We examined whether timing of known risk factors for schizophrenia may influence the development of schizophrenia with primary negative symptoms.
MethodThis cross-sectional single-centre study in England used a clinical cohort of 167 clozapine-treated schizophrenia patients. Deficit and nondeficit schizophrenia models were used as clinical proxies of patients with and without primary negative symptoms respectively. Patients were assessed using the Schedule for the Deficit Syndrome. We examined previously replicated risk factors (family history of psychosis, advanced paternal age, male gender, birth weight <3000g, summer birth, cannabis use, exposure to physical or sexual abuse and/or bullying) as well as other traumatic events for deficit and nondeficit schizophrenia.
ResultsWe found a distinct risk factor pattern for the two groups. Compared to the nondeficit group, patients with deficit schizophrenia reported a significantly lower prevalence of cannabis use (p=0.005) at the time of first-episode psychosis (FEP), physical or sexual abuse (p=0.033) prior to FEP, less exposure to crime-related traumatic events (p=0.012) and significantly associated with summer birth (p=0.017). The groups did not differ in terms of family history of psychosis, advanced paternal age, male gender, or low birth weight. To account for multiple comparisons, a confirmatory analysis was performed using logistic regression which yielded similar results except that summer birth no longer reached statistical significance.
ConclusionOur results suggest the timing of the insult may influence the symptom presentation, with insults later in life (cannabis or traumatic events) being associated with psychotic presentation and less with primary negative symptoms.
Examinamos si la cadencia en la aparición de los factores de riesgo conocidos para la esquizofrenia podría influir en el desarrollo de la esquizofrenia con síntomas negativos primarios.
MétodoEste estudio transversal en un centro en Inglaterra utilizó una cohorte clínica de 167 pacientes con esquizofrenia tratados con clozapina. Los criterios de esquizofrenia deficitaria y no deficitaria se utilizaron como aproximación clínica para categorizar pacientes con y sin síntomas negativos primarios, respectivamente. Los pacientes fueron evaluados utilizando el Inventario para la Esquizofrenia Deficitaria. Examinamos únicamente factores de riesgo previamente replicados (antecedentes familiares de psicosis, edad paterna avanzada, sexo masculino, peso al nacer <3.000g, nacimiento en verano, consumo de cannabis, exposición a abuso físico o sexual y/o acoso escolar), así como otros eventos traumáticos para la esquizofrenia deficitaria y la no deficitaria.
ResultadosEncontramos un patrón de factores de riesgo distinto para los dos grupos. En comparación con el grupo sin déficit, los pacientes con esquizofrenia deficitaria presentaron una prevalencia significativamente menor de consumo de cannabis (p=0,005) en el momento del primer episodio de psicosis (PEP), mayor frecuencia de abuso físico o sexual (p=0,033) antes del PEP, menor exposición a eventos traumáticos relacionados con el crimen (p=0,012) y significativamente asociados con el nacimiento en verano (p=0,017). Los grupos no difirieron en términos de antecedentes familiares de psicosis, edad paterna avanzada, sexo masculino o bajo peso al nacer. Para tener en cuenta las comparaciones múltiples se realizó un análisis confirmatorio mediante regresión logística que arrojó resultados similares, excepto que el parto en verano ya no alcanzó significación estadística.
ConclusiónNuestros resultados sugieren que el momento del ataque (factor de riesgo) puede influir en la presentación de los síntomas, ya que los ataques más tarde en la vida (cannabis o eventos traumáticos) se asocian con la presentación psicótica y menos con los síntomas negativos primarios.
Schizophrenia is characterized by heterogeneity in clinical presentation, course of illness and response to treatment.1,2 Since Bleuler,3 this variation has raised the question of whether schizophrenia is a single disease or a syndrome, in which different etiological factors lead to distinct illness trajectories with some commonalities.4 The implications of successful ‘fractionation’ of schizophrenia would be major, both for the design of research studies and the development of successful and novel interventions.
Deficit and nondeficit schizophreniaStudying people with primary, enduring negative symptoms is one approach to reduce the heterogeneity in research and clinical samples.5 In 1988, Carpenter et al. introduced the concept of deficit schizophrenia to identify a more homogenous subgroup of patients with enduring negative symptoms.2 The term ‘negative symptoms of schizophrenia’ refers to normal behaviour that is absent – such as flat affect, poverty of speech, avolition and apparent lack of desire for the company of others (as distinct from ‘positive’, psychotic symptoms such as delusions and hallucinations). In this context, ‘primary’ refers to negative symptoms that are caused by the schizophrenia disease process itself and not by other factors (such as medication side effects, overwhelming psychotic symptoms, anxiety or depression) that cause ‘secondary’ negative symptoms. Patients with schizophrenia who have these enduring (lasting >1 year) and primary negative features are said to have the deficit syndrome of schizophrenia, or deficit schizophrenia (DS), while those without these features have nondeficit schizophrenia (NDS).6
Deficit and nondeficit groups differ relative to the dimensions used to distinguish diseases – signs and symptoms, risk factors, course of illness, risk factors, and treatment response, suggesting that deficit schizophrenia is a separate disease within the syndrome of schizophrenia.7 Subsequent research has supported this concept, including some double dissociations.5,8
Risk factors for deficit schizophreniaSeveral studies have examined potential risk or protective factors for DS. Male gender and family history of schizophrenia are replicated risk factors for DS, compared to NDS.8 Winter birth has been associated with an excess of schizophrenia since 1929 and replicated many times,9 but when distinguishing between deficit and nondeficit types, both a review from 20017 and a pooled analysis from 6 countries in 200410 indicate that summer birth is associated with the deficit type of schizophrenia. Interestingly, a study by Kirkpatrick et al. suggested that summer birth is not only associated with the deficit subtype of schizophrenia but also with social anhedonia and low depression score in a nonpatient group.11 Recently, it has been shown that post-traumatic stress disorder (PTSD) is associated with secondary negative symptoms in schizophrenia whereas no such association was found among patients with primary negative symptoms (DS).12 Such differences suggest that earlier insults in neurodevelopment may be linked to the deficit syndrome whereas schizophrenia patients with triggers later in life (e.g. sexual or physical abuse or substance misuse) are less prone to developing primary negative symptoms. The relevance of substance misuse and secondary negative symptoms has been considered previously with detection of symptoms such as amotivation and anhedonia among chronic cannabis users13,14 but the need remains to clarify if this is a risk factor for NDS. Similarly, although population-based studies of schizophrenia have suggested associations with preterm birth and low birth weight15–18 it is unclear if this would be applicable to DS. Specific risk factors have been associated with clinical features in both the deficit/nondeficit syndrome7 and the general schizophrenia literature.8,9 For instance, a latent class analysis of first episode psychosis suggested the existence of a ‘neurodevelopmental group’ of predominantly male patients with a higher prevalence of negative symptoms, family history of psychosis and earlier onset compared to a ‘paranoid group’ with a higher proportion of women, lower family risk and predominantly positive symptoms.19 More recently, we have found an anatomical difference between DS and NDS patients that suggested their development begins to diverge by the early second trimester.20 This evidence and the difference in season of birth suggested the hypothesis that these two groups of patients may differ with regards to the timing of risk factors. However, to date no study has measured associations between a comprehensive range of risk factors and the presence/absence of the deficit syndrome.
In this study, we used a cohort of clozapine-treated schizophrenia patients to examine multiple potential risk factors for schizophrenia with primary negative symptoms. By selecting a clozapine-treated group, we aimed to reduce further the sample heterogeneity due to illness course and treatment response. Given the extensive evidence on prenatal and childhood/adolescent risk factors for schizophrenia, developmental differences between DS and NDS were predicted. We hypothesized that there would be a distinct pattern of risk factors, with those influencing early development such as low birth weight and summer birth being risk factors for DS more than NDS, and those influencing later development such traumatic life experience and cannabis use being greater risk factors for NDS. Risk factors in the study were chosen based on the DS literature7,8 and the schizophrenia literature,9 although these were limited to those available in the study dataset.
Material and methodsStudy designCross-sectional, single-centre study of a cohort of clozapine-treated schizophrenia patients at a community mental health trust in the UK. The study included electronic clinical records of more than 200 cases and five years of follow-up. The dataset for this project was accessed on 20th May 2018. The anonymized clinical records were embedded in a database approved for research and clinical purposes (NHS Research Ethics 13/EE/0121). All cases were assessed and under the care of the same consultant psychiatrist (EF-E).
ParticipantsParticipants were included from records of patients with schizophrenia. Inclusion criteria were intentionally broad, including all clozapine-treated patients for whom the ‘deficit’ versus ‘nondeficit’ categorization was available (see below). Exclusion criteria were off-label use of clozapine (no schizophrenia), lack of consent or absence of deficit versus nondeficit categorization. The study was carried out in accordance with the latest version of the Declaration of Helsinki. Informed consent of the participants was obtained.
Clinical assessmentsRoutinely, all clinical assessments included a full psychiatric history, mental state examination, current medication list, smoking habit and use of other recreational drugs, comprehensive clozapine side effects assessment and psychopathological scales (see below). Patients have their weight measured every six months and height checked once. Once/year a physical health assessment is done by the general practitioner (GP) who also documents diagnoses such as diabetes in the GP records.
Family history, pregnancy history and first presentationThe data included details of the initial contact with this clinical service, including age (as a proxy for FEP), clinical presentation (delusions, abnormal perceptions, catatonia, depression, mania or others) as well as concomitant use of cannabis (yes/no). Birth weight (in grams) was collected as part of the developmental history. Birth weight was self-reported and only included if verified by a parent, or if the patient reported the same birth weight at a minimum of two separate consultations (Ziauddeen et al., 2016). Parents’ year of birth were recorded and used to calculate paternal and maternal age at the time of patient's birth. Any family history of mental illness and diabetes was also recorded. In the present study, we recorded a positive family history of psychosis if a first-degree relative had any history of a psychotic illness (affective or non-affective). Family history of diabetes was assessed for first-degree relatives only.
Summer birthWe chose a wider definition of summer birth (May–August)21 as compared to (June–July)10,22 as we anticipated a lower statistical power given our small study population.
Psychopathological scalesAll face-to-face assessments included Global Assessment of Functioning (GAF) and the Clinical Global Impression for Schizophrenia (CGI-SCH),23 which measures severity of positive, negative, depressive, cognitive symptoms as well as an overall severity score, all ranging from 1 (no symptoms) to 7 (extremely severe). The short version of the Warwick-Edinburg Wellbeing scale was performed annually,24 as well as the Obsessive Compulsive Inventory-Revised (OCI-R).25
As part of routine clinical practice, two new scales and instruments were included from 2016: The Schedule for the Deficit Syndrome (SDS) and the Traumatic History Questionnaire (THQ). The SDS is the gold standard and validated semi-structured clinical interview tool for ascertaining the presence of the DS in schizophrenia.6 This interview is specifically designed to assess whether negative symptoms are primary and enduring features rather than secondary or transient. The six negative symptoms listed for diagnosis of DS are assessed with each given a score between 0 (normal) to 4 (severely impaired). The categorization of DS requires a score of 2 on at least two of six negative symptoms. In addition, the patient is given a global severity score and a global deficit/nondeficit categorization.6 The rating psychiatrist (EF-E) was trained by a senior author of the SDS (BK) and had extensive experience in the use of the instrument. The THQ is a screening tool for PTSD26 designed for clinical practice. It asks about 24 potentially traumatic experiences, divided in three groups: general trauma, crime-related, and physical and sexual abuse. It does not rate PTSD symptoms, but whether the subject has experienced the traumatic event, its frequency, and the age at which the patient had the trauma. A modified version was used, adding a 25th question related to perceived bullying. This last question reads: ‘have you ever experienced physical or emotional bullying, either at school or somewhere else?’. For this study, we calculated whether the traumatic experience was suffered before the onset of the FEP.
AnalysesAnalyses were divided in two parts. First, we compared the two groups (DS and NDS) regarding symptom/outcome and sociodemographic measures known from other studies to differ between deficit/nondeficit schizophrenia: severity of negative and positive symptoms, length of illness, and general functioning. Second, we compared the two patient groups on known risk factors for DS (gender, family history of schizophrenia, summer birth). We also compared known risk factors for schizophrenia itself (compared to the general population): advanced paternal age, low birth weight, history of physical and sexual abuse, and cannabis use.27–30
Statistical analysisAll statistical analyses were conducted using SPSS v25.0 for Mac, with a two-tailed 0.05 significance level, unless otherwise specified. Data from DS and NDS groups were compared with the chi-square test, Fisher's exact test, one sample t-test, and bivariate correlation using the Pearson r coefficient, as appropriate. Given our small sample size, we calculated the power of each test to control for type II errors using univariate analysis in SPSS; results are presented as observed power. An observed power greater than 80% (0.80) was considered satisfactory. Logistic regression was used as a confirmatory analysis to test the association between different risk factors and the deficit syndrome, controlling for multiple comparisons.
ResultsElectronic records included 239 patients taking clozapine and with a primary diagnosis of schizophrenia spectrum disorder. Of these, 167 (70%) had been categorized as having DS (n=59) and NDS (n=108) and were included in the study. Of these 167 patients, 133 completed questionnaires about traumatic events; information on birth weight and family history of psychosis was available for 107 and 154 patients respectively. Table 1 shows the distribution of the study groups.
Description of the sample size and gender and deficit categorization for the main study analyses.
| Total study population(N=167) | Trauma questionnaire completed(N=133) | |
|---|---|---|
| Deficit schizophrenia, N (%) | 59 (35%)Male 49, Female 10 | 46 (78%)Male 41, Female 5 |
| Nondeficit schizophrenia, N (%) | 108 (65%)Male 83, Female 25 | 87 (81%)Male 67, Female 20 |
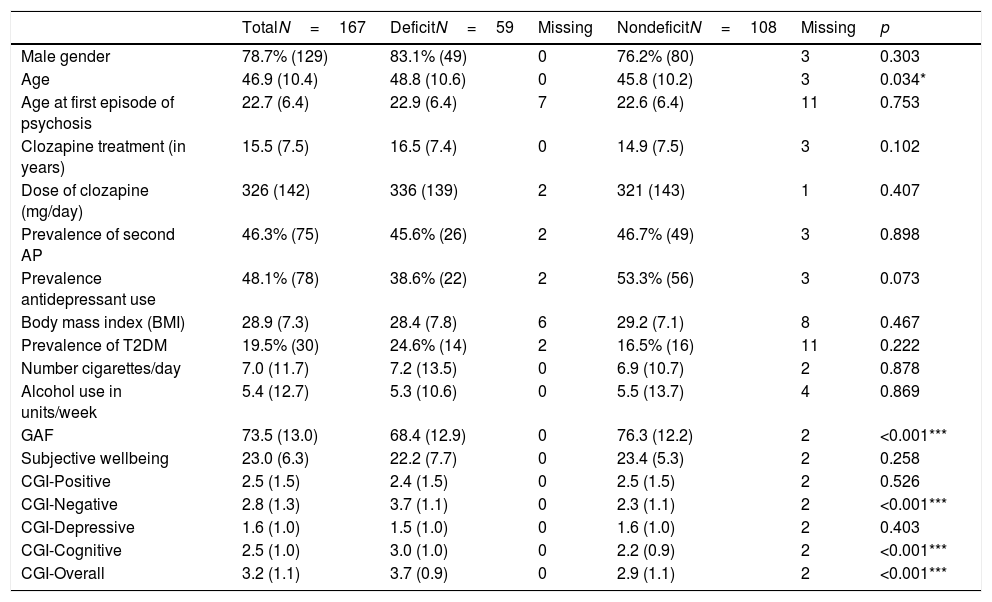
Table 2 gives the demographic and clinical characteristics of the DS and NDS groups. The deficit group had significantly lower GAF scores (p<0.01), indicating poorer overall function. As expected, the deficit group scored significantly higher on the CGI-SCH for negative symptoms, cognitive symptoms,31 and overall severity (p<0.001), but did not differ for positive or depressive symptoms (p>0.1).32 The remaining sociodemographic and clinical details did not differ significantly between the two groups (though there were non-significant trends towards those with DS being more commonly male, using less antidepressant medication, and being more likely to have type 2 diabetes mellitus [T2DM]).
Demographic and clinical characteristics of deficit and nondeficit schizophrenia, expressed as mean (standard deviation) or as percentage (number) unless otherwise specified.
| TotalN=167 | DeficitN=59 | Missing | NondeficitN=108 | Missing | p | |
|---|---|---|---|---|---|---|
| Male gender | 78.7% (129) | 83.1% (49) | 0 | 76.2% (80) | 3 | 0.303 |
| Age | 46.9 (10.4) | 48.8 (10.6) | 0 | 45.8 (10.2) | 3 | 0.034* |
| Age at first episode of psychosis | 22.7 (6.4) | 22.9 (6.4) | 7 | 22.6 (6.4) | 11 | 0.753 |
| Clozapine treatment (in years) | 15.5 (7.5) | 16.5 (7.4) | 0 | 14.9 (7.5) | 3 | 0.102 |
| Dose of clozapine (mg/day) | 326 (142) | 336 (139) | 2 | 321 (143) | 1 | 0.407 |
| Prevalence of second AP | 46.3% (75) | 45.6% (26) | 2 | 46.7% (49) | 3 | 0.898 |
| Prevalence antidepressant use | 48.1% (78) | 38.6% (22) | 2 | 53.3% (56) | 3 | 0.073 |
| Body mass index (BMI) | 28.9 (7.3) | 28.4 (7.8) | 6 | 29.2 (7.1) | 8 | 0.467 |
| Prevalence of T2DM | 19.5% (30) | 24.6% (14) | 2 | 16.5% (16) | 11 | 0.222 |
| Number cigarettes/day | 7.0 (11.7) | 7.2 (13.5) | 0 | 6.9 (10.7) | 2 | 0.878 |
| Alcohol use in units/week | 5.4 (12.7) | 5.3 (10.6) | 0 | 5.5 (13.7) | 4 | 0.869 |
| GAF | 73.5 (13.0) | 68.4 (12.9) | 0 | 76.3 (12.2) | 2 | <0.001*** |
| Subjective wellbeing | 23.0 (6.3) | 22.2 (7.7) | 0 | 23.4 (5.3) | 2 | 0.258 |
| CGI-Positive | 2.5 (1.5) | 2.4 (1.5) | 0 | 2.5 (1.5) | 2 | 0.526 |
| CGI-Negative | 2.8 (1.3) | 3.7 (1.1) | 0 | 2.3 (1.1) | 2 | <0.001*** |
| CGI-Depressive | 1.6 (1.0) | 1.5 (1.0) | 0 | 1.6 (1.0) | 2 | 0.403 |
| CGI-Cognitive | 2.5 (1.0) | 3.0 (1.0) | 0 | 2.2 (0.9) | 2 | <0.001*** |
| CGI-Overall | 3.2 (1.1) | 3.7 (0.9) | 0 | 2.9 (1.1) | 2 | <0.001*** |
AP: antipsychotic; T2DM: type 2 diabetes mellitus; BMI: body mass index; GAF: Global Assessment of Functioning; CGI: Clinical Global Impression. Associations between statistical significance is marked by stars, where p<0.05 is represented by *, p<0.01 by ** and p<0.001 by ***.
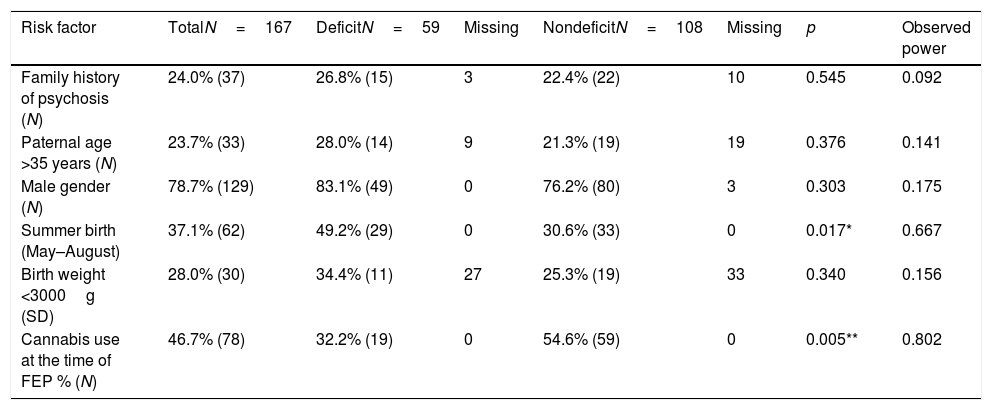
Table 3 shows the prevalence of different risk factors in their approximate chronological order. Compared to DS, NDS patients were significantly more likely to have reported using cannabis at the time of their first episode of psychosis, and similar to previous research,21 we found an association between DS and summer birth (p<0.05). We also assessed the prevalence of summer birth using the narrower definition (June–July). Although the prevalence of birth in June-July remained higher among DS patients than NDS patients (27.1% versus 18.5%), the results were not statistically significant (p=0.20) and the observed power remained low at 25%. The statistical power for risk factors that were not associated with either group, that is family history of psychosis, paternal age >35, male gender and birth weight less than 3000g had power estimates of 9.2%, 14.1%, 17.5% and 15.6% respectively.
Risk factors for deficit- and nondeficit schizophrenia.
| Risk factor | TotalN=167 | DeficitN=59 | Missing | NondeficitN=108 | Missing | p | Observed power |
|---|---|---|---|---|---|---|---|
| Family history of psychosis (N) | 24.0% (37) | 26.8% (15) | 3 | 22.4% (22) | 10 | 0.545 | 0.092 |
| Paternal age >35 years (N) | 23.7% (33) | 28.0% (14) | 9 | 21.3% (19) | 19 | 0.376 | 0.141 |
| Male gender (N) | 78.7% (129) | 83.1% (49) | 0 | 76.2% (80) | 3 | 0.303 | 0.175 |
| Summer birth (May–August) | 37.1% (62) | 49.2% (29) | 0 | 30.6% (33) | 0 | 0.017* | 0.667 |
| Birth weight <3000g (SD) | 28.0% (30) | 34.4% (11) | 27 | 25.3% (19) | 33 | 0.340 | 0.156 |
| Cannabis use at the time of FEP % (N) | 46.7% (78) | 32.2% (19) | 0 | 54.6% (59) | 0 | 0.005** | 0.802 |
FEP: first episode psychosis; N: number; SD: standard deviation.
Statistical significance is marked by stars, where p<0.05 is represented by *, p<0.01 by ** and p<0.001 by ***.
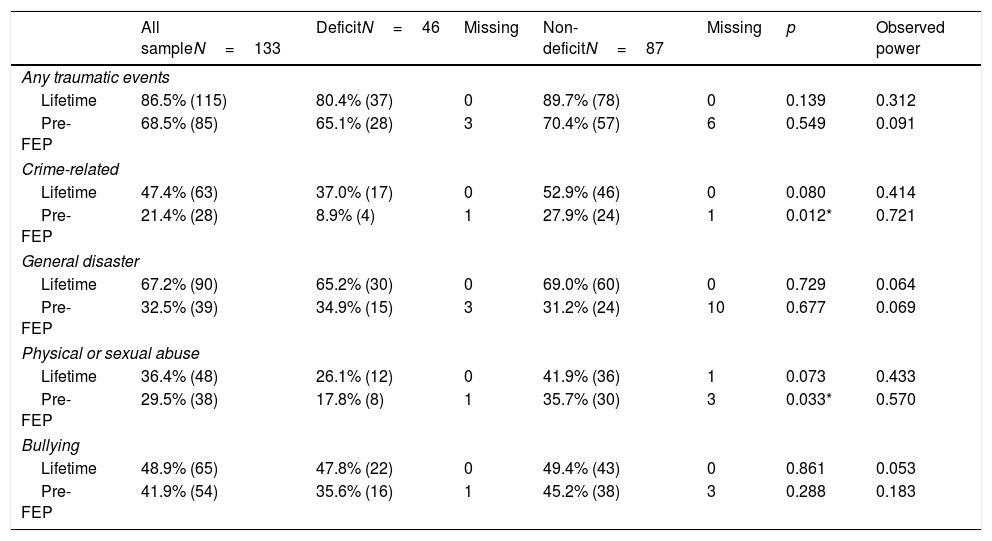
We further explored the role of traumatic events. Table 4 shows the prevalence of reported experience of range of traumatic events across the lifespan and before FEP. Both groups reported a high prevalence of trauma exposure, both lifetime exposure (86.5% in the total study population) and prior to FEP (68.5%). Crime-related events were reported significantly more often by patients with NDS, reaching statistical significance for crime-related events prior to FEP (NDS 27.9% vs. DS 8.9%; p<0.05). Nondeficit patients also had higher exposure to physical or sexual abuse, both during their entire lifetime (41.9% vs. 26.1%; p=0.07) and prior to FEP (35.7% vs. 17.8%; p<0.05).
Traumatic experiences in deficit-schizophrenia vs. nondeficit schizophrenia, before and after first episode psychosis (FEP), expressed in percentage and (total number).
| All sampleN=133 | DeficitN=46 | Missing | Non-deficitN=87 | Missing | p | Observed power | |
|---|---|---|---|---|---|---|---|
| Any traumatic events | |||||||
| Lifetime | 86.5% (115) | 80.4% (37) | 0 | 89.7% (78) | 0 | 0.139 | 0.312 |
| Pre-FEP | 68.5% (85) | 65.1% (28) | 3 | 70.4% (57) | 6 | 0.549 | 0.091 |
| Crime-related | |||||||
| Lifetime | 47.4% (63) | 37.0% (17) | 0 | 52.9% (46) | 0 | 0.080 | 0.414 |
| Pre-FEP | 21.4% (28) | 8.9% (4) | 1 | 27.9% (24) | 1 | 0.012* | 0.721 |
| General disaster | |||||||
| Lifetime | 67.2% (90) | 65.2% (30) | 0 | 69.0% (60) | 0 | 0.729 | 0.064 |
| Pre-FEP | 32.5% (39) | 34.9% (15) | 3 | 31.2% (24) | 10 | 0.677 | 0.069 |
| Physical or sexual abuse | |||||||
| Lifetime | 36.4% (48) | 26.1% (12) | 0 | 41.9% (36) | 1 | 0.073 | 0.433 |
| Pre-FEP | 29.5% (38) | 17.8% (8) | 1 | 35.7% (30) | 3 | 0.033* | 0.570 |
| Bullying | |||||||
| Lifetime | 48.9% (65) | 47.8% (22) | 0 | 49.4% (43) | 0 | 0.861 | 0.053 |
| Pre-FEP | 41.9% (54) | 35.6% (16) | 1 | 45.2% (38) | 3 | 0.288 | 0.183 |
Statistical significance is marked by stars, where p<0.05 is represented by *, p<0.01 by ** and p<0.001 by ***.
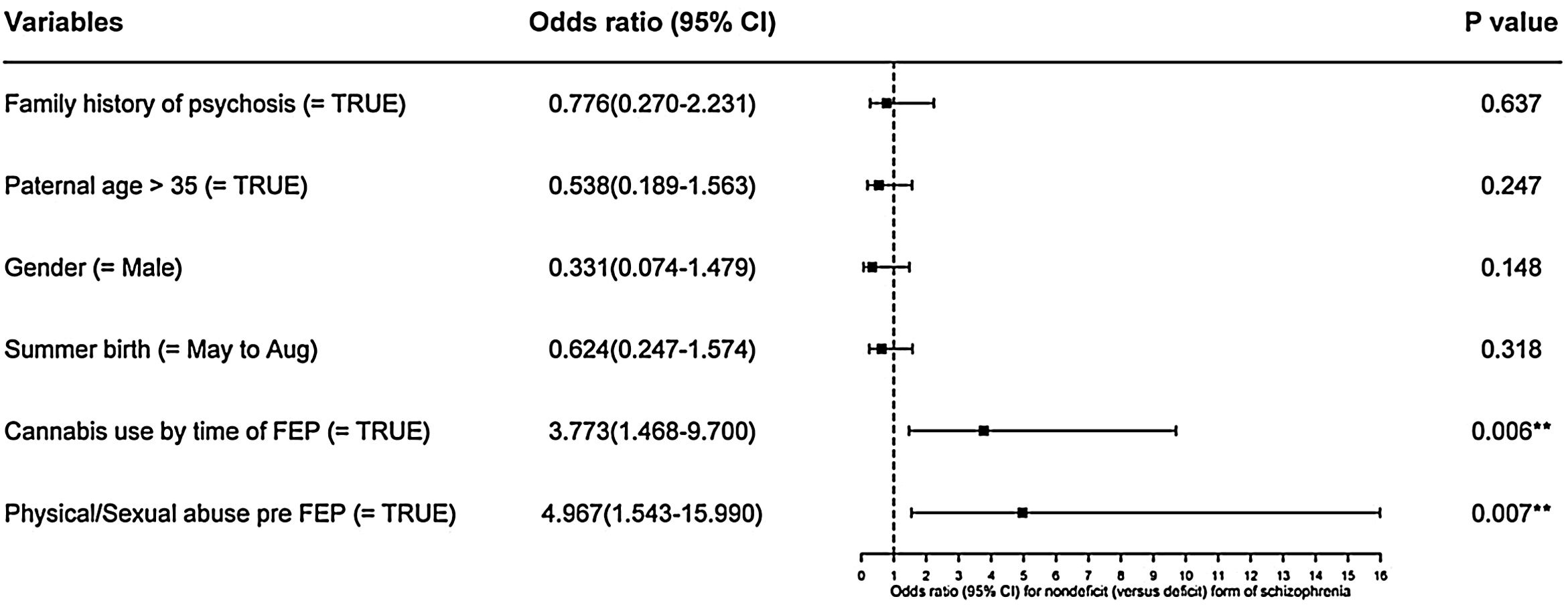
Logistic regression was performed, using deficit/nondeficit categorization as the dependent variable and the list of risk factors in Fig. 1 as independent variables, except low birth weight. We excluded birth weight as only 64% of the total sample had that information. Using this model, with a R2 of 0.25, only cannabis use at the time of FEP (p<0.01) and physical or sexual abuse before FEP (p<0.01) were significant predictors of nondeficit syndrome type.
Confirmatory logistic regression analysis, using schizophrenia categorization and the variables: family history of psychosis, paternal age >35 years old, male gender, summer birth, cannabis association and physical or sexual abuse prior to FEP in a multivariate model. Statistical significance is marked by stars, where p<0.05 is represented by *, p<0.01 by ** and p<0.001 by ***, with odds ratio (OR) and 95% confidence interval (95% CI). Abbreviation: first-episode psychosis (FEP).
In this study we found a distinct pattern of risk factors for deficit versus nondeficit schizophrenia. Compared to the DS group, patients with NDS reported a significantly higher prevalence of cannabis use at their first episode of psychosis, and more crime-related traumatic events and physical and/or sexual abuse prior to the FEP.
In our sample, we found a higher proportion of DS patients (35%) than in previous studies,33 which can be attributed to the fact that all cases came from a chronic and clozapine-treated group of patients. Nevertheless, the DS and NDS groups presented clinical differences that replicate the prior literature.8 Patients with DS had poorer global functioning and worse negative and cognitive symptoms and overall severity of illness but did not differ in terms of positive or depressive symptoms. The two groups had similar exposure to antipsychotics. The NDS group had a slightly but non-significantly (p=0.07) higher prevalence of antidepressant use; this does not necessarily reflect a higher prevalence of depression, as antidepressants are also commonly prescribed for other reasons in this population.34
Physical and sexual abuse have been associated with psychiatric disorders later in life35 and specifically with schizophrenia,30 but our study is the first reporting differential exposure to physical/sexual traumatic events prior to psychosis in DS versus NDS. The overall prevalence of these traumatic events in our sample was 29.5%, similar to epidemiological studies in schizophrenia patients.36 To our knowledge, only one study looked at the association between PTSD and primary negative symptoms of schizophrenia.12 Strauss et al. found that PTSD was associated with increased secondary negative symptoms but not with the DS (whose definition requires primary negative symptoms), despite both groups reporting equal lifetime traumatic experiences. Their results may indicate that reduced emotional life in DS patients may account for the lower risk of developing PTSD. In our study, we measured recalled exposure to trauma rather than PTSD, and examined whether the traumatic events occurred before or after the patients’ FEP. We found a higher prevalence of pre-FEP physical/sexual abuse in NDS. This suggests that the timing of trauma may influence the form of schizophrenia. Although DS patients had worse cognitive symptoms, it is unlikely that this difference in recalled traumatic events was attributable to poor recollection secondary to cognitive impairment, as the reported experience of other traumatic events (such as bullying or general disaster/trauma before psychosis onset, or the experience of physical/sexual abuse after FEP) were very similar in both groups (Table 3). Interestingly, the NDS group recalled a significantly greater exposure to crime-related traumatic events before FEP, which has not been reported before.
We also found that patients with NDS reported a significantly higher rate of cannabis use at the time of FEP. Our results replicate in part previous reports that DS patients have less lifetime use of drugs in general,37 although this original report did not find differences in the use of cannabis in particular between the two groups. In our study we only assessed the use of cannabis at the time of FEP and found a lower prevalence among deficit patients. This is supported by Ruiz-Veguilla et al. who showed that patients with the presence of high neurological ‘soft signs’ also score higher on negative symptoms and are inversely associated with heavy cannabis use prior to FEP.38 The lower prevalence is supported by research39 suggesting that clinical features associated with drug abuse are characteristic of NDS, and that the lack of social interest seen among patients with DS could lead them to fewer opportunities for substance misuse. Similarly, as reported above, our study showed an association between exposure to or involvement in criminal events and NDS prior to FEP which we again believe may be related to reduced social interest and poorer premorbid function among the DS patients.39
Our sample size (n=167) may also have contributed to the lack of replication of some risk factors. Male gender or advanced paternal age did not reach statistical significance (p=0.30 and p=0.38, respectively), although trend directions were in concordance with prior DS literature.7,21,40 Indeed, power calculations suggest a potential type II statistical error. Family history of psychosis was similar between both groups, but the definition of family history in our dataset (any psychotic disorder in first degree relative) was less specific than in prior studies, in which schizophrenia alone was found to have a greater prevalence in the families of DS probands.21 We did not find differences in low birth weight, although this has not been linked to DS previously either.
Summer birth is a replicated risk factor for DS,10 distinct to excess of winter birth associated with schizophrenia.41,42 In our study we used a wider definition of summer birth (May–August) given our limitation of a small sample size. Using the wider definition, we could replicate previous results from studies of similar power to ours, suggesting an association between summer birth and DS (p=0.02).21 Messias et al. were able to overcome sample size limitations by performing a combined analysis of studies from six countries in the northern hemisphere.10 Their study including almost 1600 subjects, showed an increase in DS births in June and July. We believe that the difference is likely secondary to low power in our study (25%) and thereby the difficulty to detect a possible difference.
Taken together, we found that in our sample of clozapine-treated patients with schizophrenia, postnatal risk factors (traumatic experiences or cannabis use) were significantly associated with NDS (versus DS), whereas DS was associated at trend level with some prenatal factors (male gender, summer birth and older father).
Although we examined risk factors in terms of the presence/absence of the deficit syndrome of schizophrenia (DS versus NDS), it may be that some risk factors are specific to particular psychopathological domains. For instance, psychotic symptoms linked to genetic or stress-related factors rather than purely neurodevelopmental insults,43,44 but there are no such studies in relation to negative symptoms.
Our study, and previous work by our team,45 suggest that timing of stressors can lead to differences in symptom presentation. Others have already suggested a more ‘negative’ form of schizophrenia in those with earlier risk factors in development.46 Further studies, perhaps using the research domains of cognition (RDoC)47 instead of a categorical diagnostic approach, might help to narrow down the different risk factors for the individual or domains of symptoms.
Nevertheless, our study should be interpreted in the context of some limitations. The group differences found are based on self-reported history and patient questionnaires, in which there is a risk of underestimation or overestimation of previous trauma or drug abuse. There are also intrinsic limitations to the Schedule for the Deficit Syndrome scale which we tried to minimize by ensuring the categorization was done by the care clinician who has a longitudinal knowledge of the patients. The sample, albeit carefully phenotyped and followed up for 6 years included chronic and clozapine treated patients and was limited in size which was reflected by low power estimates. Our findings would therefore need replication in larger samples. Importantly, these should include also patients in other phases of the disorder, such as early psychosis or before reaching treatment resistance.
ContributorsSA analyzed and interpreted the data and drafted the manuscript. EF-E designed the study, performed the clinical interviews with patients, participated in acquisition of the data and critically revised the manuscript for important intellectual content. BK discussed the results, reviewed the draft and made contributions towards final manuscript. SC contributed statistical advice and helped with design of figures and RNC critically revised the manuscript for important intellectual content. All authors edited and approved the final manuscript.
FundingEF-E was supported by intramural funding from Cambridgeshire and Peterborough NHS Foundation Trust (CPFT). This research was supported in part by the UK National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre; the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. RNC's and SC's research is supported by the UK Medical Research Council (grant MC_PC_17213 to RNC).
Conflict of interestBK receives licensing royalties from ProPhase LLC for use of the Brief Negative Symptom Scale (BNSS) by for-profit groups; these fees are donated to the Brain and Behaviour Research Foundation. He has also received honoraria and travel support from ProPhase LLC for training pharmaceutical company raters on the BNSS, consulting fees and travel support from Genentech/Roche, Minerva Neurosciences, and ProPhase LLC, consulting fees from Goldman Sachs, from anonymized pharmaceutical companies through Decision Resources, Inc., from an anonymized investment capital company through Guideposts, and fees for consulting on a legal issue involving Janssen Pharmaceutica and Wockhardt Bio. He also receives fees from Walsh Medical Media for editorial services.
RNC consults for Campden Instruments Ltd and receives royalties from Cambridge University Press, Cambridge Enterprise, and Routledge.
SA, SC and EF-E report no conflict of interest.
We would like to express our special thanks to our patients who contributed to making this research paper possible.