To describe the rare association between primary biliary cirrhosis (PBC) and Graves disease (GD).
MethodsReport the clinical, biochemical, and pathological findings of the patient and review the relevant literature.
ResultsFemale, 63 years old, sent to the endocrinologist for hyperthyroidism. The patient had a history of cholecystectomy and PBC diagnosed 9 years (previously). Before, she reported palpitations, excessive sweating, irritability and weight loss.
Physical examinationheart rate of 80bpm, coarse tremor, bosselated thyroid and non-pulsatile.
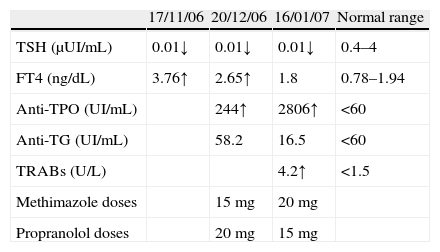
Laboratory findingsTSH 0.01μUI/mL (0.27–4.20), free T4 3.76ng/dL (0.93–1.7), anti-TPO 244UI/mL (<34), TRAbs 4.2U/L (<1.5).
Patient was treated with methimazole 15mg/day and propranolol 20mg/day. For persistence of hyperthyroidism, despite the increase in the methimazole dose, the patient underwent radioiodine therapy. Afterwards the developed hypothyroidism and therefore was treated with levothyroxine. She is currently euthyroid.
ConclusionDespite frequent association of autoimmune diseases in the same individual, the authors present this case because of the rare association between PBC and GD.
Descrever a associação rara entre cirrose biliar primária (CBP) e doença de Graves (DG).
MétodosDescrevemos os dados clínicos, bioquímicos e patológicos do doente e revemos a literatura relevante.
ResultadosSexo feminino, 63 anos, enviada à consulta de endocrinologia por hipertiroidismo. Paciente apresentava antecedentes de colecistectomia e CBP diagnosticada há 9 anos. Referia palpitações, sudorese excessiva, irritabilidade e perda de peso.
Exame físico: frequência cardíaca de 80bpm, tremor grosseiro, tiroide bosselada e não pulsátil. Dados laboratoriais: TSH 0,01μUI/mL (0,27–4,20), T4 livre 3,76ng/dL (0,93–1,7), anti-TPO 244UI/mL (<34), TRAbs 4,2U/L (<1,5).
A paciente foi medicada com metimazol 15mg/dia e propranolol 20mg/dia. Por persistência do hipertiroidismo, apesar do aumento da dose do metimazol, a doente foi submetida a terapêutica com iodo. Posteriormente desenvolveu hipotiroidismo e, por isso, foi medicada com levotiroxina. Atualmente, está em eutiroidia.
ConclusõesApesar da associação frequente de doenças autoimunes no mesmo indivíduo, os autores apresentam este caso pela associação rara entre CBP e DG.
Primary biliary cirrhosis (PBC) is most frequently a women's disease1 that occurs between the fifth and seventh decades of life.2 The etiology is unknown, although it is presumed to be autoimmune in nature.1–3 The classic pathophysiological process in PBC is damage to the biliary epithelial cells lining the small intrahepatic bile ducts (and their progressive destruction). Bile duct loss is progressive and in the end stages of the disease there can be a complete loss of small intrahepatic ducts. Fibrosis occurs within the liver as a consequence of progressive damage, and this can lead to cirrhosis over a variable time period.4
Associations between PBC and other autoimmune diseases have been reported: autoimmune thyroiditis, rheumatoid arthritis, Sjögren's syndrome and Raynaud's phenomenon.1,3,5 Although association of PBC with hyperthyroidism is quite rare,3,5–7 in this case report the authors describe a patient with PBC and hyperthyroidism by Graves’ disease (GD). Only six patients with PBC and hyperthyroidism have been reported in the literature: four with GD, one with hashitoxicosis and another with painless thyroiditis.1,3,8–10
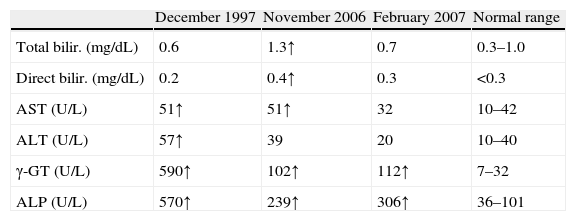
Case reportA 55-year-old woman was admitted due to liver dysfunction in February 1998. Six months earlier, she was found to have an elevated level of γ-glutamyl transpeptidase (γ-GTP) at 590U/L (normal range, 7–32), alkaline phosphatase (ALP) at 570U/L (normal range, 36–101), aspartate aminotransferase (AST) at 51U/L (normal range, 10–42) and alanine aminotransferase (ALT) at 57U/L (normal range, 10–40); hemogram with platelets and bilirrubin were normal; eritrocyte sedimentation rate (ESR) was 63 (normal range, <35). This patient was not an alcoholic, and had no history of blood transfusions or drug allergies but with a history of cholecystectomy for 22 years previously due to gallstones. She had malar eryhtema; no ascites, edema or xanthomas.
Abdominal ultrasonography: “Liver with increased size, regular borders and uniform texture; normal biliary duct system, spleen, pancreas and kidneys without changes.”
Antimitochondrial antibodies (AMAs) were positive (+++). Hepatitis C virus and hepatitis B surface antigen were both negative. Anti SSA, SSB, Sm, RNP, Scl70, centromere, nucleolar, gastric parietal cells, liver–kidney microsomal, actin, gliadin, endomisio and reticulina antibodies, were negative as well. Antinuclear antibody (ANAs) and anti smooth muscle antibody (SMA) were positive (+++).
Pathological study of the biopsy material from the liver showed PBC stage IV (“…septal fibrosis, sometimes delimited nodules of hepatocytes… portal spaces are expanded by the presence of a mononuclear infiltrate of moderate intensity, accompanied by ductular reaction and paucity of bile and interlobular ducts. There is halo effect where the periportal hepatocytes have clear cytoplasm and xanthomatous appearance of protein deposits associated with copper, indicating chronic cholestasis. Periportal inflammatory activity of type bile and mild lobular, as well as bilirrubinostasis”). The patient was diagnosed as PBC due to the clinical criteria and pathological findings. Treatment with ursodeoxycholic acid 750mg/day was initiated.
The patient was followed in the Gastroenterology department and treated with immunosuppressors and immunomodulators. Only one hospitalization, in April 2006, for decompensation (jaundice and bilirubinuria) (Table 1).
In December 2006 the patient was sent to the Endocrinology outpatient clinic for hyperthyroidism. At the time, she was being treated with ursodeoxycholic acid 500mg twice a day. Palpitations, excessive sweating, irritability and weight loss were reported in the previous month.
Physical examination revealed blood pressure of 130/80mmHg and pulse was 80beats/min; no exophthalmia. She presented coarse tremor, bosselated and non-pulsatile thyroid. Laboratory findings: TSH 0.01μUI/mL (0.27–4.20), free T4 3.76ng/dL (0.93–1.7), anti-TPO 244UI/mL (<34) and TRAbs 4.2U/L (<1.5) (Table 2).
She was then treated with methimazole 15mg/day and propranolol 10mg twice a day.
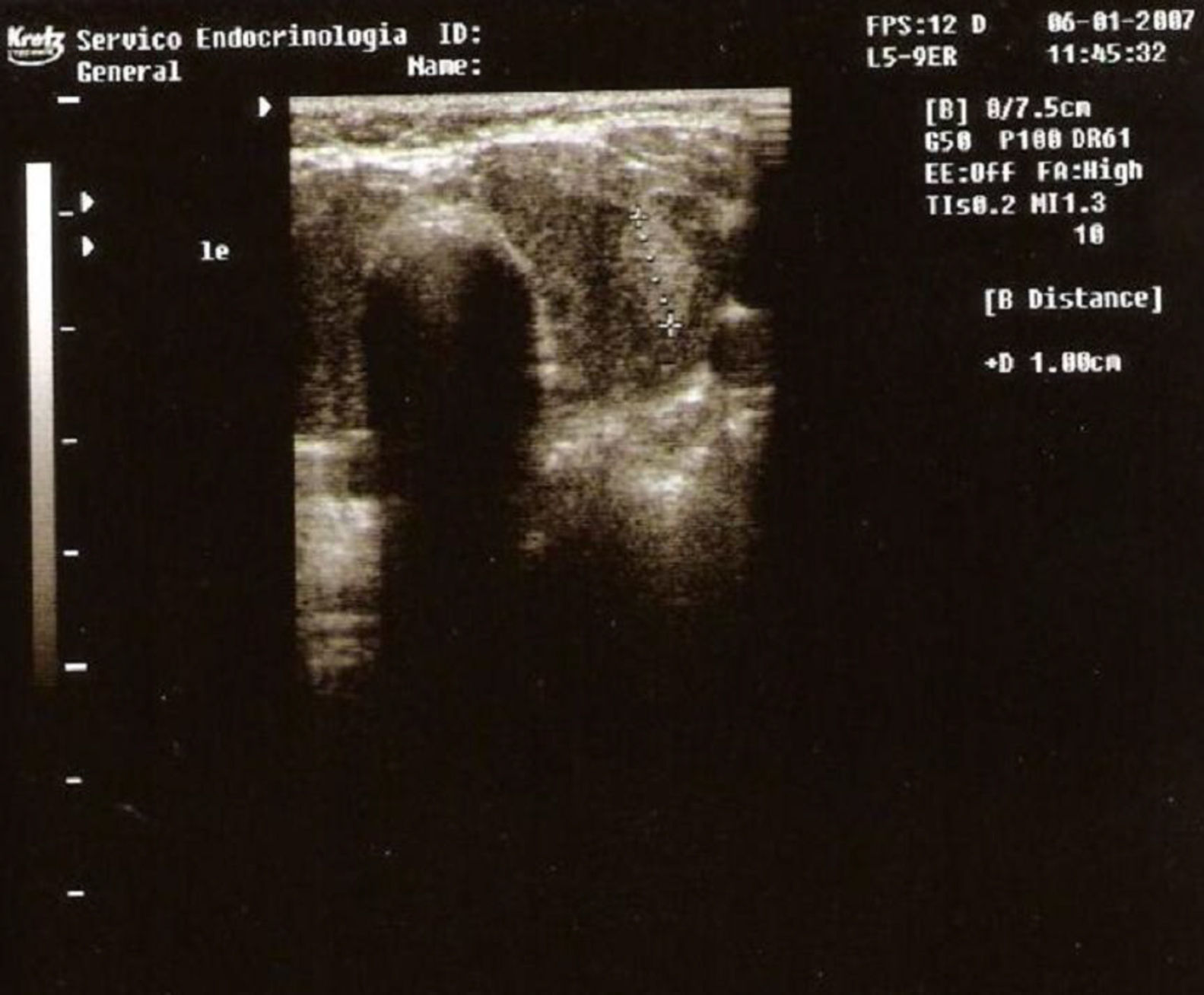
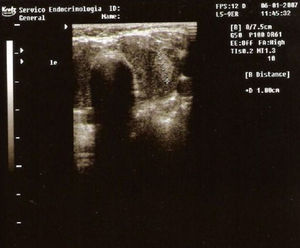
Thyroid ultrasonography showed an enlarged, lobulated, slightly heterogeneous gland and a nodule with 1cm in diameter in the left lobe (Fig. 1).
Fine-needle aspiration biopsy was performed and the diagnosis was colloid nodule.
The iodine thyroid scintigraphy showed diffusely increased uptake and distributed
almost uniformly with only a small hypoactive area located on the middle third of the left lobe; this study was highly suggestive of Graves’ disease.
The methimazole dose was carefully increased because of previous liver disease. Due to persistence of hyperthyroidism and slight worsening of liver tests, the patient underwent radioiodine therapy (7.4mCi).
Afterwards the patient developed hypothyroidism and was therefore treated with levothyroxine (0.15mg/day). She is currently euthyroid.
Discussion and review of literaturePBC is diagnosed by liver biopsy and presence of AMAs. AMAs can be found in 90–95% of patients with PBC, and have a specificity of 98% for this disease. ANAs can be identified in 20–50% of patients with PBC; SMA may also be found in this type of patients.11 Laboratory data in our patient fulfilled all diagnostic criteria for PBC: significant elevations of the ALP and γ-GT; elevated ESR is present in most cases.
It is possible that a patient with one autoimmune disease presents another one. PBC with hyperthyroidism was reported in only six patients: four with GD, one with hashitoxicosis and another with painless thyroiditis.
GD is currently accepted as an autoimmune disease of unknown etiology. The gene CTLA-4 (Cytotoxic T lymphocyte-associated antigen-4), located on chromosome 2q33 has been implicated in the onset of GD and PBC. CTLA-4 is a T cell surface molecule which interacts (in competition with the costimulatory molecule CD28) with the ligands B7-1 and B7-2 on antigen-presenting cells to influence the induction, maintenance and, particularly, termination of peripheral T cell responses.12–14 The net interaction of CD28 and CTLA-4 with their ligands may influence the nature of T-cell responses by determining the extent and kinetics of T cell activation.
A strong association between the CTLA-4 exon 1 polymorphism and PBC was reported.12 Meta-analysis involving over 13,000 subjects found significant association between polymorphisms in the A49G and CT60 alleles with GD and Hashimoto's thyroiditis.15 This supports the hypothesis that common immunoregulatory pathways may be involved in the etiology of autoimmunity.
Finally, both diseases, GD and PBC, are associated with DRB1*0801.17,18
ConclusionLike several bizarre autoimmune associations, this case shows that the connection of PBC with GD is possible (although extremely rare).
The choice of methimazole and the careful increase of dosage was due to the following facts: hepatic toxicity with methimazole is not as severe as the potentially life-threatening hepatocellular reactions that are seen with propylthiouracil; the patient had abnormal hepatic tests predominantly cholestatic – rare hepatic abnormalities associated with methimazole are typical of a cholestatic process; the side effects of methimazole are dose-related.16 In cases of previous liver disease we propose the monitoring of liver tests due to the possibility of worsening. If necessary, treatment should be made with 131I (which happened in this case).
Our recommendation for all clinicians is that when a patient has an autoimmune disease, extra attention should be paid to the signs and symptoms in order to investigate the presence of another autoimmune disease as the association of diseases with this etiologic basis is common.
Conflicts of interestThe authors declare no conflicts of interest.