Electroconvulsive therapy (ECT) is one of the main techniques available for the treatment of such serious mental illnesses as schizophrenia and drug-resistant depression. The pre-anaesthetic assessment appropriate for patients with various mental disorders or pathologies does not differ substantially from that of any patient prior to undergoing anaesthesia for a surgical procedure. The present review aims to propose guidelines to achieve a higher level of safety and effectiveness during ECT in the most frequent situations, in accordance with the current literature.
MethodsWe conducted a search on the role of anaesthesia in ECT in the Ovid MEDLINE, PubMed, and SciELO (Scientific Electronic Library Online) databases, with special attention to the populations undergoing this type of therapy. The search was carried out between 1978 and December 2016.
ResultsWe included the 96 articles that contained the most important recommendations for the preparation of this guide.
ConclusionsWe propose these guidelines in order to achieve a higher level of safety and effectiveness during ECT in special conditions. We also summarize the most important attitude to be taken into account by the anaesthesiologist in these cases.
La terapia electroconvulsiva (TEC) es una de las principales técnicas disponibles para el tratamiento de enfermedades mentales tan graves como la esquizofrenia y la depresión resistente al tratamiento farmacológico. La evaluación preanestésica adecuada en pacientes con diversos trastornos mentales o enfermedades no difiere sustancialmente de la de otros pacientes antes de recibir anestesia para un procedimiento quirúrgico. La presente revisión tiene como objetivo proponer pautas para lograr mayor nivel de seguridad y efectividad durante la TEC en las situaciones más frecuentes, de acuerdo con la bibliografía actual.
MétodosSe realizó una búsqueda sobre el papel de la anestesia en la TEC en las bases de datos Ovid MEDLINE, PubMed y Scientific Electronic Library Online (SciELO), con especial hincapié en las poblaciones que reciben este tipo de terapia. La búsqueda se realizó entre 1978 y diciembre de 2016.
ResultadosSe incluyeron los 96 artículos que contenían las recomendaciones más importantes para la preparación de esta guía.
ConclusionesProponemos estas pautas para lograr mayor nivel de seguridad y efectividad durante la TEC en condiciones especiales. También resumimos la actitud más importante que debe tener en cuenta el anestesiólogo en estos casos.
Electroconvulsive therapy (ECT) is one of the main techniques available for the treatment of such serious mental illnesses as schizophrenia, catatonia, certain cases of mania, psychotic depression and, especially, drug-resistant depression.1–4 Resistance to conventional therapies and the physical health complexity of the patient are factors that will determine the advisability of initiating ECT.
The efficacy, effectiveness,5–7 and efficiency8 of this type of therapy have been demonstrated in all the aforementioned pathologies. Moreover, the benefits of ECT are supported by clinical evidence based on the data of numerous studies and clinical trials, to the extent that patients originally excluded from this therapy due to pathologies associated with their psychiatric disorders are now accepted for treatment.
ECT is often used in patients presenting medical conditions with their psychiatric disorders (special patient populations).9 For various reasons (such as difficulties in reporting medical history or in being followed by their family doctors), such patients are not always in optimal condition for treatment. Because of this variability, appropriate measures need to be adopted to suit the individual characteristics of each patient.10
Medical comorbidities and their treatments can affect both response to ECT and its risks.11 It is thus important to consider the patient's current medical status and how it may affect the risks and benefits of ECT in evaluating whether and how to administer therapy.12
The pre-anaesthetic assessment appropriate for patients with various mental disorders or pathologies does not differ substantially from that of any patient prior to undergoing anaesthesia for a surgical procedure. It should include an adequate medical history, the complementary tests required for the individual patient, and consultations with any other specialists that the anaesthesiologist deems appropriate. This will make it possible to optimize treatment, minimize potential adverse effects and encourage direct interaction between the members of the team (anaesthesiologist, psychiatrist, nurse, and other specialists), who will jointly weigh the risks and benefits of administering ECT. A consideration specific to the evaluation of candidates for ECT, however, is the need for repeated treatment, initially days apart and then, probably, repeatedly over many years of the patient's life. Thus patients will be exposed to the risk, if any exists, on multiple occasions. This risk may change over time, because the patient's health status may change. Safety during the procedure depends to a large extent on measures taken before treatment in function of the patient's pathology and on the proper monitoring of the treatment as it progresses.
The present manuscript aims to review the literature regarding anaesthesia under the most frequent special conditions and propose guidelines to achieve a higher level of safety and effectiveness during ECT in these cases.
Materials and methodsWe conducted a search on the role of anaesthesia in ECT in the Ovid MEDLINE, PubMed, and SciELO (Scientific Electronic Library Online) databases, with special attention to the populations undergoing this type of therapy. The search was carried out between 1978 and December 2017 and the keywords used were all valid MeSH terms: ‘electroconvulsive therapy’, ‘electroshock’, ‘ECT’, ‘anaesthesia’, ‘evaluation’, ‘adolescence’, ‘childhood’, ‘pregnancy’, ‘aged’, ‘treatment’, ‘depression’, ‘schizophrenia’, ‘bipolar disorder’, ‘neurologic diseases’, ‘postpartum’, ‘breastfeeding’, ‘stroke’, ‘cerebrovascular trauma’, ‘infection’, ‘tumour’, ‘pulmonary disease’, ‘morbidity’, ‘mortality’, ‘heart diseases’, ‘safety’, ‘diabetic’ and ‘pathological conditions’.
The search was limited to studies done in humans, published in English and/or Spanish, without regard to the level of evidence. Initially, the search yielded 13,757 articles with the search keywords ‘electroconvulsive therapy.’ Once further refined with the addition of the keyword ‘anaesthesia,’ the number of results was reduced to 116. We reviewed the abstracts of all these studies and, when necessary, read the complete articles. Finally, we included the 112 articles that contained the most important recommendations for the preparation of this guide. All the articles included were reviewed by at least 2 members of our working group. Publications dealing with ECT in special situations are scarce and most of the literature is based on clinical cases, usually short series and reviews based on expert opinions. In the areas where evidence was insufficient to make concrete recommendations, we reviewed expert opinions.10
Elderly patientsECT can be used independently of age. In the elderly, ECT shows 70–80% effectiveness in patients in whom drug therapy has failed.13,14 Clinical experience suggests that ECT may even carry a lower risk of complications than some forms of pharmacological treatments.15,16 A patient's seizure threshold increases with age. The bilateral ECT does not seem to increase the convulsive threshold.17 In this subgroup in particular, special attention should be paid to the presence of possible medical conditions and/or the use of multiple drug therapies.
During treatment, it is advisable to evaluate changes in the patient's cognitive state and to consider other factors, such as electrode placement,18,19 stimulus intensity,20 and treatment frequency. In some cases, an increase in the number of falls has been reported, probably in the context of transitory cognitive alterations, although the relationship remains unclear.21
Children and adolescentsThe use of ECT in adolescents and children is infrequent. As the existing studies in the literature have been limited to short series of case reports, further studies in this area are certainly called for. However, within the established indications, among which are such life-threatening situations such as catatonic states, psychotic depression, and self-injury in autism, the use of ECT is considered to be both beneficial and safe.22–25 The relative contraindications for paediatric ECT are central nervous system tumours, increased intracranial pressure, recent intracerebral haemorrhage, cerebral vascular aneurysm, cerebral arteriovenous malformation, retinal detachment, pheochromocytoma and chest infection.26 The seizure threshold in children and adolescents tends to be lower.27 For children scheduled for several weeks of sequential outpatient treatments, a peripherally inserted central catheter (PICC) may be considered. Inhalational techniques using potent volatile anaesthetic agents may be useful for the child with needle phobia.26
PregnancyThe pharmacological treatment of psychiatric disorders in pregnant women can represent a serious problem due to the teratogenic nature of most of the drugs used and to the scarcity of studies in this subgroup of the population.
Some systematic reviews, however, have suggested that the health risks to the foetus associated with anaesthetics and ECT appear to be lower than those resulting from psychopharmacological treatment or from leaving the mental illness untreated.28 ECT can be administered across all 3 trimesters of the pregnancy and in the postpartum period with a good therapeutic outcome, especially in the management of severe depression and bipolar disorder.29–33 Nevertheless, precaution is advised when ECT is administered during the first trimester, as it increases the risk of miscarriage, placental insufficiency, and placental abruption.34,35 An obstetric consultation should be obtained before ECT is initiated.36–38
Informed consent should include the risk to the foetus associated with the procedure. The most important objective is to avoid neonatal hypoxia. If gestational age is more than 14–16 weeks, foetal heart rate should be monitored noninvasively during each treatment. In the units where ECT is administered to pregnant women, there should be easy access to the resources needed to respond to any obstetric or neonatal emergency.
The most frequent complication for the foetus following the administration of ECT is bradyarrhythmia,39 while for the mother it is the appearance of symptoms of premature labour.31 There may also be an increased risk of cerebral haemorrhage, bronchospasms, and cardiac symptoms.30 Some authors have found a foetal mortality rate of about 7%.35
Puerperium and breastfeedingNormally, it is not necessary to interrupt breastfeeding because of ECT.1 The anaesthetics generally used for the ECT procedure carry a very small risk for the breastfeeding infant.
Neurological disordersWhile intracranial tumours are not an absolute contraindication for ECT, an increased risk of intracranial hypertension does exist due an increase in cerebral blood flow.9 In such cases, treatment should be postponed.40 A further recommendation is to place the electrodes at some distance from the lesions, unilaterally, to reduce stimulus intensity to the lowest effective level, to space out the treatments, and to avoid anticholinergics. In the case of normal pressure hydrocephalus or ventriculoperitoneal shunt, there is no apparent increase in risk.10
Intracranial vascular masses are also not an absolute contraindication,41 although there does exist an increased risk of rupture. Therefore the possibility of embolization should be considered and strict monitoring of arterial blood pressure is essential.9
In patients with ictus, ECT is effective and generally well tolerated. Administration in the acute post-stroke period is only recommended if there is consensus among the different specialists (internists, neurologists and/or radiologists).42 The greater the extent of the cerebrovascular accident or the greater the intracranial hypertension, the longer the administration of ECT should be delayed, especially in cases of haemorrhagic stroke. Before ECT is initiated, an imaging test should be performed to confirm that the episode has resolved. While there are no clear guidelines regarding how soon to begin ECT, it is generally recommended that treatment should be delayed for at least 30 days.42
With regard to epilepsy, it should be pointed out that ECT has antiseizure properties,43 although it may in some cases cause seizures.44,45 Bilateral application and hyperventilation46 are recommended. Although ECT can raise the seizure threshold, it is usually preferable to maintain the patient's usual antiseizure medications.43,44 The abrupt withdrawal of antiseizure medications may increase the risk of provoking status epilepticus following ECT. In such cases, short-acting benzodiazepines (i.e. midazolam) may prove useful.47–50
In patients with dementia, ECT may increase the risk of transient post-treatment confusion, which usually improves spontaneously after 6–8 weeks.50–52 If the patient requires ECT with greater frequency and the confusion does not resolve, the medical staff should consider switching to unilateral application,53 lowering stimulus intensity, or perhaps even reconsider administering ECT altogether. The benefits obtained in psychiatric patients with dementia are comparable to those in patients without dementia.52 Subcortical dementias (e.g. Parkinson's disease) respond better than cortical dementias (e.g. Alzheimer's disease). Patients presenting cognitive deterioration require specific evaluation of their condition. Unilateral administration is preferable as is the possible spacing of ECT (2 sessions per week), and using the lowest possible effective electrical charge.
In patients with Parkinson's disease, ECT is especially recommended when there is either severe disability or in cases of intolerance or poor response to antidepressants.54,55 ECT increases dopaminergic function and its benefits persist for days or even months, making it possible to reduce the dose of dopaminergic medications in some cases.55
In cases of traumatic head injury, it is advisable to wait for the resolution of the acute phase. The risk–benefit evaluation should be based on the severity of the injury.56 If treatment is absolutely necessary, the electrodes should be placed equidistantly as far as possible from the area of the lesion in order to avoid an excessive concentration of local electrical stimulation. The available literature indicate that ECT is safe and effective therapeutic method usable in patients after traumatic brain injury.57 ECT is also safe in patients with intracranial metallic foreign bodies.56,58
In infections of the central nervous system, the priority is to treat the infection aggressively, as the increased permeability of the blood–brain barrier and cerebral blood flow can favour the spread of the infection. ECT should not be administered until the acute phase has passed.
Patients with multiple sclerosis should be advised of the risk, albeit low, of recurrence.59,60
Deep Brain Stimulators (DBS) devices are at risk for damage or generator reprogramming as well as they may interfere with monitoring and other devices. Because of that, DBS should be switched off prior to ECT. ECT is contraindicated in patients with cochlear implants despite there is some report of well tolerated unilateral ECT on the side contralateral to the implant, without device malfunctions.61 RNS system (targeted responsive neurostimulation device approved for refractory epilepsy) should be temporarily disabled before ECT.41
Respiratory diseasesPatients with chronic bronchitis can receive ECT safely if special care is taken with peri-procedural treatment.1,62 Lung function tests may be useful, since they allow a better evaluation of the pulmonary functional reserve and the identification of cases in which optimization of bronchodilator treatment would be indicated. Prophylactic bronchodilator administration is recommended the morning before ECT1,62 and during the recovery period, although there is no strong evidence in the literature to support this as routine practice.
Airway assessment, in which the difficulty of ventilation with face masks is a fundamental concern, becomes even more important in such patients due to poor respiratory reserve (especially obstructive sleep apnoea syndrome and obesity). For this reason, airway management strategies to prevent hypoxaemia should be implemented. Ventilatory monitoring may be useful, specially in patients with OSAS. In these procedures outside the operating theatre, it is strongly recommended that non-anaesthesiologist medical professionals should be trained to collaborate, if necessary, in the event of possibly serious problems involving the airway.
Patients with preexisting pulmonary disease may be at increased risk for posttreatment sequelae (rupture of preexisting pulmonary blebs related to aggressive hyperventilation; patients with chronically elevated PCO2 in whom delayed return of spontaneous ventilation may affect posttreatment management).41
Cardiovascular diseasesCardiovascular complications are the most common during ECT,9 although incidence is low (0.9%).63–65 The following are the primary clinical predictors of increased cardiovascular risk: unstable coronary syndrome, heart failure, clinical arrhythmias, and severe valvular dysfunction.66 In a retrospective study, patients with increased cardiovascular risk were found to develop more minor (mainly non-life-threatening arrhythmias), but not major (permanent morbidity or death) complications compared to patients with no apparent cardiac risk.63 In order to avoid such complications, an appropriate clinical evaluation should be performed to stabilize and optimize patients with cardiovascular diseases.64
In the frequent case of arterial hypertension, blood pressure should be carefully monitored and care should be taken to ensure that the patient is normotensive during ECT. This is of the utmost importance, since ECT is associated with haemodynamic effects in 2 very distinct phases: Initially, there is parasympathetic stimulation caused by the electric shock, which lasts seconds and is mediated by the vagus nerve, ending when the convulsion begins. At this stage, bradycardia, which may be mild to very severe, appears. The bradycardia responds to doses of atropine 0.01mg/kg. Some authors have reported that at doses of up to 0.4mg of atropine there are no significant changes in heart rate.67 Routine use of anticholinergics has been criticized as unnecessary.68 However, they may be useful in patients who are receiving sympathetic blocking agents, such as beta-blockers.69 For these patients at risk for bradycardia during treatment, glycopyrrolate 0.2mg may be administered IV immediately before ECT.41 After the seizure, the second phase begins, where episodes of arterial hypertension and tachycardia can occur in response to the strong sympathetic stimulation caused by the seizure. This sympathetic stimulation, amplified by the release of catecholamines, can last up to several minutes, increasing both heart rate and blood pressure to dangerous levels, and subjecting predisposed patients to an extremely high risk of coronary ischaemia. For this reason it is of the utmost importance to detect and optimize such patients prior to ECT.
Management of hypertension and tachycardia with IV beta-blockers or other agents may be recommended in patients at risk for cardiac ischaemia, whereas most healthy patients will not require any intervention for transient increases in blood pressure and heart rate. Esmolol (1mg/kg) has lesser effect on seizure duration than labetalol (0.3mg/kg). Calcium channel blockers and alpha 2 agonists can be also used.41 Labetalol, on the other hand, has been shown to be superior to esmolol in treating haemodynamic effects after the first 5min.70,71 For all the reasons stated above, a recent acute myocardial infarction (AMI) represents an increased risk of reinfarction during ECT.64 Although there is no study that demonstrates it objectively, it has been suggested that after an AMI it is advisable to leave three months before beginning the treatment with ECT.10,64,72 The vegetative response during and after ECT should be strictly controlled to prevent the onset of ischaemia, which is initially detected clinically, but when suspected, may require additional tests. Dexmedetomidine (1μg/kg over 10min before induction of anaesthesia) and esmolol produced significant improvement of cardiovascular response to ECT without affecting seizure duration.73,74
In case of heart failure, it is essential to evaluate whether or not it is compensated, as decompensated heart failure has a high risk of being aggravated by ECT and must therefore be compensated before ECT is initiated. If baseline capacity is <4 METs, an echocardiogram prior to the procedure is recommended to assess whether there is decompensation.75 It is important to know if the origin of the condition is an ischaemic heart disease, as this is another factor to consider in the use of atropine. In cases of advanced valvular heart disease, ECT may trigger heart failure. Volume status, as well as drugs and doses, should be adjusted to the type and degree of the disease. Clinical signs of angina, syncope, or arrhythmias may also occur. For these reasons, a pre-treatment assessment by the cardiologist is recommended to evaluate the degree of valvulopathy and its possible repercussions. Patients with a correctly-functioning prosthesis should be at no particular risk with ECT.
Patients with pacemakers often tolerate ECT well, and current devices are not affected by the electric current because they are protected by a high voltage circuit.76 In fact, in some cases, they may protect against asystole.9 However, pacemakers may still become damaged or reprogrammed under certain circumstances. Although it is possible that the ECT stimulus could interfere with the device function, these sources usually are far enough away (greater than 15cm) from the device so as to have minimal impact.41 Even so, a magnet should be available to treat arrhythmias if necessary,66,77,78 since in this way the device can be converted from a demand-mode to a fixed-rate pacemaker, thus preventing tachycardia.79 For patients with pacemakers who are to undergo ECT, the recommendations are as follows76: consulting with the cardiologist and becoming familiar with the device and its programming parameters prior to ECT, ensuring availability of a magnet during ECT, and checking the device and monitoring the patient following treatment.
With regard to implantable cardioverter defibrillators, some authors argue that they should be temporarily disabled prior to ECT to prevent activation during the procedure.66 It is important to remember that if a patient with an ICD is pacemaker dependent, placing a magnet over the pulse generator will only deactivate the ICD, it will NOT put the pacemaker into an asynchronous mode.41 Although specific devices vary, the following are generally recommended: consulting with the cardiologist and evaluating device functioning and programming parameters prior to ECT, disabling the device during ECT, and checking the device and monitoring the patient after the session.76
In patients with heart transplants, it must be remembered that this organ is denervated and therefore may not have the response to parasympathetic stimulation that usually occurs after application of the electric charge. Similarly, they do not respond to atropine. For this reason, such patients do not usually present tachycardia with pain or insufficient sedation.80 All induction agents used for ECT prolong the QT interval. Propofol, etomidate and thiopental can be used safely. Takotsubo cardiomyopathy (left ventricular hypokinesis and apical ballooning triggered by severe physical or emotional stress) has been described following ECT. Retreatment with ECT after Takotsubo cardiomyopathy, managed with beta-blockers, has been reported.41
Other pathologiesIn patients with unstable or insulin-dependent diabetes mellitus, appropriate adjustments should be made prior to ECT, especially with respect to pre-procedure fasting. Before ECT, blood sugar should be stabilized as much as possible. Capillary glycaemia should be monitored during the hour prior to ECT and after treatment to prevent hypoglycaemia. A specific serum and insulin schedule should be prescribed for each case. ECT itself can modify glucose levels through the elevation of ACTH and/or cortisol.81
The vast majority of patients with mild or well-controlled Gastroesophageal reflux disease are not at increased risk for aspiration during ECT, and intubation is not performed. These patients should continue their medications. If the patient is experiencing symptoms despite medical treatment, gastric acid-neutralizing agents (sodium citrate) should be administered immediately before ECT. If gastroesophageal reflux is severe, histamine (H2) receptor antagonists or promotility agents (metoclopramide) may be administered intravenously 30min before ECT.41
Some patients have a deficiency of pseudocholinesterase, the enzyme that metabolizes succinylcholine. The deficiency may be heterozygous or, much more commonly, homozygous82 and is best determined by the dibucaine number test.83 In addition, it should be noted that some medications (such as neostigmine, edrophonium, and pancuronium) may inhibit the action of pseudocholinesterase.82 Due to the infrequency of this anomaly, along with economic and logistic considerations, this test is not routinely administered to patients undergoing anaesthesia.82 In the few cases published in the literature, some authors recommend using low doses of succinylcholine once the deficiency has been detected,84,85 while others suggest using a non-depolarizing neuromuscular blocking agent.86,87
In patients with pheochromocytoma, a risk–benefit analysis should be done, as there is a high risk of uncontrolled hypertension and potentially life-threatening arrhythmias.88 Patients with systemic mastocytosis may suffer severe life threatening attacks (palpitations, tachycardia, hypotension and syncope, or shock, leading to cardiac arrest) that can be triggered by several factors including all histamine-releasing medications (many of which are commonly used in anaesthesia). The patients with systemic mastocytosis are commonly prescribed prophylactic H1 and H2 receptor antagonists, with diphenhydramine. These medications are typically continued during the course of ECT.89
If a fracture is suspected (in the event, for example, of a recent fall), radiographic confirmation is indicated. A joint assessment by the orthopaedist (to assess the risk of displacement following seizure and to determine optimal position), the anaesthesiologist (to adjust the dose of succinylcholine for complete neuromuscular block), and the psychiatrist (to determine stimulus intensity) is recommended.90,91 It is very important to maintain a good level of post-ECT analgesia.
In neuroleptic malignant syndrome, it is important to consider caffeine intake, smoking habit, and the presence of other drugs that may interact with antipsychotics, as these can lead to a metabolic change that may trigger the syndrome.92,93 Lastly, some authors argue that ECT should not be automatically ruled out as a treatment option in patients receiving palliative care.94
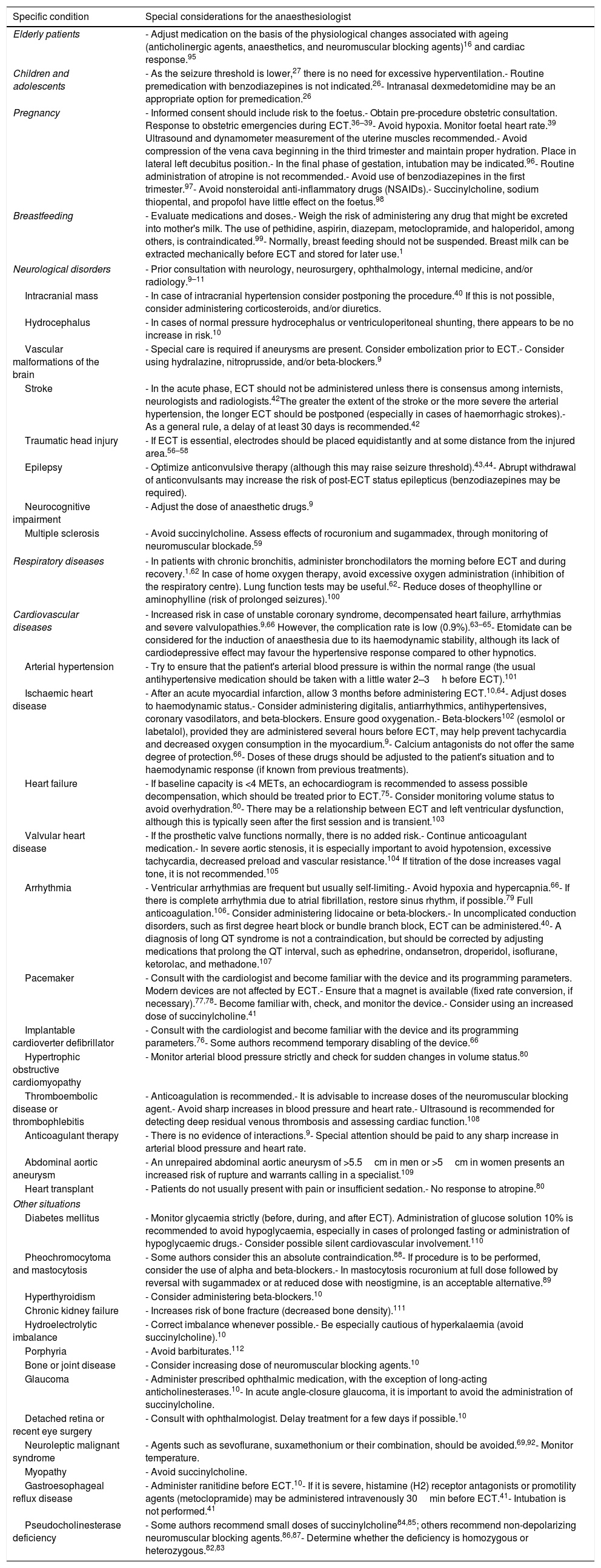
Table 1 summarizes the most important considerations for the anaesthesiologist to bear in mind in the specific situations described above.
Special considerations for the ECT anaesthesiologist for various specific conditions.
| Specific condition | Special considerations for the anaesthesiologist |
|---|---|
| Elderly patients | - Adjust medication on the basis of the physiological changes associated with ageing (anticholinergic agents, anaesthetics, and neuromuscular blocking agents)16 and cardiac response.95 |
| Children and adolescents | - As the seizure threshold is lower,27 there is no need for excessive hyperventilation.- Routine premedication with benzodiazepines is not indicated.26- Intranasal dexmedetomidine may be an appropriate option for premedication.26 |
| Pregnancy | - Informed consent should include risk to the foetus.- Obtain pre-procedure obstetric consultation. Response to obstetric emergencies during ECT.36–39- Avoid hypoxia. Monitor foetal heart rate.39 Ultrasound and dynamometer measurement of the uterine muscles recommended.- Avoid compression of the vena cava beginning in the third trimester and maintain proper hydration. Place in lateral left decubitus position.- In the final phase of gestation, intubation may be indicated.96- Routine administration of atropine is not recommended.- Avoid use of benzodiazepines in the first trimester.97- Avoid nonsteroidal anti-inflammatory drugs (NSAIDs).- Succinylcholine, sodium thiopental, and propofol have little effect on the foetus.98 |
| Breastfeeding | - Evaluate medications and doses.- Weigh the risk of administering any drug that might be excreted into mother's milk. The use of pethidine, aspirin, diazepam, metoclopramide, and haloperidol, among others, is contraindicated.99- Normally, breast feeding should not be suspended. Breast milk can be extracted mechanically before ECT and stored for later use.1 |
| Neurological disorders | - Prior consultation with neurology, neurosurgery, ophthalmology, internal medicine, and/or radiology.9–11 |
| Intracranial mass | - In case of intracranial hypertension consider postponing the procedure.40 If this is not possible, consider administering corticosteroids, and/or diuretics. |
| Hydrocephalus | - In cases of normal pressure hydrocephalus or ventriculoperitoneal shunting, there appears to be no increase in risk.10 |
| Vascular malformations of the brain | - Special care is required if aneurysms are present. Consider embolization prior to ECT.- Consider using hydralazine, nitroprusside, and/or beta-blockers.9 |
| Stroke | - In the acute phase, ECT should not be administered unless there is consensus among internists, neurologists and radiologists.42The greater the extent of the stroke or the more severe the arterial hypertension, the longer ECT should be postponed (especially in cases of haemorrhagic strokes).- As a general rule, a delay of at least 30 days is recommended.42 |
| Traumatic head injury | - If ECT is essential, electrodes should be placed equidistantly and at some distance from the injured area.56–58 |
| Epilepsy | - Optimize anticonvulsive therapy (although this may raise seizure threshold).43,44- Abrupt withdrawal of anticonvulsants may increase the risk of post-ECT status epilepticus (benzodiazepines may be required). |
| Neurocognitive impairment | - Adjust the dose of anaesthetic drugs.9 |
| Multiple sclerosis | - Avoid succinylcholine. Assess effects of rocuronium and sugammadex, through monitoring of neuromuscular blockade.59 |
| Respiratory diseases | - In patients with chronic bronchitis, administer bronchodilators the morning before ECT and during recovery.1,62 In case of home oxygen therapy, avoid excessive oxygen administration (inhibition of the respiratory centre). Lung function tests may be useful.62- Reduce doses of theophylline or aminophylline (risk of prolonged seizures).100 |
| Cardiovascular diseases | - Increased risk in case of unstable coronary syndrome, decompensated heart failure, arrhythmias and severe valvulopathies.9,66 However, the complication rate is low (0.9%).63–65- Etomidate can be considered for the induction of anaesthesia due to its haemodynamic stability, although its lack of cardiodepressive effect may favour the hypertensive response compared to other hypnotics. |
| Arterial hypertension | - Try to ensure that the patient's arterial blood pressure is within the normal range (the usual antihypertensive medication should be taken with a little water 2–3h before ECT).101 |
| Ischaemic heart disease | - After an acute myocardial infarction, allow 3 months before administering ECT.10,64- Adjust doses to haemodynamic status.- Consider administering digitalis, antiarrhythmics, antihypertensives, coronary vasodilators, and beta-blockers. Ensure good oxygenation.- Beta-blockers102 (esmolol or labetalol), provided they are administered several hours before ECT, may help prevent tachycardia and decreased oxygen consumption in the myocardium.9- Calcium antagonists do not offer the same degree of protection.66- Doses of these drugs should be adjusted to the patient's situation and to haemodynamic response (if known from previous treatments). |
| Heart failure | - If baseline capacity is <4 METs, an echocardiogram is recommended to assess possible decompensation, which should be treated prior to ECT.75- Consider monitoring volume status to avoid overhydration.80- There may be a relationship between ECT and left ventricular dysfunction, although this is typically seen after the first session and is transient.103 |
| Valvular heart disease | - If the prosthetic valve functions normally, there is no added risk.- Continue anticoagulant medication.- In severe aortic stenosis, it is especially important to avoid hypotension, excessive tachycardia, decreased preload and vascular resistance.104 If titration of the dose increases vagal tone, it is not recommended.105 |
| Arrhythmia | - Ventricular arrhythmias are frequent but usually self-limiting.- Avoid hypoxia and hypercapnia.66- If there is complete arrhythmia due to atrial fibrillation, restore sinus rhythm, if possible.79 Full anticoagulation.106- Consider administering lidocaine or beta-blockers.- In uncomplicated conduction disorders, such as first degree heart block or bundle branch block, ECT can be administered.40- A diagnosis of long QT syndrome is not a contraindication, but should be corrected by adjusting medications that prolong the QT interval, such as ephedrine, ondansetron, droperidol, isoflurane, ketorolac, and methadone.107 |
| Pacemaker | - Consult with the cardiologist and become familiar with the device and its programming parameters. Modern devices are not affected by ECT.- Ensure that a magnet is available (fixed rate conversion, if necessary).77,78- Become familiar with, check, and monitor the device.- Consider using an increased dose of succinylcholine.41 |
| Implantable cardioverter defibrillator | - Consult with the cardiologist and become familiar with the device and its programming parameters.76- Some authors recommend temporary disabling of the device.66 |
| Hypertrophic obstructive cardiomyopathy | - Monitor arterial blood pressure strictly and check for sudden changes in volume status.80 |
| Thromboembolic disease or thrombophlebitis | - Anticoagulation is recommended.- It is advisable to increase doses of the neuromuscular blocking agent.- Avoid sharp increases in blood pressure and heart rate.- Ultrasound is recommended for detecting deep residual venous thrombosis and assessing cardiac function.108 |
| Anticoagulant therapy | - There is no evidence of interactions.9- Special attention should be paid to any sharp increase in arterial blood pressure and heart rate. |
| Abdominal aortic aneurysm | - An unrepaired abdominal aortic aneurysm of >5.5cm in men or >5cm in women presents an increased risk of rupture and warrants calling in a specialist.109 |
| Heart transplant | - Patients do not usually present with pain or insufficient sedation.- No response to atropine.80 |
| Other situations | |
| Diabetes mellitus | - Monitor glycaemia strictly (before, during, and after ECT). Administration of glucose solution 10% is recommended to avoid hypoglycaemia, especially in cases of prolonged fasting or administration of hypoglycaemic drugs.- Consider possible silent cardiovascular involvement.110 |
| Pheochromocytoma and mastocytosis | - Some authors consider this an absolute contraindication.88- If procedure is to be performed, consider the use of alpha and beta-blockers.- In mastocytosis rocuronium at full dose followed by reversal with sugammadex or at reduced dose with neostigmine, is an acceptable alternative.89 |
| Hyperthyroidism | - Consider administering beta-blockers.10 |
| Chronic kidney failure | - Increases risk of bone fracture (decreased bone density).111 |
| Hydroelectrolytic imbalance | - Correct imbalance whenever possible.- Be especially cautious of hyperkalaemia (avoid succinylcholine).10 |
| Porphyria | - Avoid barbiturates.112 |
| Bone or joint disease | - Consider increasing dose of neuromuscular blocking agents.10 |
| Glaucoma | - Administer prescribed ophthalmic medication, with the exception of long-acting anticholinesterases.10- In acute angle-closure glaucoma, it is important to avoid the administration of succinylcholine. |
| Detached retina or recent eye surgery | - Consult with ophthalmologist. Delay treatment for a few days if possible.10 |
| Neuroleptic malignant syndrome | - Agents such as sevoflurane, suxamethonium or their combination, should be avoided.69,92- Monitor temperature. |
| Myopathy | - Avoid succinylcholine. |
| Gastroesophageal reflux disease | - Administer ranitidine before ECT.10- If it is severe, histamine (H2) receptor antagonists or promotility agents (metoclopramide) may be administered intravenously 30min before ECT.41- Intubation is not performed.41 |
| Pseudocholinesterase deficiency | - Some authors recommend small doses of succinylcholine84,85; others recommend non-depolarizing neuromuscular blocking agents.86,87- Determine whether the deficiency is homozygous or heterozygous.82,83 |
The authors declare no conflict of interests.