety and depression through key modulators, such as the glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenases (11β-HSDs).95,96 So-called neurosteroids, synthesized in the brain, are involved in complex interactions within and outside the brain.97,98 In addition, steroids also enter the brain from the peripheral circulation. Patients with chronic hypercortisolemia frequently have depression, which appears to be related to dopamine synthesis in certain brain areas.99-101 Dopamine plays an important role in addictive behaviors, including compulsive shopping, eating, sodium appetite, gambling, and hypersexuality.102-106 Enkephalin surges in an anteromedial quadrant of the dorsal neostriatum have recently been found to contribute to generating intense consumption of palatable food.107
The relationship between hormonal and neural pathways (hypothalamus; arcuate nucleus; ventromedial, dorsomedial, and paraventricular nucleus) that regulate energy homeostasis and body fat are well shown and reviewed in (6). Involved are glucagon-like peptide 1, neuropeptide Y, oxyntomodulin, proopiomelanocortin, pancreatic polypeptide, peptide YY, orexin, cholecystokinin, alpha-melanocyte stimulating hormone, Agouti-related protein, cocaine-and amphetamine-regulated transcript, melanin-concentrating hormone, and others. There is obviously a complex imbalance between the hunger hormone ghrelin and various satiety hormones including glucagon-like peptide 1, peptide Y, cholecystokinin, amylin.108 Given the global explosion of fast food restaurants over the last several decades and the dramatic rise in obesity, especially since 1990, it is worthwhile asking the question on whether fast food is addictive in addition to being unhealthy, with inorganic phosphate in food additives causing vascular damage.109-111
Taste perception
One of the fundamental senses in humans is the taste system, with each taste being represented in its own separate cortical field, making up the gustotopic map in the brain.112 Manipulating this fundamental taste system, for instance, by masking of unwanted taste, can lead to commercial gains.113 So-called diet drinks with less sugar content but ingredients such as non-nutritive sweeteners like aspartame can affect perceived taste.114 German researchers have recently shown that obese children (age 6 to 18 years) have less sensitive taste buds when undergoing a paper strip test with the taste qualities, sweet, sour, salty, bitter and umami (savory). In that study, obese children rated sweet samples consistently as less sweet than did the normal-weight children.115 A sixth basic taste component, one that helps to detect dietary fat, has recently been suggested for obese subjects. The lipid taste receptor apparently is less able to detect triolein and oleic acid due to the intake of orlistat, a lipase inhibitor and weight loss medication.116 Salt consumption and salt appetite play an important role in blood pressure regulation and hypertension. Sodium salts often are added for flavor enhancement (for instance, to block or mask unpleasant tastes such as bitterness in cheese), or for functional purposes (i.e., preservation of food that is not immediately consumed). As the perceived intensity of sodium chloride shows a wide inter-individual variation, which in part is explained by individual taste function, the general over-consumption of salt / sodium chloride is mainly related to hedonic behavior.117,118 Sex hormones appear to play a role in the salt preference of men and women, as we know from studies including children and throughout pregnancy.119,120 During pregnancy, total serum cortisol concentrations increase, ovaries and maternal decidua produce early rises in renin levels causing 5-20 fold increases in plasma aldosterone levels in the third trimester.121 Further stimulation of aldosterone production occurs due to the increased estrogen and subsequent angiotensin II levels.122 Progesterone furthermore stimulates the production of another potent mineralocorticoid, deoxycorticosterone, which rises by 2-3 fold.123
Normal salt discrimination is important for blood pressure control, and is dependent on the taste receptor field origin, nerve transmission and respective circuit in the brain. Oral amiloride, an epithelial sodium channel blocker that is frequently used in patients with hypertension, can severely affect the discrimination between sodium chloride and potassium chloride taste in rats.124 Syndromes of mineralocorticoid excess typically result from overactive amiloride-sensitive sodium channels.8 These are located in the distal tubule and collecting ducts of the kidney, as well as other tissues, including vascular smooth muscle, sweat glands, and distal colon.125-128
In normotensive young men with a positive family history of arterial hypertension, salt loading with 5 g of sodium chloride daily for one week (which is far below the daily consumption of most Americans) did not result in an adequate suppression of aldosterone, likely increasing their risk of becoming hypertensive in the future if overconsumption of salt were continued, and if the central effects of aldosterone and stimulation of the MR were taken into account.92,129
Bitter taste receptors have the function to protect against ingestion of potentially toxic substances and belong to the G-protein coupled receptor superfamily.130 If cheese were less salted, it would taste more bitter, and would likely lead to less consumption of both salt and calories from cheese, and concomitantly consumed food items and/or alcohol (wine etc.). While the bitter taste is usually masked with increasing salt content, sour / acidic taste (commonly found in preser vatives) is compensated by an admixture of sweeteners or sugar, thereby increasing the consumption of such products.131 The cephalic phase insulin release is characterized by insulin release before increasing plasma glucose levels by tasting sweet food. In Wistar rats, the non-nutritive sweetener saccharine can elicit this cephalic phase insulin release, but non-sweet starch, which is nutritive, cannot.132 In humans, diurnal variation of sweet taste recognition thresholds is correlated with plasma leptin levels, with the lowest thresholds in the morning and the highest thresholds at night in non-obese subjects.133 It would be interesting to know the sweet taste recognition thresholds of obese individuals with leptin resistance and often insulin resistance. Ghrelin, a 28-amino acid peptide hormone, stimulates the release of growth hormone, appetite, food-seeking behavior, and olfactory sensitivity, as well as inf luencing taste responsiveness, with plasma ghrelin levels being elevated before meals and reduced after eating.134 Data from ghrelin cognate receptor (GHSR) null mice that showed significantly reduced taste responsivity to sour (citric acid) and salty (sodium chloride) tastants, suggest that ghrelin, which is present within the taste buds of the tongue, plays a local modulatory role in taste bud signaling and function.135 In Dahl-Iwai salt-sensitive rats, continuous administration of the ghrelin receptor antagonist, [D-Lys3]-GHRP-6, can induce salt-sensitive hypertension, probably by increasing autonomic sympathetic nervous activity.136
Ghrelin plasma levels are decreased in obese individuals, unless Prader Willi syndrome is present, in which case circulating ghrelin levels are high.137 One could wonder how the physiology of ghrelin, satiety hormones, and hypothalamic functions in general (food intake, thirst perception, osmostat, reproductive behavior, etc.) could be manipulated to generate the perfect storm for obesity and its consequences, including hypertension with a variety of hedonic and addiction behaviors, perhaps facilitated by an evil cocktail of food additives and drugs, including antidepressive agents.138,139
Conclusion
In addition to obesity, type 2 diabetes, and metabolic syndrome, hypertension represents a major public and global health problem, most of which could probably be improved by lifestyle changes, including changing dietary habits with a lower consumption of the processed and preserved foods that generally contain higher amounts of salt than freshly preserved food items. A Cochrane Database systematic review in 2011 showed that sodium reduction reduced blood pressure by 1% in normotensives and by 3.5% in hypertensives.140 In addition, an increase in physical activity can improve overall health dramatically and certainly would help achieve the ancient Greek and Roman model "mens sana in corpore sano".141 Endocrine hypertension is on the rise when considering non-traditional factors such as obesity-associated hypertension, testosterone, GH, and vitamin D deficiency and such like. The rare syndromes of mineralocorticoid excess are linked to overactive amiloride-sensitive sodium channels, and are becoming more common in an environment promoting sodium chloride intake. The treatment for most of these conditions consists of restricting dietary salt intake. In addition, some require special therapies including dexamethasone / hydrocortisone for patients with congenital adrenal hyperplasia, spironolactone / eplerenone for apparent mineralocorticoid excess, epithelial sodium channel inhibitors amiloride / triamterene for Liddle syndrome and for familial and sporadic hyperkalemic hypertension.
Disclosures
Prof. Christian A. Koch has served as consultant for Novo Nordisk and Ipsen, and as Principal Investigator of the SEISMIC and the TR321 trials. Together with Prof. GP Chrousos, Prof. Koch has edited a book entitled "Endocrine Hypertension" published by Springer / Humana Press, in 2012. Parts of this manuscript stem from previous reviews, including the ones from the online textbook of Prof. Leslie De Groot (Chapter 7 under the CAK Section, and Chapters 25, 26, and 35 under the GPC Section).
E-mail:ckoch@umc.edu
ARTICLE INFO
Article History:
Received 7 January 2012
Accepted 20 November 2012