Pseudohypoparathyroidism type 1b (PHP1b) results from methylation defects in the maternal GNAS locus. It is implied that the observed hypocalcemia results from impairment of renal vitamin D 1α hydroxylase. This study was undertaken to clarify: (1) How serum concentrations of calcium, phosphate, PTH and 1,25 dihydroxy vitamin D (1,25OH2D) relate to each other in patients with PHP1b; (2) how a mathematical model of calcium metabolism could reproduce the observed findings.
PatientsThe study included 139 untreated patients, retrieved from the literature, with simultaneous measurements of Ca, P and PTH of whom 25 had measurements of 1,25OH2D.
ResultsMean values and standard errors of the mean (SEM) were: PTH=43pM (3.66); P=2.16mM (0.056); Ca=1.69mM (0.038); 1,25OH2D=84pM (10.9). Phosphate correlated negatively with calcium (R=−0.590; p<0.001) and with age (R=−0.623; p<0.001). No other correlations were observed. The mathematical model simulated closely the biochemical pattern of PHP1b when the clearance of phosphate was reduced to 20%, the clearance of calcium was doubled and the response of 1α hydroxylase was reduced to 30%.
Conclusions(1) In PHP1b the concentrations of 1,25OH2D are mostly normal. Therefore, they cannot explain the observed hypocalcemia; (2) hypocalcemia can only be explained by increased fractional excretion of calcium resulting from GNAS haplo-insufficiency at the level of the distal tubule; (3) the use of mathematical models to simulate complex metabolic systems allows the extraction, from clinical data, of insights onto physiopathology that are not accessible to intuition alone.
O pseudohipoparatiroidismo tipo 1b (PHP1b) resulta de defeitos de metilação no locus materno do GNAS. É aceite que a hipocalcémia que se observa nestes casos resulta da consequente inactivação da hidroxilase 1α. Este estudo foi feito para caracterizar: 1) As relações entre as calcémias, fosfatémias e concentrações de PTH e de 1,25 dihidroxi vitamina D (1,25OH2D) nos doentes com PHP1b; 2) Se um modelo matemático do metabolismo do cálcio poderia reproduzir os dados observados.
DoentesO estudo incluiu os casos de 139 doentes não medicados, extraidos da literatura, e que tinham medições simultâneas de Ca, P e PTH. Em 25 deles tinham também sido medidos os valores da 1,25OH2D.
ResultadosOs valores médios e o erro-padrão (SEM) foram: PTH=43pM (3,66); P=2.16mM (0,056); Ca=1.69mM (0,038); 1,25OH2D=84pM (10,9). Os valores da fosfatémia estavam fortemente correlacionados com os da calcémia (R=-0.590; p<0.001) e com a idade age (R=-0.623; p<0.001). Não se encontraram outras correlações significativas. O modelo matemático simulou com grande aproximação o padrão hormonal global do PHP 1b quando a depuração de fosfatos foi reduzida para 20%, a depuração de cálcio foi duplicada e resposta da 1α hidroxilase foi reduzida a 30%.
Conclusões1) As concentrações de1,25OH2D são normais na maior parte dos casos de PHP1b. Por consequência, não podem explicar a hipocalcémia; 2) A hipocalcémia só pode ser explicada pelo aumento da excreção fraccional de cálcio resultante da haplo-insuficiência do GNAS ao nível do túbulo distal; 3) O recurso a modelos matemáticos que simulam sistemas metabólicos complexos permite extrair, a partir de dados da rotina clínica, informações sobre fisiopatologia que não são acessíveis ao raciocínio intuitivo.
Patients with pseudohypoparathyroidism type 1b (PHP1b) present resistance to parathyroid hormone (PTH) at the proximal renal tubules. In this tissue, the G-protein alpha-subunit (Gsα), from the PTH receptor-coupled G-protein, is not transcribed by the paternal allele, being expressed mainly by the maternal allele, due to imprinting.1,2 Therefore, disruption of the GSα encoding locus involving silencing of the maternal allele results in reduced or absent GSα activity. The consequent renal resistance to the actions of PTH is believed to increase the threshold for phosphate excretion and to reduce the synthesis of 1α hydroxylase. Hyperphosphatemia, hypocalcemia and high PTH levels are expected, and observed, consequences. Most encountered serum levels of 1,25 dihydroxyvitamin D (1,25OH2D) are within the normal range.
The present study was designed with two purposes: (1) To define the relationships between the serum concentrations of the observed variables (Ca, P, PTH and 1,25OH2D) in a population of patients with PHP1b, extracted from the literature; (2) to find out if and how feeding the primary abnormalities of PHP1b (resistance to the effects of PTH on the threshold for phosphaturia and on the synthesis of 1α hydroxylase) into a previously published mathematical model3,4 of calcium metabolism could reproduce the observed findings on the patients.
Materials and methodsAll articles selected in PubMed under the heading “pseudohypoparathyroidism” until August 2012 and their cross-references were screened for descriptions of actual cases of PHP1b. All cases with simultaneous, pretreatment, measurements of serum calcium, phosphate and PTH were selected for the study.1,2,5–22 Two additional cases without measurements of PTH but in which 1,25OH2D had been measured17 were included. Care was taken to avoid duplications of cases published on different occasions by the same groups.
PatientsA total of 139 cases were selected (80 females, 52 males, and 7 with non-specified sex). Sixty-three of the cases were familial, 38 were sporadic and this information was missing in 38. Twenty-five of the patients also had measurements of 1,25OH2D. In 14 cases PTH had been measured by RIA directed at the amino terminal part of the PTH molecule. In these cases the values were converted to the equivalent values for the assays of “intact” PTH using a factor – 0.0165 – calculated from the reference ranges reported. Reference data for all the variables were checked for conformity with those published by Kratz et al.23 for adults, generally accepted by the medical community when local references are not available. As exploratory analysis revealed that serum phosphate was correlated with age this variable was included in the correlation analysis.
For the correlations with 1,25OH2D only 23 patients were used, because in 2 of them there was no information on PTH.
Statistics – The significance of the differences between the averages of groups were calculated by the t-test. Two tailed Pearson's correlations between variables were calculated by bivariate analysis and then by stepwise multiple regression analysis considering each of the variables (except age) as a dependent variable. The calculations were made using the software SPSS, version 19. Significance was defined at the level of 5%.
SimulationsIn order to compute the expected results of the pertinent variables (serum concentrations of calcium, phosphorus, PTH and 1,25OH2D) when the known defects observed in PHP1b were considered, we used an upgraded version3 of a mathematical model of calcium metabolism published by our group.4 A source code of the program can be obtained from h.g.ferreira3@gmail.com. The parameters determining all the functions dependent on PTH were relaxed so that: (1) The postulated abnormalities described in PHP1b were included in the equations; (2) the outcoming values for the pertinent variables at steady state would mimic as much as possible the average values from the observed series.
ResultsDescriptive statistics of the variablesThe serum levels of calcium, phosphorus and 1,25OH2D had a Gaussian distribution. Age and the concentrations of PTH had a log normal distribution. All variables had unimodal distributions.
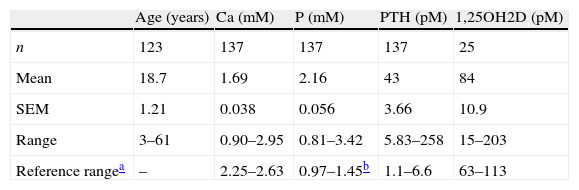
The number of cases (n), mean, standard error of the mean (SEM) and range of each of the variables is shown in Table 1.
Number of patients with PHP-1b and mean, SEM and range of the variables.
| Age (years) | Ca (mM) | P (mM) | PTH (pM) | 1,25OH2D (pM) | |
| n | 123 | 137 | 137 | 137 | 25 |
| Mean | 18.7 | 1.69 | 2.16 | 43 | 84 |
| SEM | 1.21 | 0.038 | 0.056 | 3.66 | 10.9 |
| Range | 3–61 | 0.90–2.95 | 0.81–3.42 | 5.83–258 | 15–203 |
| Reference rangea | – | 2.25–2.63 | 0.97–1.45b | 1.1–6.6 | 63–113 |
When the patients were grouped by sex it was found that the mean age of the males was significantly lower than that of the females (13.3 vs. 21.5 years; p=0.001) and the phosphate levels were significantly higher in males than in females (2.29 vs. 2.01mM; p=0.007). No significant differences were observed for the other variables. When the patients were grouped as sporadic (n=38) vs. familial (n=63) cases no differences were found in sex (36/27 females in familial cases vs. 21/17 in sporadic cases), age (16 vs. 19.5 years), calcium (1.61 vs. 1.75mM), phosphate (2.08 vs. 2.07mM) or PTH (47.7 vs. 44.4pM).
CorrelationsPhosphate levels correlated negatively with calcium levels (t=−7.668; p<0.001) and with age (t=−7.253; p<0.001) on multiple regression analysis considering age, serum calcium concentrations and PTH as independent variables. No other significant correlations were observed. In patients aged 39 years or older the concentrations of PTH were positively correlated with that of calcium (t=2.501; p=0.041). This subgroup was selected because 39 years was the age of the youngest patient with PHP and documented tertiary hyperparathyroidism.20 The correlations between the serum concentrations of PTH and those of calcium and phosphate remained not significant even after this subgroup of patients were removed.
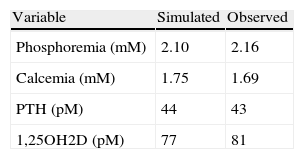
SimulationsThe model could simulate the observed hypocalcemia only on the condition that the clearance of calcium was increased above the reference value. The best simulation of all the observed variables was obtained introducing in the model the adjustments in the parameters shown in Table 2.
Changes in the parameters introduced in the model so that the presumed consequences of the genetic defects characteristic of PHP1b are included and the outcomes, at steady state, mimic the empirical results observed in the present series.
| Parameter | Change introduced in the model |
| Clearance of phosphate by the kidney | Reduced to 20% of the reference value |
| Clearance of calcium by the kidney | Increased by 100% of the reference value |
| Responsiveness of 1α hydroxylase to PTH | Reduced to 30% of the reference value |
The magnitude of the reduction in the responsiveness of 1α hydroxylase to PTH is difficult to anticipate considering that this enzyme is also expressed at the level of the distal tubule.24 It seemed, therefore reasonable to consider that this defect was less intense than that on the clearance of phosphate.
Modifications on the parameters describing the effect of PTH on the production of fibroblast growth factor 23 (FGF 23) and on the fluxes of calcium to and from the bone were also relaxed but the effects on the pertinent variables were small. The predicted values of the variables at steady state and the average of the actually observed values in the patients are shown in Table 3.
DiscussionHypoparathyroidism type 1b is an interesting model to study the translation of well defined abnormalities at the genetic/cellular level into their systemic repercussions. An epigenetic abnormality consisting of methylation defects at the maternally derived GNAS locus, paternally imprinted at the proximal renal tubule1,2,25 is present in these patients. It has been generally accepted that this abnormality is responsible for the observed resistance to the actions of PTH resulting in hyperphosphatemia and hypocalcemia due to increased threshold for phosphate excretion and reduced synthesis of 1α hydroxylase. Yet, no quantitative studies on the excretion of phosphate or on the activity of 1α hydroxylase have been reported in these patients or even in animal models.
An increase in the threshold for phosphate can explain the observed hyperphosphoremia, leading to increased PTH, while the impaired synthesis of 1α hydroxylase should result in reduced levels of 1,25OH2D, a lowering of serum calcium concentration and a further increase in the concentrations of PTH. The finding of a normal concentration of 1,25OH2D in one of our patients16 raised doubts about the validity of this explanation and prompted us to perform a meta-analysis of the available literature on this matter considering that PHP1b is a rare condition.
In a series of 7 untreated patients with pseudohypoparathyroidism type 1, subtype not specified (PHP1?), the authors reported average 1,25OH2D levels below normal (37pM/l vs. 100 in controls).26 This series was not included in our analysis since the authors provided only average values for 1,25OH2D. However, in the 27 patients collected for the present study it was found that the scatter of values of 1,25OH2D contained the normal range. This might be explained by the high values of PTH, but cannot explain the observed hypocalcemia. Hypocalcemia could be due to some degree of resistance to parathyroid hormone at the level of the distal renal tubule and consequent increase in the calcium excretion when normalized for the corresponding glomerular load. The responsiveness to PTH at this level is believed to be preserved, since an infusion of PTH in patients with PHP1? reduces the urinary excretion of calcium24,27 and, for similar levels of calcium, patients with PHP1? excrete less calcium than patients with idiopathic hypoparathyroidism, both groups being under treatment with 1α hydroxyl vitamin D.24,28
While reduction in the excretion of calcium after an infusion of PTH indicates that the distal nephron responds to PTH it does not establish that the response is quantitatively normal. Evidence that the distal nephron is affected in patients with PHP1? is provided by the finding that the urinary excretion of cathepsin D (a marker of distal tubular function) following an infusion of PTH is significantly reduced in patients with PHP1? as compared to normal controls or to patients with idiopathic hypoparathyroidism.26 This observation probably reflects haplo-insufficiency of GNAS in non imprinted tissues of patients with PHP1?, a finding described in mice knocked-out for the gene GNAS.25 The only way to quantitatively assess the re-absorption of calcium by the distal nephron is by calculating the fractional excretion of calcium. To the extent of our knowledge only one report in the literature contains data in untreated patients with PHP1?24 that allow such calculation. In the five patients with PHP1? in which this ratio could be computed it was 0.0195±0.0046 while in 10 patients with idiopathic hypoparathyroidism reported in the same publication it was 0.0316±0.0059. This was compared with 0.0109±0.0009 in our own series of 41 normal controls. Although only the difference between controls and hypoparathyroid patients reached a level of statistical significance (p<0.001), most likely due to the small sample size of patients with PHP1?, these observations suggest that patients with PHP1? live in a steady state such that the fractional excretion of calcium by the kidney is higher than normal but lower than that observed in idiopathic hypoparathyroidism. This is what could be expected considering the GNAS haplo-insufficiency at the level of the distal nephron.25,26 The possibility that, as a result of a positive balance of phosphate, a direct physical effect of the hyperphosphatemia could contribute to the observed hypocalcemia is suggested by the observation that when increased quantities of phosphate are added to aliquots of the same blood sample a perfect negative correlation between concentrations of ionized calcium and phosphorus is observed.29 However, living organisms exist in a steady state of fluxes, not in equilibrium, as in a flask. For the product of solubility to be a determinant factor of hypocalcemia one would expect continuous accretion of calcium in the bone or somewhere else as occurs in familial tumoral calcinosis a condition wherein, despite the extensive calcinosis, serum calcium concentrations are usually kept within normal limits. Cases of osteosclerosis30 or of extensive soft tissue calcification31 in PHP1b have been reported but are exceptional and most patients with PHP1b present lower bone mass density than normal controls or patients with hypoparathyroidism.32,33
The simulations performed with the mathematical model of calcium metabolism provided support to the formulation that haplo-insufficiency of the GSα encoding locus at the level of the distal tubule results in a reduction of its responsiveness to the calcium sparing function of PTH.
When the fractional excretion of calcium by the kidney was increased to twice as much its reference value (a factor calculated from the reported cases by Yamamoto,24 and reasonable to expect given the haplo-insufficiency) the model closely reproduced the empirical observations extracted from the reported literature, including the somewhat bizarre findings of hypocalcemia in the presence of high values of PTH and normal serum concentrations of 1,25OH2D.
No differences in the metabolic phenotype were found between sporadic and familial cases. The genetic basis of the familial forms is rather homogeneous, in most cases consisting of a deletion of about 3kb in the STX16 gene. Unidentified genetic causes account for most sporadic forms although, along with familial forms, the abnormalities converge to the same final defect – loss of the maternal epigenotype within the exon 1A DMR. Considering the small sample size and the different methods used by different authors at different times no attempts were made to further sub divide the population according to the genetic abnormalities.
Phosphate levels were negatively associated with age, a finding already described in healthy people.34 This association may be responsible for the significantly higher phosphate levels observed in males (2.29 vs. 2.00mM) as the average age of the males in the sample was significantly lower than that of the females (13.3 vs. 21.5 years).
It is interesting that the serum concentrations of PTH, although consistently high did not correlate with calcium, phosphorus or 1,25OH2D except in older patients in whom documented20 or presumed tertiary hyperparathyroidism may occur. Factors responsible for the elevation of PTH are hypocalcemia, hyperphosphatemia and, when present, low concentrations of 1,25OH2D. The absence of correlations between these variables is certainly due to the complexity of the non-linear multi-loop system regulating calcium metabolism.
Conclusions: (1) In PHP1b the concentrations of 1,25OH2D are inappropriately normal, scattered over an interval that contains the normal range. Therefore, reduced activity of 1α hydroxylase cannot explain the consistently observed hypocalcemia; (2) hypocalcemia is most likely due to an increased fractional excretion of calcium resulting from GNAS haplo-insufficiency at the level of the distal tubule; (3) although the genetic defects in PHP1b affect predominantly the excretion of phosphate and the activity of 1α hydroxylase, the physiopathology of the condition is ultimately determined by the altered fractional excretion rate of calcium, besides that of phosphate. The reduced responsiveness of 1α hydroxylase to PTH is offset by the high levels of PTH; (4) the use of mathematical models to simulate complex metabolic systems allows to extract, from clinical data, information on physiopathology that is not accessible to intuition alone.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare no conflicts of interest.