Dental-biomaterials loaded with drugs are a promising strategy for local infection treatment. In this context, some studies suggest the incorporation of chlorhexidine (CHX), as an antifungal drug, in acrylic reline resins for the treatment of Candida albicans associated dental infections. The purpose of this study was to evaluate the release of CHX from one acrylic reline resin, Kooliner (K). Furthermore, the effect of different media conditions and the drug loading on the CHX release was assessed.
MethodsResin specimens were prepared from a hard denture reline material based on poly(ethylmethacrylate) (PEMA) and isobutylmethacrylate (IBMA) containing 2.5, 5.0, 7.5 and 10wt% of CHX. The CHX-loaded resins were incubated in water and artificial saliva (at pH 7) for up to 28 days at 37°C. Chlorhexidine content was determined by UV-spectroscopy (255nm).
ResultsCHX release was influenced by media composition and drug loading. CHX demonstrated a similar release profile in the two media, however the water showed the higher amount of release in comparison with the inorganic mimetic saliva. CHX release increased proportionally to the concentration of the CHX added, in a linear trend line. Moreover, a high percentage of CHX was not release up to 28 days.
ConclusionsDespite the limitations of the present investigation, results from the present study highlight the need of evaluating drug release not only in water but also in saliva. Strategies that increase the complete release of drug from the material should be investigated in order to prevent the development of multiresistant strains.
A veiculação localizada de fármacos em biomateriais dentários é uma estratégia que tem vindo a ser considerada para tratamento de infeções designadamente por Candida albicans. Neste contexto, surgiu o interesse em avaliar a incorporação do fármaco clorhexidina (CHX) em resinas acrílicas de rebasamento. O principal objetivo deste estudo foi avaliar o perfil de libertação da CHX a partir de diferentes formulações de uma resina acrílica de rebasamento direto, Kooliner (K), constituídas por diferentes percentagens de fármaco e em diferentes meios de libertação.
MétodosForam preparados espécimes da resina K com 2,5; 5,0; 7,5 e 10% (m/m) de CHX. Os ensaios de libertação foram realizados em água e saliva artificial (pH=7). A concentração de CHX no meio foi quantificada através de Espectroscopia UV-Visível (255nm).
ResultadosOs ensaios realizados permitiram concluir que a libertação é influenciada pela composição do meio e pela percentagem em massa de CHX incorporada nos espécimes. A maior concentração de CHX foi verificada na água e maiores percentagens de CHX contribuíram para a libertação de maiores quantidades de fármaco. Verificou-se ainda que apenas uma pequena percentagem de CHX inicialmente incorporada no material foi libertada para os meios.
ConclusãoOs resultados obtidos neste estudo vêm reforçar a ideia de que é necessária a realização dos ensaios não só em água mas também em meios que mimetizem a saliva. Estratégias que permitam a libertação completa de CHX a partir de resinas acrílicas devem ser investigadas para diminuir a probabilidade de desenvolvimento de estirpes multirresistentes.
Despite dramatic improvements in tooth retention around the world, a substantial proportion of older adults have lost natural teeth and many wear removable partial or complete dentures.1 The Candida-associated denture stomatitis is a common condition among people who wear complete dentures, characterized by generalized inflammation of the palatal mucosa covered by the denture.2,3 It is a harmless form of oral candidiasis and it is associated with a quantitative increase of yeasts on the mucosa and the denture's fit surface.4 Although denture stomatitis appears to be caused by a multiplicity of predisposing factors, Candida albicans has been claimed to be the principal pathogen.4,5
Materials for prostheses, such as acrylic resins, represent a perfect support for biofilm formation. C. albicans has been found on both hard and soft denture base acrylic resins in vitro and in vivo.4 The chemical and physical characteristics of the surface of these materials support biofilm formation through reversible and then irreversible adhesion to the surface.6 Microorganisms in the biofilm can reduce metabolic activities, form extracellular polymer matrix to defend against harmful environmental physical and chemical factors, evade host immunological surveillance and hinder the diffusion and permeation of antibiotics.7
A number of antimicrobial agents are available for the management of fungal infections; however, the choice of antifungals suitable for the treatment of oral candidosis is limited.8 Among those, chlorhexidine (CHX) has been used as an adjunctive therapeutic option for topical application due to its broad-spectrum antimicrobial activity, including C. albicans.5,8 Furthermore, in comparison to another common prescribed antifungal drug as fluconazole, CHX has shown to develop less resistant strains. In fact, despite the good short-term fluconazole efficiency in the treatment of Denture Related Stomatitis (DRS) the fluconazole generalized prescription should be avoided to reduce the emergence of resistant strains of C. albicans and the increasing episodes of DRS recurrences.9
The mode of action of CHX on Candida spp. is still unclear but it has been suggested that the mechanism for fungi is very similar to bacteria.8,10 CHX is a positively-charged molecule that binds to the negatively-charged sites on the candidal cell wall; it destabilizes the cell wall and interferes with osmosis. The fungus uptakes CHX in a short amount of time and impairs the integrity of the cell wall and the plasma membrane entering the cytoplasm resulting in leakage of cell contents and cell death.10
There are many oral delivery formulations for CHX. It is used primarily as 0.2% mouthwash with a topical mode of action. However, in this case most of the agent is removed from the oral cavity during the first hour due to the diluent effect of saliva and the cleansing effect of the oral musculature, possibly reducing its therapeutic efficacy.5 Alternatively, CHX-impregnated acrylic-based drug delivery systems have been suggested for treatment of DRS due to the controlled release of the drug in the oral cavity.4,5,8,11–14
Although showing promising results most of these studies evaluate CHX release from the acrylic resin devices just in water. However, in order to simulate the denture-mucosa interface it is important to study the CHX release in a media solution more similar to the organic saliva.15 With this in mind a study was designed aiming to compare the CHX release from one acrylic reline resin loaded with different drug amounts (2.5, 5.0, 7.5 and 10%, w/w) in water and in artificial saliva.
The acrylic resin selected for this purpose was a hard chairside reline resin. This material is supplied as a power and liquid, the power consisting of poly(ethylmethacrylate) and the liquid containing isobutylmethacrylate. A direct relining system is not only simple and practical to perform but also time- and cost-effective. Advantages of the hard chairside reline resins include ease of manipulation and low exothermic heat of reaction.16–18
The tested hypotheses of the study were: first, increasing drug resin loading increases CHX liberation; and second, drug release is influenced by media composition.
Materials and methodsOne direct reline resin, Kooliner (K) (GC America Inc, Alsip, IL, USA) was used and represents a pre-polymerized poly(ethylmethacrylate) (PEMA) based material which has isobutylmethacrylate (IBMA) as the monomer. The mixing ratio and the conditions for processing and polymerization were manipulated according to the manufacturer's instructions. These were 2.5g/1.79mL polymer powder/monomer liquid (ratio for mixing).
Fifteen specimens (10×2.5mm) of K material were prepared from cylinder shaped stainless steel molds.19 On the experimental specimens, chlorhexidine diacetate monohydrate (Panreac Applichem, Darmstadt, Germany) was incorporated and mixed using a mortar and pestle for homogenization. Five groups (n=3) of samples of K were prepared: a control group (without CHX) and 4 experimental groups with different concentrations of CHX (2.5, 5.0, 7.5 and 10%, w/w) powder.
For each specimen preparation, the stainless steel mold was placed on a polyester sheet. The material was prepared and placed into the mold. A new polyester sheet was positioned on top of the mold and the set was maintained under compression into an incubator at 37±2°C (Memmert incubator) during polymerization, in order to simulate the intraoral polymerization of direct reline resin.
After polymerization, the samples were removed from the molds and the edges of each sample were polished with a 600-grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., Lake Bluff, IL) in order to remove any irregularities.
A standard stock solution of 1000μg/mL was prepared by dissolving 10mg of CHX in 10.0mL of deionized water. The solution was then kept out of the light, at room temperature.
The releasing solutions used in this study were: deionized water and artificial saliva at pH=7. The artificial saliva used in the present study was prepared according to the formula, with the following qualitative composition: phosphate buffer pH=7.0 (anhydride disodium phosphate, monosodium phosphate anhydride and deionized water), xanthan gum, calcium chloride dihydrat, sodium chloride, potassium chloride and propylene glycol.20,21
The determination of CHX was conducted using polystyrene flat-bottom microplate wells (96-well microplates) in a microplate reader (FLUOstar Omega – BMG LABTECH).
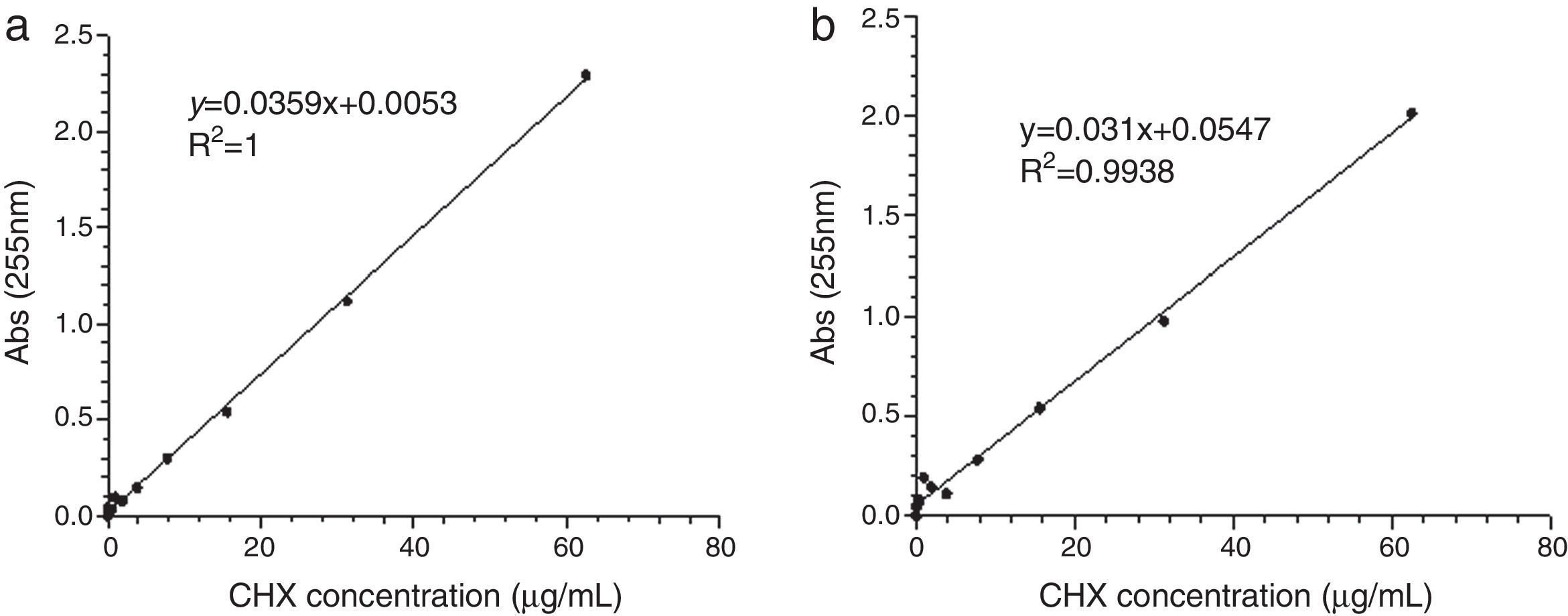
Using an Ultraviolet-Visible Absorbance Spectra detection mode, three absorbance peaks were detected for the standard stock solution of CHX at 230nm, 250nm and 255nm. The wavelength used in this study was 255nm that corresponds to the literature mentioned wavelength.22–24
The measurements were performed at room temperature (25°C). The experiments were run with repeat series using graded dilution series of CHX from 0.06 to 1000μg/mL. Then, 200 or 300μL of a standard stock solution with 0, 0.06, 0.12, 0.24, 0.49, 0.98, 1.95, 3.91, 7.81, 15.62, 31.25 and 62.5μg/mL of CHX was applied into the wells. Two calibration curves were constructed for both media solution. As the capability of the method to discriminate small differences in concentration of CHX (sensitivity) was higher to a volume of 300μL, this was the selected value for further analysis (after the optimization step). The calibration curves were repeated in each new measurement of CHX.
Three K specimens without CHX (control group) and three K specimens with CHX 2.5, 5.0, 7.5 and 10% (w/w) (experimental groups) were placed separately in a graduated Falcon tube and then covered with deionized water and artificial saliva at pH=7 with a ratio of 1g/5mL.
The 15 tubes which accommodated the samples were left into an incubator (Memmert) at 37°C, with continuous gentle shaking (300rpm). During appropriate time intervals of 2, 4, 7, 26, 50, 75, 171, 339, 507 and 672h (28 days), aliquots of 300μL were collected from each tube. The 300μL of media solution in the 15 containers was renewed at each test interval, in order to simulate the clinical situation regarding the dilution of the thin salivary film at the denture-mucosa interface. All the collected samples were analyzed for CHX content as previously described.
One-way analysis of variance (ANOVA) with a post hoc Tukey's test was applied to the results for the cumulative concentration and maximum cumulative release of CHX. The level of statistical difference was defined as p<0.05. Data were statistically analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
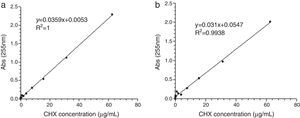
ResultsLinear relationship between absorbance peak areas at 255nm via a microplate reader UV-Visible Spectrophotometer and the CHX concentrations was established for each release media (Fig. 1). The absorbance peak areas of the samples at 255nm were converted to the CHX released concentrations by the respective linear relationships.22 The analytical method showed good linearity in the two evaluated media.
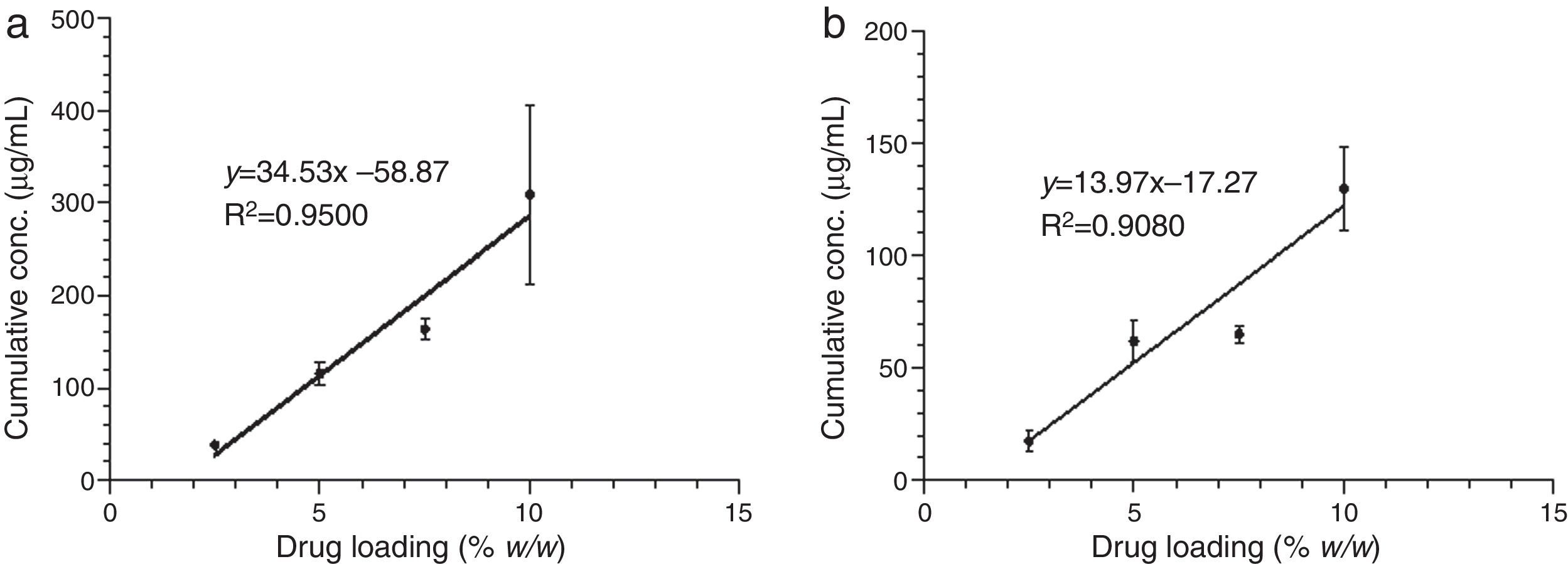
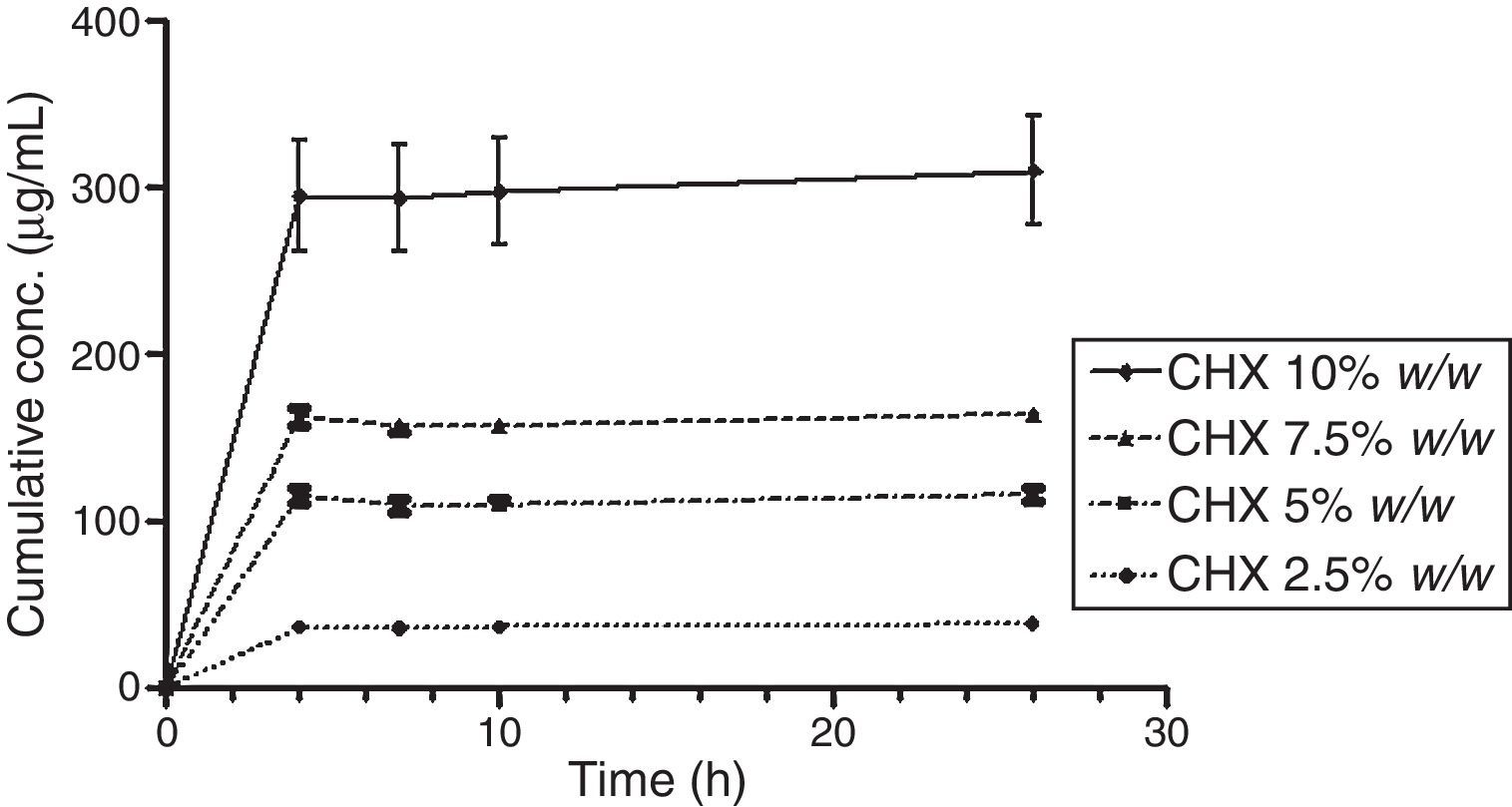
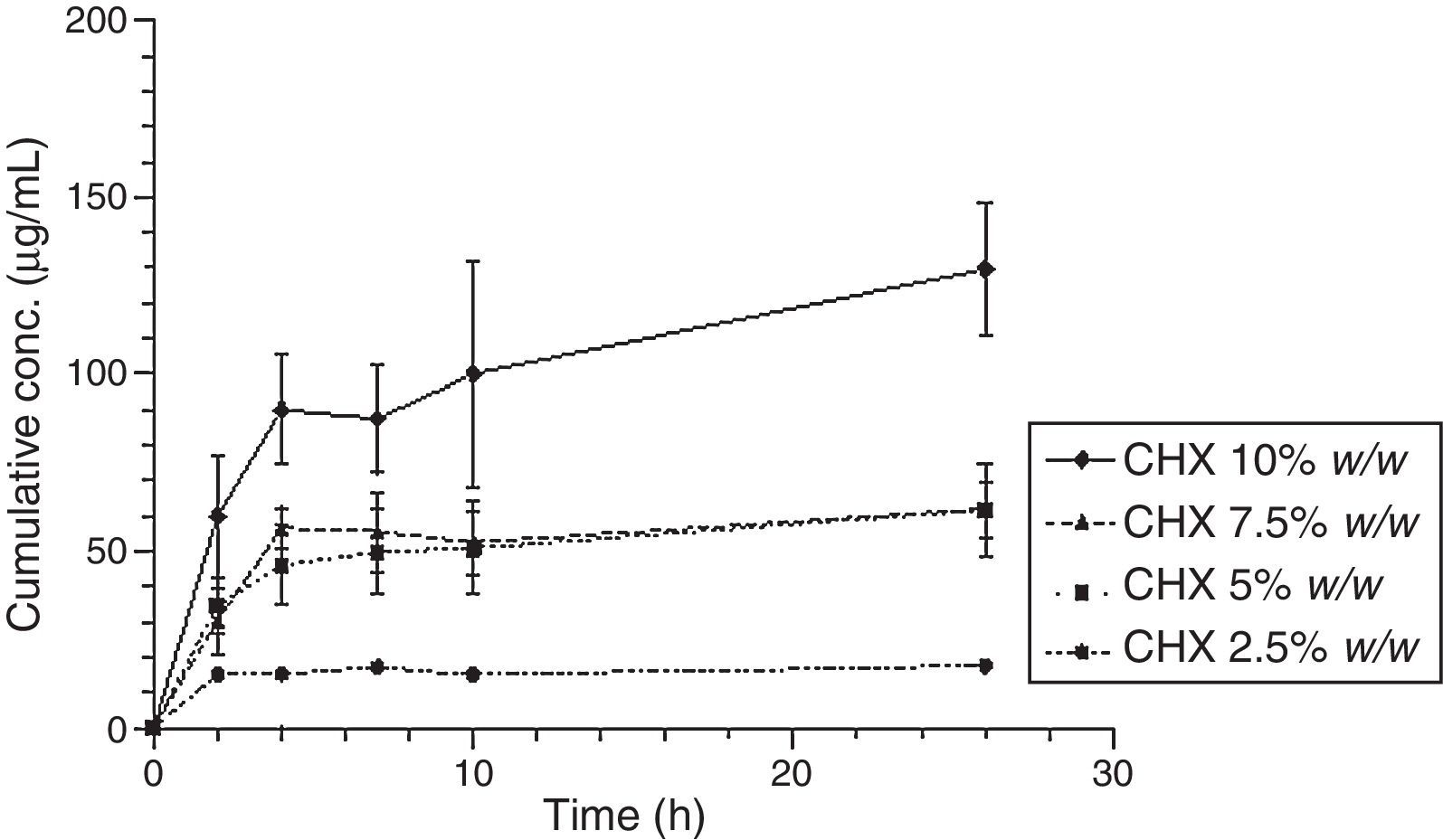
The release of CHX to the media (water or saliva) was properly measured; it increased proportionally to the concentration of the added CHX, in a linear trend line (Fig. 2). Considering the same media release composition, there is an effect of drug loading on drug release. In both media, the release concentrations are significantly different (p<0.05), except for 5.0 vs. 7.5% drug loading.
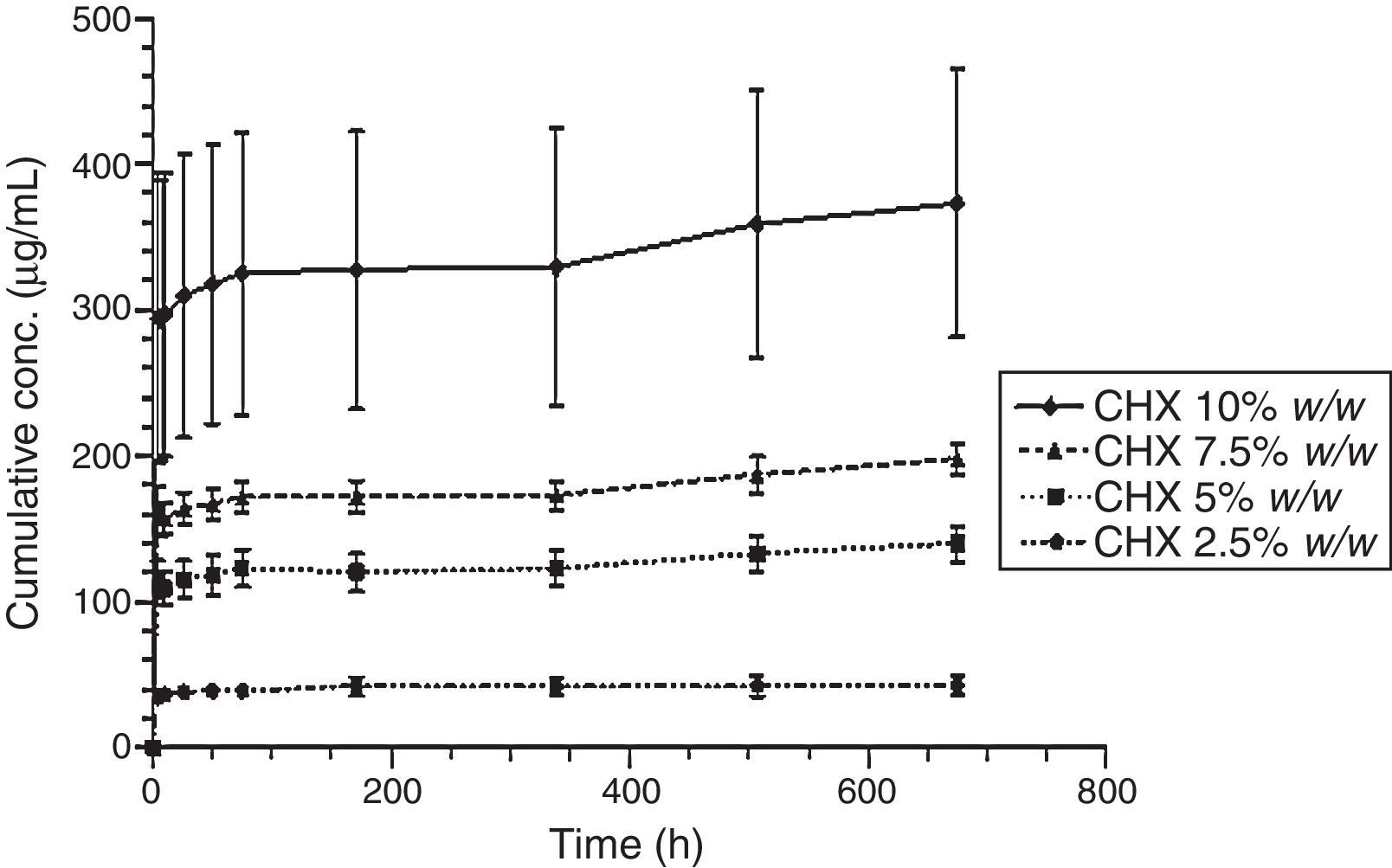
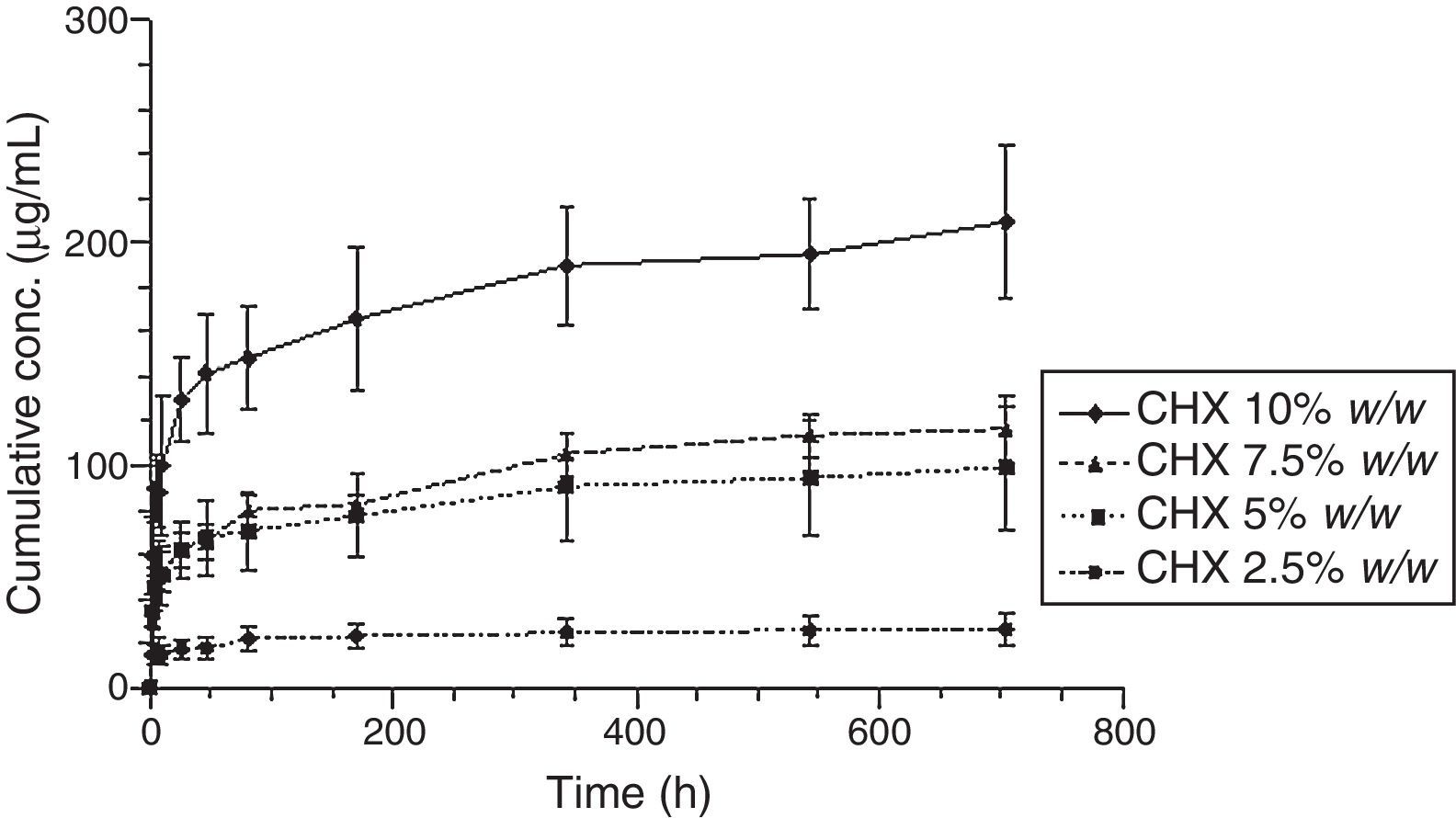
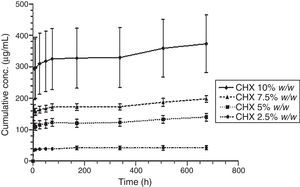
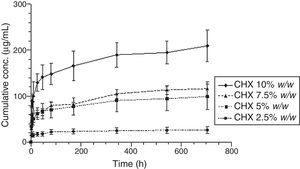
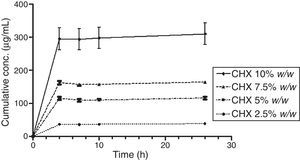
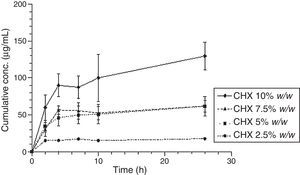
Figs. 3 and 4 show the representative release profiles of CHX in both media. CHX demonstrated a similar release profile in the water and saliva. In all studies CHX release stops after an initial burst phase (Figs. 5 and 6).
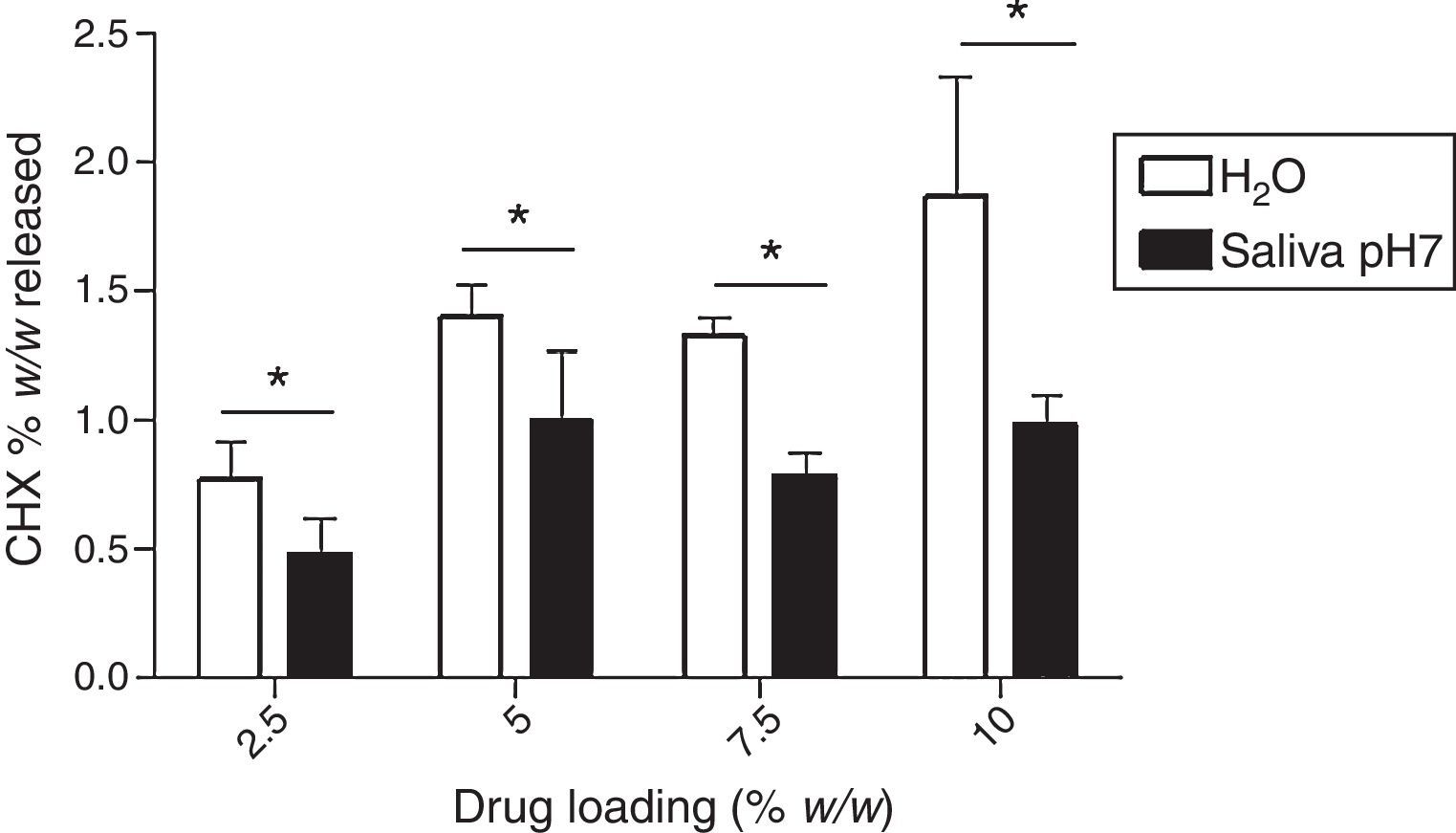
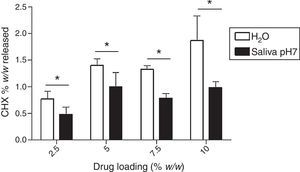
Fig. 7 shows the maximum cumulative release (% w/w), as function of drug loading (theoretical values: 2.5%, 5.0%, 7.5% and 10%) in both media. The higher amount of CHX release was observed in water and the specimens group of 10% led to a higher drug release. However, the maximum cumulative release is only 1.87±0.46%, meaning that only a small amount of initial loaded CHX is liberated to the media.
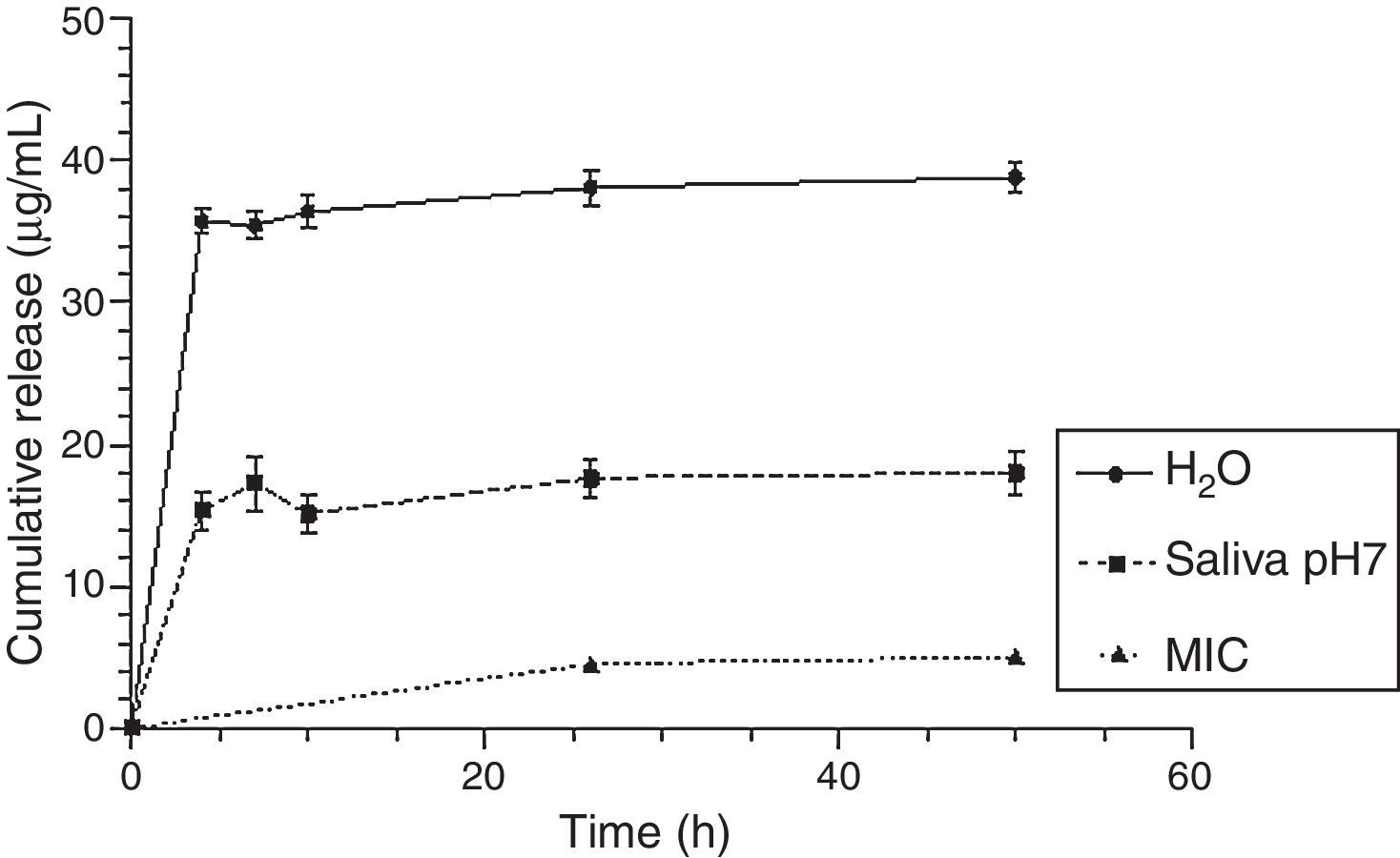
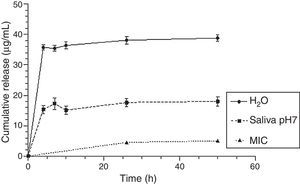
Fig. 8 shows that the CHX released concentrations are effective against Candida albicans, even with a lower loading of CHX (2.5%), in both release solutions.
DiscussionThe effect of drug loading and release media composition on drug liberation from methacrylate systems was investigated in K cylinder specimens with different loading concentrations of CHX.
The release of CHX increased proportionally to the concentration of the loaded drug in the polymer matrix in water and saliva, as shown in Fig. 2. This fact is in agreement with previous studies in which the CHX was incorporated to hard PEMA resin and drug release was evaluated in water.14,25,26 Once the materials are immersed in the release media, the liquid diffuses into the polymeric matrix and dissolve the drug upon contact. The drug particles, when dissolved, leave behind pores and channels in the polymer structure. The drug molecules can then diffuse out through the interconnecting pores. As the drug loading increases, the pores created by the occupied drug molecules would be larger and/or greater in number and the release of the drug would be in high amount.25–27 Release profiles in both media showed that CHX release stops after an initial burst (Figs. 3–6). Studies with other acrylic resins in water showed different results.4,5,13 In the present study, the amount of CHX released in water was markedly lower than previously reported results with a poly-ethyl methacrylate and tetrahydro-furfuryl methacrylate (PEM/THFM) drug-release device.13 Moreover, a burst release was also described for an auto-polymerized poly(methyl methacrylate) (PMMA) based resin but CHX leach steadily out of the resin into water and sustained drug release continued throughout the 28 day-test period.4,5 These dissimilar conclusions in comparison with our investigation may be due to the differences in the experimental protocol, namely, the composition of the tested acrylic resin and type of specimens. Concerning acrylic resin composition, there are many variables influencing polymer matrix structure after setting, as the type of monomer, polymer molecular weight, polymerization reaction, among others. These differences will directly affect material porosity and consequently drug release.19,28 The dimensions and surface area of the tested specimens may also affect the results as release is influenced by surface area. In the mentioned studies,4,5,13 discs were used whereas in our study the tested model was a cylinder. A proportionally larger surface area of the disks exposes more drug to the soaking solution and thus may enhance its release.
A direct comparison between the amount of CHX release in water and saliva show that it is significantly influenced by media composition (Fig. 7). In fact, for all the different tested drug-loaded percentages, CHX release was always lower in saliva. A similar conclusion was observed in a study of CHX embedded into methyl (methacrylate) and 2-hydroxyethyl(methacrylate) copolymer device.29 It was shown that the release rates in an inorganic saliva were significantly lower than those observed from similar devices in water. It was hypothesized that the chloride in the saliva media had diffused into the core of the device, and converted the acetate salt of chlorhexidine (solubility≈23g/L at 37°C) to the less soluble chloride salt (solubility≈1g/L at 37°C). The lower amount of release of CHX in saliva observed in this study is consistent with the assumption that a dramatic reduction in drug release rates are to be expected in human saliva by the same mechanism.
Finally, our results show that even for the lowest loaded CHX concentration (2.5%), drug release concentrations were always higher than the minimum inhibitory concentration of C. albicans reported in the literature30 as illustrated in Fig. 8. However, a lot of drug remains in the device (the highest percentage was around 2% in relation to the total amount of loaded drug) as seen in Fig. 7. This is an overlooked aspect in the published papers and it is highly important when considering a candidate material for local drug delivery. In fact, a total release of CHX from the device is essential as the presence of the drug after a long-term implantation period will contribute to induce and/or select resistant strains.19,31
Future investigations should be carried out aiming to develop systems that allow the total drug release. Inert porosity enhancers as lactose that has been successfully tested for acrylic bone cements could be a valuable strategy to be evaluated in forthcoming work.19,32
ConclusionsIn the present study the effect of drug loading and media composition on CHX release from a hard chairside reline resin was evaluated. Results showed that increasing drug loading enhances the release of CHX in water as well as in an artificial saliva. Also, drug release was always lower in a more complex media as inorganic artificial saliva in comparison with water. Data of the present investigation suggests that release behavior of an antifungal drug, CHX, contained within a drug-release device is influenced by polymer composition, tested specimen and release media. Standardization of experimental protocols must be a priority in order to compare the results among different studies. Furthermore, as a high percentage of CHX was not release up to 28 days, other strategies should be considered for the development of local drug acrylic devices, for example, including release enhancers in the material to allow the total drug release in a measurable length of time and avoid the development of multiresistant Candida strains.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingFundação para a Ciência e a Tecnologia (FCT), Portugal: project iMed.ULisboa (UID/DTP/04138/2013).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would also like to express their thanks to Prof. António Almeida, MsC Rosana Pinto, MsC Joana Marto and PhD Ana Matos of the Faculty of Pharmacy, University of Lisbon.