At least 10% of patients with Obsessive-compulsive Disorder (OCD) are refractory to psychopharmacological treatment. The emergence of new technologies for the modulation of altered neuronal activity in Neurosurgery, deep brain stimulation (DBS), has enabled its use in severe and refractory OCD cases. The objective of this article is to review the current scientific evidence on the effectiveness and applicability of this technique to refractory OCD.
MethodWe systematically reviewed the literature to identify the main characteristics of deep brain stimulation, its use and applicability as treatment for obsessive-compulsive disorder. Therefore, we reviewed PubMed/Medline, Embase and PsycINFO databases, combining the key-words ‘Deep brain stimulation’, ‘DBS’ and ‘Obsessive-compulsive disorder’ ‘OCS’. The articles were selected by two of the authors independently, based on the abstracts, and if they described any of the main characteristics of the therapy referring to OCD: applicability; mechanism of action; brain therapeutic targets; efficacy; side-effects; co-therapies. All the information was subsequently extracted and analysed.
ResultsThe critical analysis of the evidence shows that the use of DBS in treatment-resistant OCD is providing satisfactory results regarding efficacy, with assumable side-effects. However, there is insufficient evidence to support the use of any single brain target over another. Patient selection has to be done following analyses of risks/benefits, being advisable to individualise the decision of continuing with concomitant psychopharmacological and psychological treatments.
ConclusionsThe use of DBS is still considered to be in the field of research, although it is increasingly used in refractory-OCD, producing in the majority of studies significant improvements in symptomatology, and in functionality and quality of life. It is essential to implement random and controlled studies regarding its long-term efficacy, cost-risk analyses and cost/benefit.
Al menos el 10% de pacientes con trastorno obsesivo-compulsivo (TOC) son refractarios al tratamiento psicofarmacológico. La aparición de nuevas tecnologías neuroquirúrgicas (estimulación cerebral profunda[ECP]) de modulación de la actividad neuronal alterada está posibilitando su extensión a casos graves y refractarios de TOC en los que anteriormente se utilizaban técnicas quirúrgicas no reversibles. El objetivo de este artículo es revisar la evidencia científica existente sobre la eficacia y aplicabilidad de esta técnica en este grupo de pacientes.
MétodoSe ha realizado una revisión sistemática de la literatura en las bases de datos PubMed/Medline, Embase y PsycINFO usando las palabras clave relacionadas con «deep brain stimulation», «DBS» y «obsessive-compulsive disorder», «OCD». Dos de los autores seleccionaron los artículos, de manera independiente, a partir de sus abstracts y en función de si describían alguno de los aspectos principales de la técnica en el TOC: aplicabilidad; mecanismo de acción; dianas terapéuticas cerebrales; efectividad; efectos secundarios, y coterapias. Toda la información fue sistemáticamente extraída y evaluada.
ResultadosEl análisis crítico de la evidencia señala que la aplicación de la ECP en el tratamiento del TOC refractario está aportando resultados satisfactorios, con rangos asumibles de efectos secundarios. Sin embargo, todavía no hay evidencia suficiente que permita priorizar el uso de una determinada diana cerebral. La selección de pacientes ha de seguir un análisis de riesgo/beneficio, debiéndose individualizar la decisión de mantener un tratamiento concomitante farmacológico/psicoterapéutico.
ConclusionesLa ECP se encuentra todavía en el ámbito de la investigación, pero su aplicación en el TOC-refractario es cada vez más frecuente, produciendo en la mayoría de los estudios una significativa mejoría de los síntomas, y también del funcionamiento y calidad de vida. Es preciso realizar más estudios controlados y aleatorizados sobre su efectividad a largo plazo, y sobre su relación riesgo/beneficio y costes.
Deep brain stimulation (DBS) is a technique that stimulates the subcortical regions of the brain and has been increasingly used in recent years in the treatment of motor neurological disorders, since the paper by Benabid et al.1 It involves stereotaxically implanting electrodes that emit short electrical pulses, generally high-frequency, to modulate the functioning of specific brain circuits. The main areas of the brain that have been stimulated targets are: thalamus; subthalamic nucleus; nucleus accumbens; anterior limb of internal capsule (ALIC); bed nucleus of stria terminalis (BNST), and cingulum. Its aim is to eliminate, or at least modulate, cerebrally transmitted pathological processes in these areas, by reconfiguring of the activity of the neuronal circuits and/or networks.
The mechanisms of action of DBS are not yet clear. Various options have been postulated: (i) reversible functional inhibition of the stimulated brain structures, which would be secondary to a depolarisation block of the neurons surrounding the electrode, or modifications to the ion channels of the cell membrane; (ii) synaptic depression secondary to stimulation of the afferent axons, with the consecutive inhibition of transmission by transmitter exhaustion, and (iii) synaptically-mediated neuronal inhibition by inhibitory GABAergic afferents.2 In any case, biochemical, neurohormonal, cellular neurophysiological and neuroplasticity mechanisms have been implicated that eventually modulate the activity of neuronal circuits. It should be highlighted, however, that, although changes can be seen in emotional symptoms in a few seconds or minutes, with DBS, as with drug treatments, the effect usually takes one or several weeks to manifest. This cannot be explained by direct stimulation or inhibition mechanisms; we consider a more likely explanation to be long-term modulation processes of the neuronal systems through plastic adaptation processes.
Mechanisms of action of deep brain stimulation in severe treatment-resistant obsessive compulsive disorderThe scientific justification for applying DBS to obsessive compulsive disorder is based on accepting the neurobiological hypothesis that these disorders are associated with hyperactivity and hyperconnectivity in the “cortico-striato-pallido-thalamo-cortical loop”. This neuronal dysfunction would also occur, for example, in specific structures such as the nucleus accumbens, which acts as an entry port to the basal ganglia, constituting an interface for the motor and limbic systems and playing a significant role in the onset of OCD. In this context DBS would act as a functional modulation mechanism of the pathological hyperactivity of the circuits and impaired structures.3,4
Even though the mechanisms of action of DBS in refractory OCD are not fully known and its detailed analysis would require a specific, systematic literature review, it seems appropriate to mention the possible alternative mechanisms of action that are most frequently cited in the literature. (i) “functional lesioning” mechanism with local inhibition. The initial theories regarding the mechanisms of action of DBS were based on comparing them with the effects of neurosurgical ablation techniques used to treat these disorders.5 According to these theories, the lesion, whether anatomical or “functional”, would produce local neuronal inhibition via mechanisms such as, for example, nerve conduction block, neurotransmitter depletion, or the activation of inhibitory impulses.6–9 (ii) Mechanisms of local excitation. It has been observed, by means of computational models and experimental studies, that DBS depends on mechanisms of neuronal excitation of the axon that are associated with inhibition of the soma, in the stimulated nuclei.9 Thus, for example, the study by Tsai et al. demonstrated that DBS of the subthalamic nucleus causes an increase in the firing of specific neurons, which is believed to be due to the activation of the glutamatergic afferent pathways.10 Similarly, Li et al. demonstrated an antidromic activation of the deep neuronal layers of the subthalamic nucleus during DBS.11 It has also been observed that stimulation of the nucleus accumbens causes inhibition of the orbitofrontal cortex.9 (iii) Mechanisms of modulation of temporal firing. Various studies have demonstrated that in these disorders the neuronal areas that appear to be involved, the subthalamic nucleus or globus pallidus, for example, tend to show abnormal firing patterns.12 In this situation it is postulated that the effect of DBS, more than causing local inhibition, is to modulate an impaired neuronal response in these nuclei.9,13,14 (iv) Mechanisms of neurogenesis and plasticity. As occurs with drug treatment, the effectiveness of treatment with DBS takes weeks, even months, to manifest.15 This suggests that its mechanism of action cannot simply be based on acute modification of the excitation or inhibition processes or patterns of neuronal response. Therefore, the presence of neurogenesis and plasticity processes that would take place essentially at the level of the hippocampal structures has been posited as an alternative, or at least complementary, mechanism9; this theory has also been supported by animal experimentation.16,17
The application of DBS has recently extended from motor disorders, such as Parkinson's disease, to psychiatric disorders, principally severe refractory OCD. It has been used also in a preliminary way for other disorders such as severe depressive disorder, Tourette's syndrome, eating disorders and substance abuse disorders.
Application of deep brain stimulation for severe treatment-refractory compulsive-obsessive disorderOCD, with a lifetime prevalence of between 2% and 3%, is a psychiatric condition characterised by obsessions and compulsions. Obsessions include recurring thoughts, images or impulses that the patients recognise as their own but that they cannot control, causing them marked anxiety and distress. They try to neutralise the anxiety caused by these obsessions by developing repetitive behaviours, termed compulsions. These symptoms exacerbate distress, and significantly affect quality of life and level of functionality, resulting in considerable disability.18,19
Despite the best drug and cognitive-behavioural treatments, at least 10% of patients with OCD can be considered resistant or refractory to treatment.20 Neurosurgical interventions have frequently been performed for these patients, such as anterior bilateral capsulotomy or cingulotomy, that have demonstrated success rates, expressed in reductions in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores ranging between 26% and 60%.21–23 However, the disadvantage of these interventions are irreversible changes to brain structure. Therefore, the therapeutic emphasis has currently been placed on the development of therapeutic strategies based on neuronal neuromodulation, which involves the administration of electrical stimuli that cause changes to neuronal function.24 DBS is very specific among these strategies, which include electroconvulsive therapy or magnetic transcranial stimulation. Based on the knowledge gained in treatments for neuronal damage, DBS is used to cause “functional ablation” via electrical stimulation, initially used in the brain structures where surgical neural ablation treatments had shown effectiveness, and more recently in other brain targets studied. The advantages of applying this therapeutic strategy lie not so much in improved effectiveness, but in its fewer side effects and better acceptability.25,26 Along these lines, Nuttin et al. decided in 1999 – with considerable success- to use DBS in the ALIC, for the treatment of severe treatment-refractory OCD.5 Since then various authors have used DBS for the treatment of refractory OCD with good results. So that, in recent years, various systematic reviews and meta-analysis studies20,24,26 have provided data to support the effectiveness of DBS, which, by acting on various brain targets, achieves a proportion of response (reductions of Y-BOCS scores of more than 35%) in patients with OCD close to 60%, and with mean reductions in the severity of obsessive- compulsive symptoms (measured using the Y-BOCS) close to 50%.
DBS was approved in 2009 by the USA Food and Drug Administration as a therapeutic option for OCD, and awarded the EU's Certificate of Approval in the same year.
Definition of treatment-resistant/refractory obsessive- compulsive disorderAlthough some authors propose that the terms “resistant” and “refractory” should be clearly differentiated for OCD, and suggest that the concept of refractory implies a greater degree of therapeutic resistance,27 there is a clear tendency in the literature reviewed to use both terms as synonyms. This occurs, often associated in the various studies with variations in the clinical criteria that define the different therapeutic response categories. The International Treatment Refractory OCD Consortium attempted to resolve this situation, which has clear negative implications for both clinical activity and the research process, by establishing operational criteria of “treatment non-response”.28 To that end, the authors established, firstly, the different possible levels of therapeutic response, defining “full response” as a 35% or more reduction of symptoms on the Y-BOCS, and a Clinical Global Impression (CGI) score of 1 or 2; “non-response” as a reduction in Y-BOCS score greater than 25% but less than 35%; and “non-response” as a reduction of less than 25% on the Y-BOCS and a GCI score of 4. Secondly, the group established the operational criteria to define 10 progressive levels of severity of “non-response”. These levels require therapeutic trials of progressive complexity with (i) up to 3 distinct selective serotonin reuptake inhibitors at sufficient doses and over a sufficiently long period of time (at least 12 weeks), alone and in combination with a strategy of cognitive-behavioural psychotherapy (CBT) that includes exposure and prevention of response over a minimum of 20 sessions; (ii) implementing a therapeutic trial with, at least, 3 selective serotonin reuptake inhibitors, including clomipramine (with intravenous trials) in combination with CBT, and/or with other antidepressants (NSRI, IMAO); (iii) the use of augmentation strategies with neuroleptics alone and/or in combination with mood stabilisers, and with psychostimulants for sufficiently long periods of time.
Finally, it is important to mention that when establishing the category “treatment-resistant/refractory OCD”, some authors suggest including a time criterion of a minimum period of 5 years.29–31
The aim of this systematic review was to analyse the viability and appropriateness of using DBS for OCD.
MethodsWe performed a systematic review of the literature, following the PRISMA model, to identify scientific articles that provide data on the use of DBS for OCD. To that end we performed a search of the electronic databases, including PubMed/Medline, Embase and PsycINFO, using a combination of key terms included under “deep brain stimulation”, “DBS” and “obsessive compulsive disorder”, “OCD”. The reference lists of previous reviews were also inspected in the search of additional articles.
The literature review was limited to articles in English or Spanish, performed on humans from January 2000 until December 2016. Studies were chosen for this review if they met the following criteria: (1) original articles, such as clinical trials, comparative studies, or multi-centre studies, presenting data from more than one patient, and (2) studies contributing quantitative or qualitative data on the use of DBS for OCD on (i) eligibility criteria for using the technique; (ii) assessment of efficacy and outcome using the Y-BOCS, pre- and postoperatively, and/or quality of life/function, (iii) brain therapeutic targets, and/or (iv) side effects.
However, for the review of other significant aspects of DBS on OCD, as well as for drawing up the introduction and discussion of results, we also used scientific studies on the use of DBS for OCD that did not meet some of the abovementioned criteria, as well as previous systematic reviews and meta-analyses.
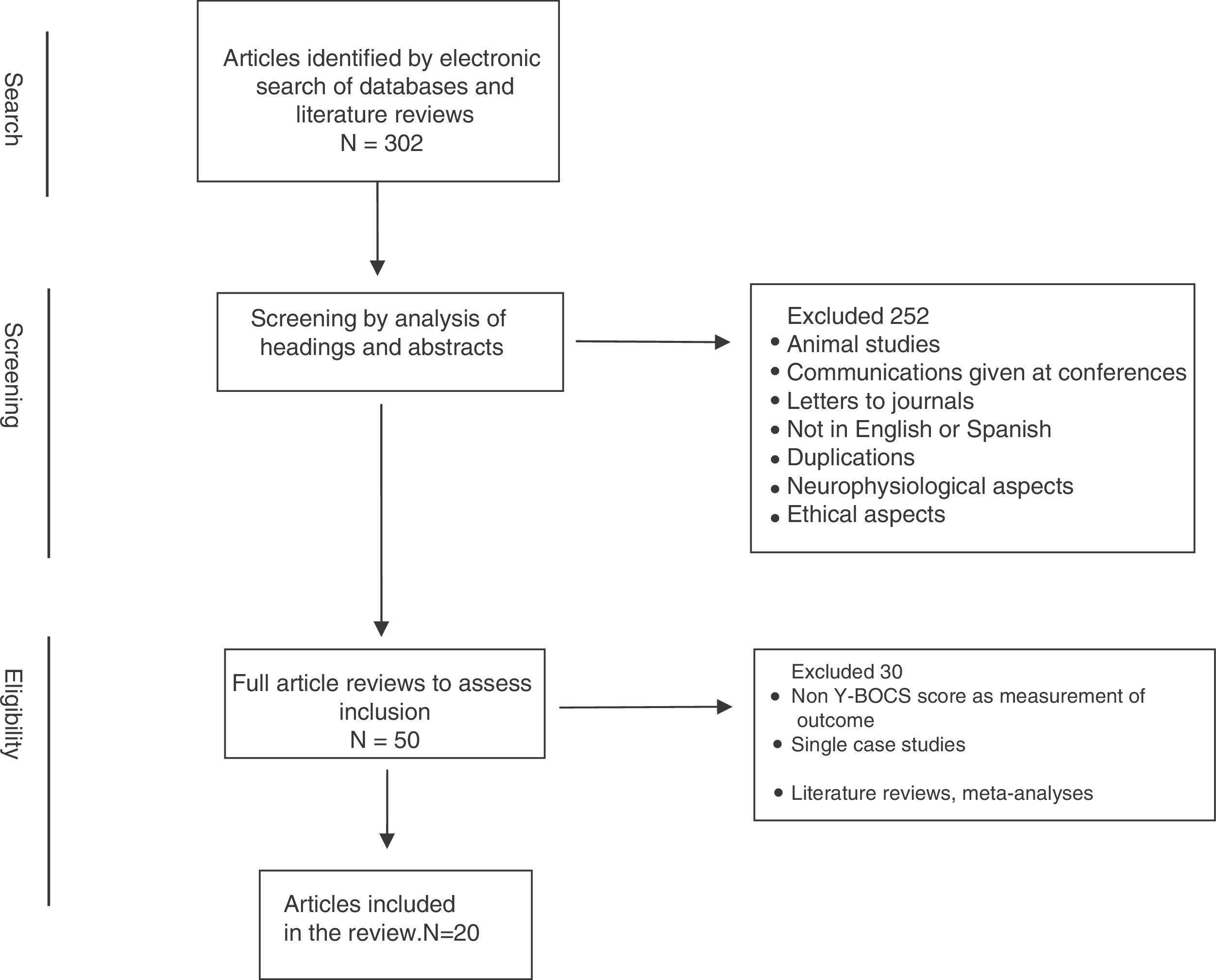
The literature search initially yielded a total of 302 articles, which we put through a multi-phase analysis process (Fig. 1). The abstracts of the pre-selected articles from this literature search were reviewed independently by 2 of the authors (JVB and JM), selecting those that had concordance with the established selection criteria. Discrepancies between the 2 reviews were resolved through a further evaluation process and joint decision-making. Thus 252 articles were removed, because they were: animal studies, abstracts from conferences and meetings, duplications, not in English or Spanish, focus on neurophysiological or ethical aspects, etc. In a second phase, through a complete review of the chosen articles we performed a qualitative analysis of the information contained in them. From this analysis 30 articles were eliminated that did not include more than one patient and/or did not present assessments using the Y-BOCS in the pre- and postoperative periods, and/or of quality of life/functionality, as well as literature reviews and meta-analyses.
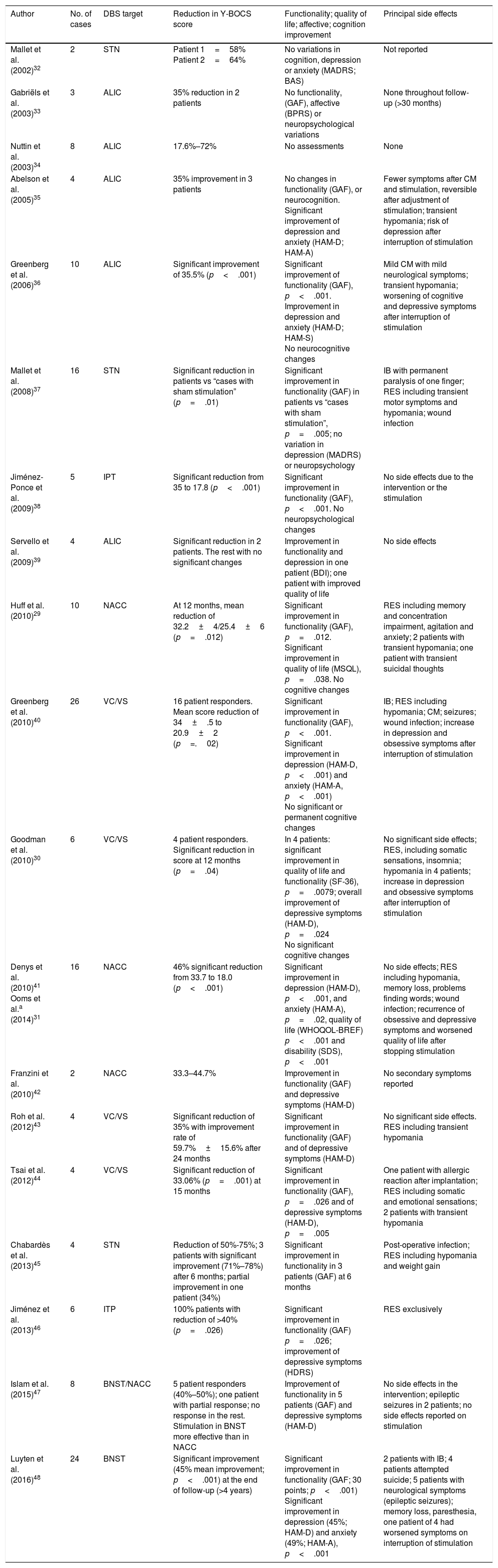
Thus 20 articles were finally included for review. Table 1 shows a summary of the articles included in this review.
Summary of the articles selected.
| Author | No. of cases | DBS target | Reduction in Y-BOCS score | Functionality; quality of life; affective; cognition improvement | Principal side effects |
|---|---|---|---|---|---|
| Mallet et al. (2002)32 | 2 | STN | Patient 1=58% Patient 2=64% | No variations in cognition, depression or anxiety (MADRS; BAS) | Not reported |
| Gabriëls et al. (2003)33 | 3 | ALIC | 35% reduction in 2 patients | No functionality, (GAF), affective (BPRS) or neuropsychological variations | None throughout follow-up (>30 months) |
| Nuttin et al. (2003)34 | 8 | ALIC | 17.6%–72% | No assessments | None |
| Abelson et al. (2005)35 | 4 | ALIC | 35% improvement in 3 patients | No changes in functionality (GAF), or neurocognition. Significant improvement of depression and anxiety (HAM-D; HAM-A) | Fewer symptoms after CM and stimulation, reversible after adjustment of stimulation; transient hypomania; risk of depression after interruption of stimulation |
| Greenberg et al. (2006)36 | 10 | ALIC | Significant improvement of 35.5% (p<.001) | Significant improvement of functionality (GAF), p<.001. Improvement in depression and anxiety (HAM-D; HAM-S) No neurocognitive changes | Mild CM with mild neurological symptoms; transient hypomania; worsening of cognitive and depressive symptoms after interruption of stimulation |
| Mallet et al. (2008)37 | 16 | STN | Significant reduction in patients vs “cases with sham stimulation” (p=.01) | Significant improvement in functionality (GAF) in patients vs “cases with sham stimulation”, p=.005; no variation in depression (MADRS) or neuropsychology | IB with permanent paralysis of one finger; RES including transient motor symptoms and hypomania; wound infection |
| Jiménez-Ponce et al. (2009)38 | 5 | IPT | Significant reduction from 35 to 17.8 (p<.001) | Significant improvement in functionality (GAF), p<.001. No neuropsychological changes | No side effects due to the intervention or the stimulation |
| Servello et al. (2009)39 | 4 | ALIC | Significant reduction in 2 patients. The rest with no significant changes | Improvement in functionality and depression in one patient (BDI); one patient with improved quality of life | No side effects |
| Huff et al. (2010)29 | 10 | NACC | At 12 months, mean reduction of 32.2±4/25.4±6 (p=.012) | Significant improvement in functionality (GAF), p=.012. Significant improvement in quality of life (MSQL), p=.038. No cognitive changes | RES including memory and concentration impairment, agitation and anxiety; 2 patients with transient hypomania; one patient with transient suicidal thoughts |
| Greenberg et al. (2010)40 | 26 | VC/VS | 16 patient responders. Mean score reduction of 34±.5 to 20.9±2 (p=.02) | Significant improvement in functionality (GAF), p<.001. Significant improvement in depression (HAM-D, p<.001) and anxiety (HAM-A, p<.001) No significant or permanent cognitive changes | IB; RES including hypomania; CM; seizures; wound infection; increase in depression and obsessive symptoms after interruption of stimulation |
| Goodman et al. (2010)30 | 6 | VC/VS | 4 patient responders. Significant reduction in score at 12 months (p=.04) | In 4 patients: significant improvement in quality of life and functionality (SF-36), p=.0079; overall improvement of depressive symptoms (HAM-D), p=.024 No significant cognitive changes | No significant side effects; RES, including somatic sensations, insomnia; hypomania in 4 patients; increase in depression and obsessive symptoms after interruption of stimulation |
| Denys et al. (2010)41 Ooms et al.a (2014)31 | 16 | NACC | 46% significant reduction from 33.7 to 18.0 (p<.001) | Significant improvement in depression (HAM-D), p<.001, and anxiety (HAM-A), p=.02, quality of life (WHOQOL-BREF) p<.001 and disability (SDS), p<.001 | No side effects; RES including hypomania, memory loss, problems finding words; wound infection; recurrence of obsessive and depressive symptoms and worsened quality of life after stopping stimulation |
| Franzini et al. (2010)42 | 2 | NACC | 33.3–44.7% | Improvement in functionality (GAF) and depressive symptoms (HAM-D) | No secondary symptoms reported |
| Roh et al. (2012)43 | 4 | VC/VS | Significant reduction of 35% with improvement rate of 59.7%±15.6% after 24 months | Significant improvement in functionality (GAF) and of depressive symptoms (HAM-D) | No significant side effects. RES including transient hypomania |
| Tsai et al. (2012)44 | 4 | VC/VS | Significant reduction of 33.06% (p=.001) at 15 months | Significant improvement in functionality (GAF), p=.026 and of depressive symptoms (HAM-D), p=.005 | One patient with allergic reaction after implantation; RES including somatic and emotional sensations; 2 patients with transient hypomania |
| Chabardès et al. (2013)45 | 4 | STN | Reduction of 50%-75%; 3 patients with significant improvement (71%–78%) after 6 months; partial improvement in one patient (34%) | Significant improvement in functionality in 3 patients (GAF) at 6 months | Post-operative infection; RES including hypomania and weight gain |
| Jiménez et al. (2013)46 | 6 | ITP | 100% patients with reduction of >40% (p=.026) | Significant improvement in functionality (GAF) p=.026; improvement of depressive symptoms (HDRS) | RES exclusively |
| Islam et al. (2015)47 | 8 | BNST/NACC | 5 patient responders (40%–50%); one patient with partial response; no response in the rest. Stimulation in BNST more effective than in NACC | Improvement of functionality in 5 patients (GAF) and depressive symptoms (HAM-D) | No side effects in the intervention; epileptic seizures in 2 patients; no side effects reported on stimulation |
| Luyten et al. (2016)48 | 24 | BNST | Significant improvement (45% mean improvement; p<.001) at the end of follow-up (>4 years) | Significant improvement in functionality (GAF; 30 points; p<.001) Significant improvement in depression (45%; HAM-D) and anxiety (49%; HAM-A), p<.001 | 2 patients with IB; 4 patients attempted suicide; 5 patients with neurological symptoms (epileptic seizures); memory loss, paresthesia, one patient of 4 had worsened symptoms on interruption of stimulation |
ALIC: anterior limb of internal capsule; BAS: Brief Anxiety Scale; BDI: Beck Depression Inventory; BNST: bed nucleus of stria terminalis; BPRS: Brief Psychiatric Rating Scale; CGI: Clinical Global Impression; CM: complications deriving from material failure; DBS: deep brain stimulation; RES: reversible effects of the stimulation; GAF: Global Assessment of Functionality; HAM-A: Hamilton Anxiety Scale; HAM-D: Hamilton Depression Scale; HDRS: Hamilton Depression Rating Scale; IB: intracranial bleeding; ITP: inferior thalamic peduncle; MADRS: Montgomery and Asberg Depression Rating Scale; MSQL: Modular System of Quality of Life; NACC: nucleus accumbens; SDS: Sheehan Disability Scale; SF-36: 36-Item Short Form Health Survey; STN: subthalamic nucleus; VC/VS: ventral capsule/ventral striatum; WHOQOL-BREF: WHO Quality of Life Brief; Y-BOCS: Yale-Brown Obsessive Compulsive Scale.
Since 1999, numerous studies have been published describing the use of DBS for cases of OCD. From these studies we can conclude that the target structures commonly selected for DBS have been: (i) the ALIC; (ii) the nucleus accumbens; (iii) the subthalamic nucleus; (iv) the ventral capsule/ventral striatum; (v) the inferior thalamic peduncle; and (vi) the BNST. Below we shall review the effectiveness of DBS in each of these brain structures.
Anterior limb of the internal capsule (ALIC)This is the anterior part of the internal capsule, which is situated between the head of the caudate nucleus and the lenticular nucleus, and whose fibres connect the prefrontal cortex and the mediodorsal thalamus. This is one of the structures on which the neurosurgical ablation techniques were performed in the past; Nuttin et al. were the first to substitute, with good outcomes, the use of these ablation techniques for DBS in this structure.5 Since then this structure has been chosen for DBS in other studies.20,26,33–36,39,48,49 These studies show improvement percentages varying between 35% and 79% of reduction in Y-BOCS scores. The mechanisms of action through which DBS of the ALIC acts in the treatment of OCD are not clear, but they might include modulation of the thalamocortical circuits and the basal ganglia.50 However, a recent study attributes part of the effect of stimulation in the ALIC to stimulation of the BNST, and even suggest that this therapeutic target is more effective than the ALIC.48
Nucleus accumbensIts site below the ALIC, between the anterior portion of the putamen and the head of the caudate nucleus, enables the nucleus accumbens and the ALIC to be stimulated at the same time. Moreover, often the terms nucleus accumbens and ventral striatum are used interchangeably to refer to the confluence between the putamen and the caudate. This nucleus has been chosen as a “target” for DBS due to its role in the origin of OCD, through its mediation in the dysfunction of the reward system.49
We identified 4 studies that included 32 patients with refractory OCD treated with DBS of this nucleus.29,41,42,47 These studies found that DBS causes a significant reduction in obsessive symptoms. A reduction, in the study by Denys et al. for example, by 46% on the Y-BOCS score.41 This study has also been catalogued as the only one to show the effectiveness of bilateral DBS of the nucleus accumbens with a Level II of scientific evidence.15 And it is also particularly interesting because it also shows an increased response when DBS is combined with CBT.
Ventral capsule/ventral striatumThe ventral striatal region includes the ventral caudate nucleus and the nucleus accumbens, which together with the ventral capsule have been termed “ventral capsule/ventral striatum”. It is believed to be involved in the psychological mechanisms of “reward-punishment” and motivation. Four studies were identified that included 38 treatment-refractory patients treated with DBS of this brain structure,30,40,43,44 observing a mean of 50% responders and achieving a mean reduction of the initial Y-BOCS score of 41.5%, after 12–36 months’ follow-up. Moreover, it has recently been observed that this improvement in symptoms is maintained long term.51
Subthalamic nucleusThe subthalamic nucleus is a structure that forms part of the basal ganglia and is situated near to the thalamus, substantia nigra and the corticospinal tract. It has traditionally been argued that this structure was only involved in the control of voluntary motor movements, however, it has been observed that its stimulation by DBS is also effective in the treatment of OCD. In this case, however, the electrodes are placed more medially than for the treatment of Parkinson's disease, which shows that it is appropriate to place these electrodes in the limbic part of this nucleus.50 We found 3 studies that included 22 patients, and which achieved reductions in obsessive symptoms of between 50% and 78% of Y-BOCS scores.32,37,45 Among these studies, that of Mallet et al. is particularly relevant in that it used a “double-blind crossover randomised” design on a sample of 16 patients with OCD who received “sham DBS” and “active DBS” alternately.37 This study was assessed as the only one to demonstrate the effectiveness of DBS for refractory OCD with a Level I of scientific evidence.15
Inferior thalamic peduncleThis is a connection structure between the orbitofrontal cortex and the thalamus. Only 2 studies were identified with a total of 11 patients, which was also confirmed in 2 recent reviews.38,46 In these studies, the level of improved symptoms after DBS, measured using the Y-BOCS, was between 40% and 82.5%. Nonetheless, the authors put these forward as tentative results given the limited number of patients treated.
Bed nucleus of stria terminalis (BNST)The BNST is a structure of the amygdala that has been implicated in compulsive behaviours, stress and anxiety, in previous studies on animal models. We identified 2 studies that include a total of 28 patients.47,48 Luyten et al. (2016)48 describe the stimulation of the ALIC/BNST region in 24 patients with refractory OCD, observing significant reductions long term (4 years; n=17) in Y-BOCS scores (mean reduction of 66%), as well as in symptoms of anxiety and depression measured by the Hamilton Anxiety and Depression Rating scales (58% in HAM-A and 67% in HAM-D). These levels of improvement were maintained longer term,52 with response percentages of 65% and 60% after 6 years (n=17) and 9 years (n=10) of follow-up, respectively, concluding in addition that the BNST might be a better target than ALIC in the treatment of refractory OCD.48 The paper by Islam et al. describes the treatment outcomes of 8 patients with resistant OCD using DBS to the BNST (n=4) and nucleus accumbens (n=4), observing a response rate of 62.5% (improvements of above 35% in Y-BOCS scores),47 and they also concluded after individual case analysis that the patients with BNST stimulation obtained better results that those with DBS of the nucleus accumbens.
Overall assessments and comparison of the effectiveness of deep brain stimulation according to the target neuronal structureHamani et al. performed a critical analysis of the existing evidence on the use of DBS for refractory OCD.15 Of the 353 studies initially reviewed, they extracted those of the greatest scientific evidence (n=7), and of those they only found 3 with a Level I or II29,37,41; they concluded that:
- i)
There is a Level I of scientific evidence, based exclusively on the study by Mallet et al.,37 for the use of bilateral DBS of the subthalamic nucleus.
- ii)
There is a Level II of scientific evidence, based exclusively on the study by Denys et al.,41 for the use of bilateral DBS of the nucleus accumbens.
- iii)
There is no scientific evidence to demonstrate the effectiveness of unilateral DBS of the nucleus accumbens.29 And unilateral stimulation of the nucleus accumbens compared to bilateral stimulation was demonstrated as less effective in the paper by Blomstedt et al.53
However, we must mention that we can conclude from the various systemic literature reviews performed in recent years that we do not have sufficient data to establish whether one brain target is better than another in terms of effectiveness in reducing the symptoms of OCD after DBS.20,26,49,50,53 The question must also be raised whether there might be different optimal targets for different patients with resistant OCD.54 Significant reductions in the severity of symptoms are described in the papers we reviewed which, for example, in the case of stimulation of the subthalamic nucleus and of the striatum are around 40% of Y-BOCS scores, as with stimulation of the ALIC.20 Interestingly, De Koning et al. describe that the effectiveness of stimulation of the nucleus accumbens was related with stimulation of the electrodes that were close to the ALIC, which leads us to believe that both structures were stimulated in that study.49 All of this supports the idea that, as suggested by Lipsman et al.,50 the various structures selected for DBS for OCD participate in the same cortico-striatal-thalamic-cortical circuit. Therefore, even though from a structural and histological perspective these structures are different, not only are they clearly close to one another, but they are also functionally linked via a rich system of afferent and efferent fibres. This fact, as suggested by Alonso et al.,26 might explain the “distance effect” that DBS has on the abnormal neuronal connectivity that occurs in the cortico-striatal-thalamic-cortical circuit involved in OCD, and also the similarity of clinical response obtained when stimulating each of them.
The recent meta-analysis by Kisely et al.,24 is of particular interest, using the Cochrane assessment strategy specifically aimed at analysing the effect of DBS on OCD, in studies that analyse, through a double-blind design, the comparison between “active DBS” and “sham DBS”. This analysis states that DBS has a significant effect in reducing the symptoms of OCD measured using the Y-BOCS. However, this reduction of a mean 9 points has to be viewed as a partial reduction in symptoms in the opinion of the authors.24 Therefore the conclusions on the effectiveness of DBS must be taken with due caution, largely due to the scarcity of papers that use a double-blind, randomised methodology and the methodological limitations that currently persist in this area.15,55 Among these we should highlight the current imprecise knowledge of:
- i)
The criteria that define the OCD cases that are “candidates” for DBS.
- ii)
The effectiveness of DBS to the different neuronal nuclei and structures, from which to select suitable target structures for DBS, according to the clinical profile of patients with OCD.
- iii)
The most efficient stimulation parameters.
- iv)
Criteria of “response”, “remission” and “recovery” if symptoms that should be applied in these clinical trials.
Due to all of the above factors, a large part of the reviews that analyse the effectiveness of DBS in the treatment of refractory OCD suggest the need to accurately standardise this therapeutic strategy, and regard it as a “very promising treatment but still in the experimental stage”.15,20,24,49,50,53,55 In view of this situation, it is essential to establish a standard registry of cases undergoing DBS to include the key variables relating to the patients’ clinical histories and the procedure for the technique, and the measurement and assessment of outcomes, and consequences of the intervention.26,55
Effect on the functionality and quality of life of patients and the acceptability of deep brain stimulationAs Katschin (2006)56 specifies, the success of a therapeutic intervention should not be limited exclusively to the elimination or reduction of clinical symptoms, but should also include improved functionality and quality of life. This is particularly relevant for long-term treatments where, as with DBS, it is important to assess the extent to which improved symptoms impact quality of life weighed against the potential disadvantages of the therapeutic intervention. Accordingly, and as we can see in Table 1, practically all the studies included in this review have assessed the impact of DBS on the quality of life and functionality of patients with resistant OCD. Thus, for example, Huff et al.29 obtained a significant increase in quality of life, measured using the Modular System of Quality of Life (MSLQ), of 41.3±15.8 to 53.2±19.8 in a series of 9 patients who underwent stimulation of the nucleus of the ventral capsule/ventral striatum for a year. Similarly, Goodman et al.30 found a significant increase in quality of life expressed in SF-36 score, after one year of stimulation of the ALIC. Finally, Ooms et al. (2014)31 found, in a series of patients who underwent stimulation of the nucleus accumbens over a period of 3–5 years, a significant improvement (90%) in the overall quality of life score measured by the WHO Quality of Life Scale (WHOQOL); they also achieved significant improvements in various domains of this scale for these patients. Similarly, most studies include the assessment of functionality as an additional measurement of the outcome and effectiveness of DBS, using the Global Assessment of Functionality (GAF) scale, and practically all of them observe a significant improvement in functionality (Table 1). Therefore, for example, in 2 of these studies, comparing the effectiveness of implanting in the BNST compared to other targets (internal capsule [IC], ALIC),48,52 a significant improvement in functionality measured using the GAF scale was observed overall and maintained over time, with mean improvements of 30 points (over a baseline mean of 35).
The effectiveness of DBS, both in terms of symptoms and quality of life, for resistant OCD does not by itself justify the greater popularity of this therapeutic strategy when compared with neurological ablation techniques, for which it was also possible to demonstrate not only similar effectiveness, but also that their secondary effects could in general be considered equivalent to those of DBS.25 Therefore, as the review paper by Pepper et al.25 comparing both techniques was able to demonstrate, the preference for DBS over ablation techniques is not due to superior clinical effectiveness, but rather to its greater acceptability by patients and practitioners alike. One of the main elements determining this greater acceptability is that DBS does not cause permanent damage, and therefore has a reversibility profile.54
Response and related aspectsDefinition of responseThere is no precise definition or reliable indicators of response and recovery. The literature reviewed describes different definitions of response depending on whether the study was “open non-randomised” or “double-blind”.
Most “open, non-randomised” studies define response in OCD as an improvement of 35% or more in Y-BOCS scores compared to the baseline scores. A partial response is defined as an improvement between 20% and 35% of the Y-BOCS score.15,49
“Double-blind” studies tend to define a response as when the improvement in the treated group achieves at least a 25% reduction in Y-BOCS score and is also significantly different to the score of those receiving the placebo.15
There are also studies that include in the definition of response to DBS other measurements of outcome such as quality of life, extent of functional recovery, or level of disability.26,29,31,41
Delayed response to deep brain stimulationThe time frame for the effect of DBS to become established has not yet been specified. The results of the reviews of the different studies tend to show that, as with drug treatment, the response to DBS takes time to manifest (from a week to months) and increases over time.15 As we have already indicated, this leads us to believe that its mechanism of action might be based on mechanisms of neurogenesis and neuronal plasticity. This is also relevant when making comparisons between studies and between the phases of trials that include a crossover design with on-off intervention (intervention/suspension/re-starting), since contamination of results can occur in these trials due to a “carry-over effect” of the treatment in the phase without treatment.
Response predictionAs yet no predictive factors of response to DBS for refractory OCD have been described. Nevertheless, some authors have described data in their studies that seem to support a possible response prediction. Thus, for example, it has been described that unmotivated laughter during the surgical procedure for implantation and during the initial electrical stimulation appears to act as a predictor of a good response.49,57 Furthermore, a correlation has been seen between the onset of intense unmotivated laughter and a greater reduction in Y-BOCS scores 2 years after implantation of the electrodes. It was also found, in a study on DBS of the ventral capsule/ventral striatum, that the pre-implantation metabolism in certain areas of the cingulate cortex was significantly related to the therapeutic outcome.58 Also, the frequency of the pulse in the electrical stimulation appears to be a predictive factor that can determine the effectiveness of DBS.49 Finally, a recent study describes a better response associated with onset of OCD at an older age, and the presence of sexual/religious obsessions and compulsions.26
Assessment of the effectiveness of deep brain stimulation on severe refractory obsessive-compulsive disorderScales for measuring the severity of obsessive-compulsive disorderAs confirmed in the systematic reviews undertaken, most of the studies use the Y-BOCS scale20,21,24–26,59,60 to measure the severity of obsessive symptoms, and to analyse the response to treatment.
Other psychopathological scalesDue to the frequent comorbidity with affective disorders, most studies (Table 1) include scales that measure symptoms of depression and anxiety.
Cognitive function scalesA battery of neuropsychological tests are used systematically in the different studies: intelligence quotient; visual and verbal learning; conceptual reasoning; mental flexibility, processing speed.29,32,33,37,38
Quality of life scalesThe effectiveness of interventions should also be measured by changes in quality of life and functionality.56 In line with this approach, studies that currently analyse the effectiveness of DBS for refractory OCD also include measures to assess these domains,26,29–31,41 such as the WHO Quality of Life Scale-Brief Version (WHOQOL-BREF).
Psychosocial functionality and disability scalesGeneric measures of the level of psychosocial functionality are also generally used, such as the GAF, CGI or Sheeham Disability Scale.24,31 Thus, as we can see in Table 1, almost all the studies use the GAF to assess functionality.
Concomitant therapiesOn general lines, when DBS is used for refractory OCD, both pharmacological and psychological treatments tend to be maintained and doses assessed according to patient response.
Pharmacological treatmentPharmacological treatment is usually gradually reduced until totally discontinued around 2 weeks before the surgical intervention.31 Thus reducing the possible risk of bleeding that has been associated with the peri-operative use of serotonergic antidepressants.61–63 Treatment can be restarted after the intervention up to its usual level. The drug treatment is subsequently adjusted depending on the response to DBS and clinical needs.31,36 In some cases previous treatment can even be completely discontinued.
Psychotherapeutic treatmentSome authors have described in their series the maintenance of previous psychotherapeutic interventions, essentially CBT.36 Furthermore, some studies have shown that the combined use of DBS with CBT is associated with a significant increase in therapeutic response.31,41 Given that the criterion of “refractory OCD” also implies that CBT has lacked effectiveness, CBT being effective after DBS seems to suggest that this stimulation will sensitise the neuronal system, promoting the effectiveness of the psychological intervention in modifying obsessive behaviour.20
Other recent studies have described that the combined administration of CBT and DBS of the nucleus accumbens is associated with an improvement at 3 years, expressed essentially in the physical and psychological domains, and seems to relate more to reduced symptoms of anxiety and depression than the obsessive symptoms themselves.31 Similarly, the combination of DBS and CBT was demonstrated as the ideal strategy to treat these patients, and that DBS also acts as a mechanism to promote the effectiveness of CBT.64 Based on these results the authors conclude that both therapeutic strategies complement each other, 64 and therefore their combined use is recommended in the treatment of these patients.
Side effects of deep brain stimulation on severe refractory obsessive-compulsive disorderSide effects are frequently described with the use of DBS for psychiatric disorders, but are usually reversible. They can be classified under the following sections: (i) complications of the surgical intervention; (ii) complications associated with the instruments; (iii) complications deriving from the stimulation itself.
Complications of the surgical interventionNowadays secondary complications of the surgical intervention are usually rare. Even so, they can present as a consequence of the insertion of the electrode and cause, in the worst of cases, bleeding that causes focal neurological symptoms; the various studies describe the incidence of these side effects at between .2% and 5% of the surgical interventions.2,20,49,65 In this regard, a greater risk of bleeding associated with serotonergic antidepressants, due to a possible anti-aggregant effect,61–63 has been described based on observational cohort and case-control studies, therefore it is recommended that these drugs should be discontinued prior to the surgical procedure. Postoperative infections associated with the implanted electrode, immunological reactions to the electrodes or errors in implanting the electrodes are also rare.2,20,49
Complications associated with the instrumentsThis refers to the complications relating to technical faults of the material itself, such as breakage of parts of the DBS apparatus or faults in the neurostimulator. Although these complications used to appear in up to 8% of cases, thanks to better techniques they are now very rare.2,20,49 Vora et al. reviewed the consequences of neurostimulator battery failure, describing symptoms such as anxiety, fatigue, mood disturbances and panic attacks.66
Complications deriving from the stimulation itselfThese types of complications are common, although they vary and depend on the structure selected for stimulation. They are generally reversible by adjusting the stimulation parameters. They can be divided into (a) complications of acute or immediate stimulation, and (b) chronic stimulation complications.
- a)
Complications of acute or immediate stimulation. Occasionally mild neurological symptoms such as dyskinesia, dysarthria etc., appear immediately after the stimulation, which remit after adjusting the stimulation pattern. Similarly, taste, smell and motor responses have been described, as well as physiological responses significantly associated with the ventral position of the electrode.67Acute affective changes have been reported during the first days after the electrical stimulation, especially in the nucleus accumbens, the ALIC and the subthalamic nucleus, which are generally transient and reversible. This is also the case for the onset of behavioural alterations, emotional lability, and symptoms of depression or anxiety.2,20,37,49Transient episodes of hypomania are the most common side effect after the electrical stimulation, with a frequency of between 50% and 67% of cases of DBS of the ALIC and nucleus accumbens, lowering to 4% and 8% of cases of DBS of the subthalamic nucleus.2,20,37,49 One case has also been described of progression to mania after stimulation of the BNST.68
- b)
Long term complications after the stimulation. Complications secondary to the stimulation can be included in 2 categories of affective and cognitive disorders.Affective disorders. Chronic DBS for refractory OCD can cause mood changes that, although not directly sought, can benefit the patient's mental state. This occurs with mood improvement, which is particularly beneficial because a great many patients with treatment-refractory OCD have comorbid clinical depression. Therefore, for example, various authors have obtained an improvement in depression after stimulation of the ALIC, of the ventral striatum/ventral capsule and of the nucleus accumbens.30,36,39–42,49 This antidepressant effect appears to be related with stimulation of the ventral striatum, and does not occur with stimulation of the subthalamic nucleus.37,41,49 As occurs with the side effects of acute stimulation, a common side effect of chronic stimulation is hypomania.20 The onset, or increase, of symptoms of depression and suicidal thoughts has been observed in some studies.20It has also been observed in some patients that stopping the stimulation can cause a worsening or reappearance of obsessive symptoms or of the affective state which recover when the DBS is restarted.35,36,40,48,69,70 Acute increases in symptoms of anxiety and irritability have also been described secondary to changes in the stimulation parameters, or when the battery runs down, which also resolve after readjusting the parameters or after changing the battery. It is not clear, as indicated by Ooms et al. (2014),31 whether this worsening of symptoms would also occur if the stimulation were to be interrupted gradually rather than suddenly.Cognitive effects. The possible presence of cognitive disorders secondary to the use of DBS for psychiatric disorders has been widely researched and no significant or permanent effects have been found.2,20,24,36,49 Some studies have described mild, transient and relatively non-specific cognitive symptoms.26,41,49
Selection of patients with severe refractory obsessive-compulsive disorder who are candidates for deep brain stimulation
A central element in the treatment of refractory OCD with DBS is the selection of candidate patients. The paper by Garnaat et al. analyses this issue very specifically, laying the foundations for the selection of candidates for DBS.71 The various clinical trials to date have used the following inclusion/exclusion criteria:
Inclusion criteriaThere are no reliable and universally accepted criteria on which to base the selection of patients with “treatment-refractory OCD” as candidates for DBS. However the studies we reviewed tended to use the following criteria for the selection of candidates:
- i)
Age: over 18.
- ii)
Severity of symptoms:
- a.
Severity of psychopathology: the minimum level of severity of psychopathology usually required is a score higher than 28 on the Y-BOCS.36,53 However, in most of the studies the mean Y-BOCS score is higher than 30 points.53
- b.
Duration of the condition: it is usually required for the refractory OCD to have lasted for more than 5 years.31,36,53 However the mean duration for the patients included in the studies tends to be more than 20 years.36,53
- a.
- iii)
Functional lesioning: some studies include the dimension “functional lesioning”, characterised by a score of less than 40 on the GAF scale, and a score higher than 4 on the CGI scale.37
- iv)
Treatment resistance (refractory OCD): even though, as we have seen earlier, no precise and universally accepted criteria have been established to define the concept of “refractory OCD”, there is some uniformity of criteria in the literature that have been used to define this nosological category and to select patients who are candidates for DBS.31,36 These criteria are:
- a)
A significant absence of clinical response, at least 3 attempts at pharmacological treatment at maximum doses, and for an appropriate period of time (more than 3 months). At least one of these therapeutic interventions must have included a selective serotonin reuptake inhibitor, and the other a therapeutic trial with clomipramine.
- b)
Failure of a drug potentiation test, for example with an atypical antipsychotic, lithium, buspirone, etc.
- c)
Absence of clinically significant response to a psychological treatment that included at least 20 sessions of CBT performed by an appropriately trained therapist.
The following clinical features, among others, are used as exclusion criteria: (i) a previous history of psychosis or mania, or at least in the past 3 years; (ii) a clear risk of suicide; (iii) substance dependence; (iv) severe personality disorder; (v) dysmorphophobic disorder; (vi) significant clinical abnormality or significant neurological disorder, and (vii) inability to establish appropriate therapeutic adherence or to follow the demands of treatment with DBS.36 It is appropriate to highlight here that the paper by Gabriëls et al. (2003)33 concludes that the presence of a somatomorphic disorder could be an additional exclusion criterion, which was not confirmed in other studies.
Procedure for applying deep brain stimulation for severe refractory obsessive-compulsory disorderThe procedure for applying DBS for patients with a psychiatric disorder is, in general, the same as that used for movement disorders. The procedure starts with a planning MRI, followed by the implantation of the stereotaxic guide using sedation and local anaesthesia accompanied by intraoperative axial computerised tomography. From the data obtained, and taking the stereotaxic atlas into account, the target neuronal structures are selected in which the electrode will be implanted. The initial setting of the stimulation parameters is decided empirically, and then subsequently adjusted depending on the clinical response. The initial parameters vary between a current amplitude from 1 to 6V; pulse duration of 60–200μs, and frequency of stimulation from 120 to 180Hz. Then, in the same surgical act, the impulse generator is implanted in the thoracic or abdominal region and connected to the brain electrodes.
Models of probe and implications for therapeutic responseMost studies describe the use of Medtronic electrode models 3389 and 3387.49 These electrodes, depending on the model, have a diameter of 1.27mm and 4 contacts 1.5 or 3mm long separated by .5mm, 1.5mm or 4mm.53 According to De Koning, model 3887 has larger contacts that make it more difficult to know the exact structure that is being stimulated.49
Configuration of electrodes and stimulation patternsAs Morishita et al. indicate, the first step in programming a therapeutic intervention with DBS involves precisely establishing the location of the electrodes and defining the configuration of the electrodes that must be applied, and includes selection of the size of the active contacts and the distance between them.55 This configuration will vary according to the neuronal structure selected for DBS and the model of electrode to be used. The next step in programming is to establish the intensity, frequency and amplitude of the electrical pulse to be applied in each “active contact” point, in the acute stimulation phase. These variables will depend on whether a positive clinical effect is achieved with the stimulation and on the side effects that present.
An intraoperative stimulation to check the correct implantation of the electrode has occasionally been reported. For example, the paper by Greenberg et al. describes an intraoperative stimulation of 130Hz, 210μs and 2–6V.36 The effects described for this stimulation were: improved mood and anxiety symptoms, increased verbal fluency, increase facial expressiveness with enhanced state of alertness, and increased heart rate.36 However, it is not always possible to detect these signs despite correct implantation, the clinical effects are usually seen later, therefore this intraoperative check is not usually undertaken in clinical practice.
We should bear in mind, as we have indicated, that there is not always an immediate favourable and permanent response in the acute stimulation phase. Some patients may even show transient psychiatric symptoms that are usually affective in type. Therefore, to establish an appropriate chronic stimulation pattern, we must frequently review the patient's clinical response, usually monthly, to readjust the electrical stimulation pattern according to the clinical response.55
In the critical review performed by Hamani et al. the electrical stimulation parameters used in the 2 structures showing the greatest levels of scientific evidence were: (i) for the subthalamic nucleus, up to 4V, 130Hz, and 60μs; (ii) for the nucleus accumbens, 5V, 130Hz, and 90μs.15
ConclusionsThis review confirms the use of DBS in the treatment of severe and refractory cases of OCD. Although there are no clear predictive factors of efficacy, and the selection of one brain target over another does not seem to provided significant differences in effectiveness, it seems clear that this type of intervention is beneficial for a relevant, although limited, number of patients. In most of the studies reviewed, its use is associated with a significant reduction in clinical symptoms and with the onset of side effects, which in any case are generally considered reversible. Some of the studies reviewed showed that its application is also associated with a clear improvement in functionality and quality of life. The decision to maintain concomitant pharmacological/psychotherapeutic treatment for these patients is not clearly established in the studies we reviewed; there is no study that shows whether it is more effective to continue or discontinue drug treatment and, as we have seen, only one study describes the usefulness of CBT after the application of DBS. We must highlight however, as a final conclusion, that further controlled and randomised studies to provide better scientific evidence of the long-term effectiveness of DBS and the risk-benefit relationship and costs of its application for these patients are required before its real efficacy can be definitively established.
In this context, the correct selection of candidate patients is still crucial to ensure a better prognosis. For all of the above reasons, as Morishita et al.55 recommends, the decision to apply DBS for a specific patient must be made after a thorough analysis of the risk-benefit relationship, and the final decision on candidates should be agreed by an institutional committee made up of specialists from the areas involved which also includes a psychiatrist who is not involved in the programme. Furthermore, the treatment must be monitored by a specialist, multidisciplinary mental health team who then take charge of the permanent and long-term follow-up of these patients.
Finally, the correct assessment of these patients, in the selection process and throughout follow-up should cover not only their symptoms (whether by psychopathological examination or using scales), but also their functionality, disability and quality of life. In this context we recommend that all the information relative to the patients included in this intervention programme should be entered in a specific and permanent “case” registry.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Vázquez-Bourgon J, Martino J, Sierra Peña M, Infante Ceberio J, Martínez Martínez MÁ, Ocón R, et al. La estimulación cerebral profunda en el trastorno obsesivo-compulsivo refractario al tratamiento: una revisión sistemática. Rev Psiquiatr Salud Ment (Barc). 2019;12:37–51.