Negative symptoms are prevalent in schizophrenia and associated with a poorer outcome. Validated newer psychometric instruments could contribute to better assessment and improved treatment of negative symptoms. The Negative Symptom Assessment-16 (NSA-16) has been shown to have strong psychometric properties, but there is a need for validation in non-English languages. This study aimed to examine the psychometric properties of a Spanish version of the NSA-16 (Sp-NSA-16).
Material and methodObservational, cross-sectional validation study in a sample of 123 outpatients with schizophrenia. Assessments: NSA-16, PANSS, HDRS, CGI-SCH and PSP.
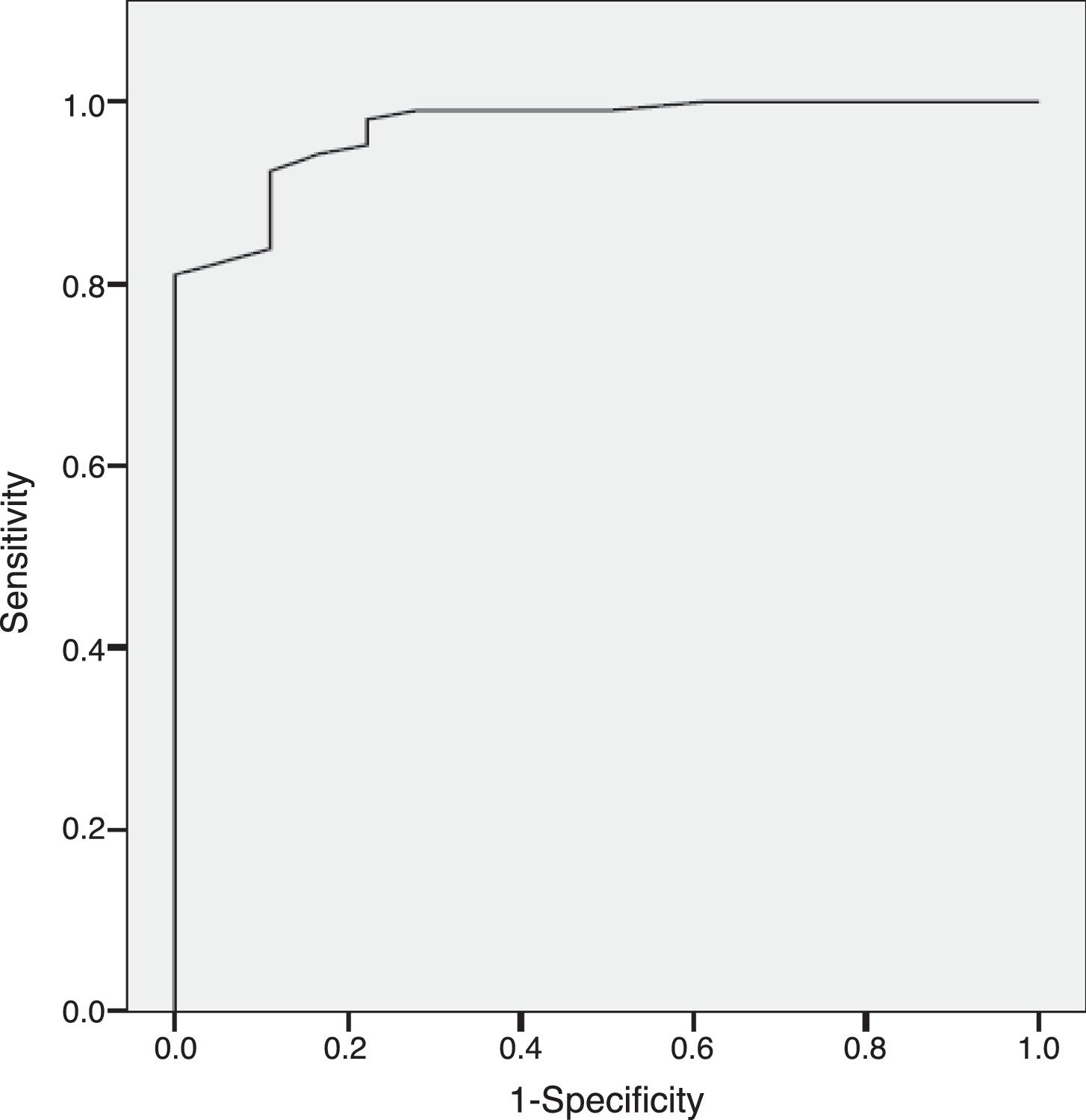
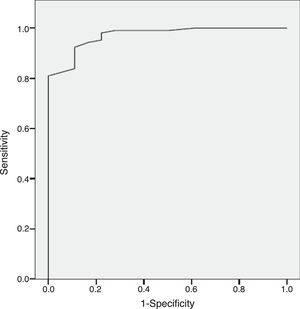
ResultsThe results indicate appropriate psychometric properties, high internal consistency (Cronbach's alpha=0.86), convergent validity (PANSS negative scale, PANSS Marder Negative Factor and CGI-negative symptoms r values between 0.81 and 0.94) and divergent validity (PANSS positive scale and the HDRS r values between 0.10 and 0.34). In addition, the NSA-16 also exhibited discriminant validity (ROC curve=0.97, 95% CI=0.94 to 1.00; 94.3% sensitivity and 83.3% specificity).
ConclusionsThe Sp-NSA-16 is reliable and valid for measuring negative symptoms in patients with schizophrenia. This provides Spanish clinicians with a new tool for clinical practice and research. However, it is necessary to provide further information about its inter-rater reliability.
Los síntomas negativos prevalecen en la esquizofrenia y están asociados con un peor resultado. La validación de nuevos instrumentos psicométricos podría contribuir a una mejor evaluación de los síntomas negativos y a un avance en su tratamiento. La escala de evaluación de los síntomas negativos-16 (Negative Symptom Assessment-16 [NSA-16]) ha demostrado adecuadas propiedades psicométricas, pero existe la necesidad de validarla en otros idiomas diferentes del inglés. Este estudio tiene como objetivo examinar las propiedades psicométricas de la versión española de la NSA-16 (Sp-NSA-16).
Material y métodoEstudio observacional, transversal de validación en una muestra de 123 pacientes con diagnóstico de esquizofrenia en tratamiento ambulatorio. Evaluación: NSA-16, PANSS, HDRS, ICG-ESQ y PSP.
ResultadosLos resultados muestran unas adecuadas propiedades psicométricas, alta consistencia interna (alfa de Cronbach=0,86), validez convergente (escala negativa de la PANSS, factor negativo de Marder de la PANSS y escala de los síntomas negativos de la ICG: valores r entre 0,81 y 0,94) y validez divergente (escala positiva de la PANSS y HDRS: valores r entre 0,10 y 0,34). Además, la NSA-16 también muestra validez discriminante (curva ROC=0,97; IC 95%=0,94 a 1,00; 94,3% de sensibilidad y 83,3% de especificidad).
ConclusionesLa Sp-NSA-16 es fiable y válida para medir los síntomas negativos en pacientes con esquizofrenia. Proveemos a los clínicos españoles con una nueva herramienta para la práctica clínica e investigación. Sin embargo, es necesario proporcionar más información sobre su fiabilidad interevaluadores.
The negative syndrome of schizophrenia, which includes social isolation, anhedonia, avolition, flat affect, and alogia,1,2 affects about 20% of patients with schizophrenia. Together with cognitive symptoms, it has a significant impact on functioning, especially in the social domain.3–6
These symptoms can occur at any stage of the illness. In fact, they can exist in a prodromal stage before the emergence of positive symptoms7,8 or co-exist with them, although they may be less impressive during acute psychosis because the clinical picture is dominated by positive symptoms.8 Furthermore, they can be present at subsequent follow-up points for up to 10 years9 and may become more prevalent over time.10 Negative symptoms are viewed as treatment-resistant, and only modest progress has been made in treating them effectively,11–13 although some evidence suggests that secondary negative symptoms may respond to pharmacologic and psychosocial interventions. Progress in these treatments is currently impeded by the limitations of available assessment instruments. For this reason, the development of instruments able to assess the severity of a patient's negative symptoms is the most important first step towards optimal treatment.1 Moreover, the commonly used negative symptom assessment tools, like the Brief Psychiatric Rating Scale (BPRS),14 the Scale for the Assessment of Negative Symptoms (SANS),15 and the Positive and Negative Syndrome Scale, Negative Subscale (PANSS-NS) 16 have been the subject of criticism.
The Negative Symptom Assessment (NSA) was initially developed by Alphs et al. in 1989.17 However, the most widely used version is the shortened 16-item version of the NSA developed by Axelrod et al. in 1993,18 which has a latent structure similar to the original instrument (five-factor dimensional structure: communication, social involvement, affect/emotion, motivation, and retardation). It was modified from 25 to 16 items to make it an easy-to-use instrument with strong psychometric properties and good clinical utility.17,18 It has demonstrated high interrater and test–retest reliability as well as high concurrent validity with similar instruments when used by English-speaking raters.18–20 At present, there is a need to validate the NSA-16 in different languages. Clinical trials for approval of new drugs involve various regions/countries. Spanish is the primary language of 20 countries, ranking as the second most widely spoken language in terms of native speakers.21 Therefore, this study aims to validate the Spanish version of the NSA-16. We hypothesise that the NSA-16 is a reliable and valid instrument for assessing negative symptoms in Spanish patients with schizophrenia. Furthermore, this new instrument could contribute to better understanding of negative symptoms and improved psychopharmacological research.
Materials and methodsParticipants and study designThe number of participants included was 123 patients with stable schizophrenia. Stability was defined as those patients who were clinically stable and had not required any change in their current pharmacological treatment during the previous month. Patient inclusion criteria were1 age ≥18 years2; ICD-10 diagnosis of schizophrenia3; currently on treatment for his/her illness; and4 written informed consent to participate in the study. Exclusion criteria were designed to be minimal, due to the nature of the study, and only individuals with intellectual developmental disorder or acquired brain injury, or who refused to participate in the study were excluded. Participants were recruited from two outpatient mental health centres in Spain. This was an observational, cross-sectional validation study. It was approved by the Clinical Research Ethics Committee of one of the centres (Hospital Universitario Central de Asturias, Oviedo, Spain) and it was in compliance with the 1975 Declaration of Helsinki, as revised in 1983. Written informed consent was obtained from all subjects before enrolment.
MeasuresDemographic and clinical data were collected and assessments were done for all subjects. Severity of illness was assessed with the Clinical Global Impression-Schizophrenia scale (CGI-SCH).22 In addition to the Spanish validation of the NSA-16,18 psychopathology was evaluated using the Spanish versions of the Positive and Negative Syndrome Scale (PANSS)23 and the Hamilton Depression Rating Scale (HDRS).24–26 Level of functioning was assessed using the Global Assessment of Functioning (GAF)27 and the Spanish version of the Personal and Social Performance scale (PSP).28
The Negative Symptom Assessment – 16 items (NSA-16)18 is a semi-structured interview containing 16 items that comprehensively assess the negative syndrome of schizophrenia, including the following five factors: communication, emotion/affect, social involvement, motivation, and retardation.18 It has been validated in inpatients and outpatients with schizophrenia.18 These five factors were extracted from the larger six-factor NSA-25.18 Each of the sixteen items and the overall global negative symptoms are rated on a 1 to 6-point scale where ‘1’ represents no reduction from normal behaviours associated with the item and ‘6’ represents a severe reduction in or absence of the behaviour, with markedly impaired functionality. The rating scale also includes a “Not Ratable” designation (denoted as “9”). Detailed anchoring criteria for the rating points are provided on the scale, along with a total score, sum of the scores on the 16 items, and scores in each of the five factors. Also, the NSA-16 has an “extra” item which provides a Global Negative Symptom Rating – Severity based on the global clinical impression of the patient's negative symptoms. The main limitation of the NSA is its high reliance on functioning or behaviours even for experiential symptoms such as reduced social drive whose severity is measured by type and frequency of social interactions.
The Spanish version of the NSA-16 (NSA-16-Sp) was developed using the translationback-translation method. The original developer accepted that we carried out the Spanish validation of the scale. The short-form scale, long-form scale, and interview were translated into Spanish by native Spanish-speaking clinicians. The translated version was back-translated into English by an English clinician fluent in Spanish. Finally, the back-translated version was reviewed and approved.
Statistical analysisThe statistical analysis was done using the SPSS 17.0. The two-tailed level of significance used was 0.05. Skewness and kurtosis were calculated to measure the shape of the distributions (skewness and kurtosis values+/−1 were considered good). Ceiling and floor effects were also determined (number of patients with scores greater than 95% and lower than 5%, respectively). The internal consistency of the Sp-NSA-16 and its factors was calculated using the Cronbach's alpha coefficient at the item level. Convergent validity was calculated using the Pearson correlation coefficient between the total Sp-NSA-16 score and total scores on the PANSS negative scale, PANSS Marder Negative Factor,29 and CGI-negative symptoms with the hypothesis that a moderate r coefficient would be found as they are related but different constructs. Pearson correlation coefficients between the total Sp-NSA-16 score and scores on the PANSS positive scale and HDRS were determined to assess divergent validity. In this case, the hypothesis was that a low r coefficient would be found as they are different constructs.
For analysing discriminant validity, patients with schizophrenia were classified into four groups based on their CGI-SCH negative symptom severity scores: doubtfully ill (CGISCH=1–2), mildly ill (CGI-SCH=3), moderately ill (CGI-SCH=4), and severely ill (CGI-SCH=5–7). An ANOVA test (Duncan post hoc) was used to identify statistically significant differences in Sp-NSA-16 scores according to severity groups. The diagnostic performance of the Sp-NSA-16 for discriminating between patients with and without negative symptoms was analysed using the receiver operating characteristic (ROC) curve analysis.
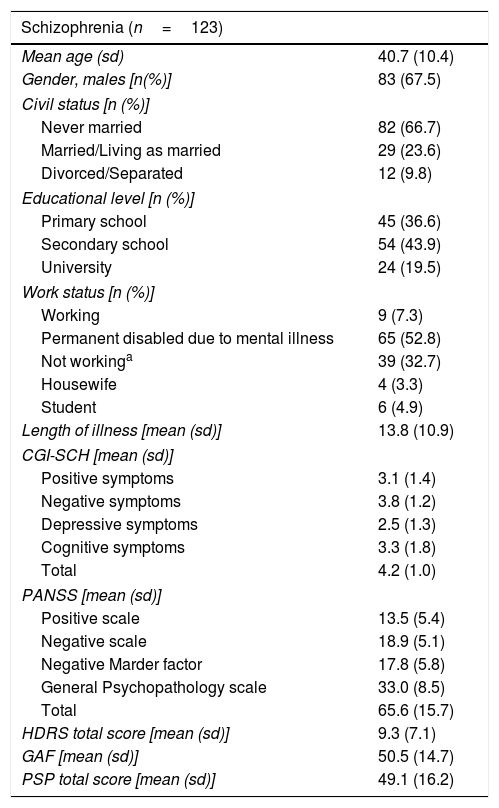
ResultsOne hundred and twenty-three subjects were included with a mean age (standard deviation) of 40.7 (10.4) years and a mean illness duration of 13.8 (10.9) years; 67.5%were male. NSA-16-Sp demographic and clinical characteristics of the full sample of patients are presented in Table 1.
Demographic and clinical characteristics.
| Schizophrenia (n=123) | |
|---|---|
| Mean age (sd) | 40.7 (10.4) |
| Gender, males [n(%)] | 83 (67.5) |
| Civil status [n (%)] | |
| Never married | 82 (66.7) |
| Married/Living as married | 29 (23.6) |
| Divorced/Separated | 12 (9.8) |
| Educational level [n (%)] | |
| Primary school | 45 (36.6) |
| Secondary school | 54 (43.9) |
| University | 24 (19.5) |
| Work status [n (%)] | |
| Working | 9 (7.3) |
| Permanent disabled due to mental illness | 65 (52.8) |
| Not workinga | 39 (32.7) |
| Housewife | 4 (3.3) |
| Student | 6 (4.9) |
| Length of illness [mean (sd)] | 13.8 (10.9) |
| CGI-SCH [mean (sd)] | |
| Positive symptoms | 3.1 (1.4) |
| Negative symptoms | 3.8 (1.2) |
| Depressive symptoms | 2.5 (1.3) |
| Cognitive symptoms | 3.3 (1.8) |
| Total | 4.2 (1.0) |
| PANSS [mean (sd)] | |
| Positive scale | 13.5 (5.4) |
| Negative scale | 18.9 (5.1) |
| Negative Marder factor | 17.8 (5.8) |
| General Psychopathology scale | 33.0 (8.5) |
| Total | 65.6 (15.7) |
| HDRS total score [mean (sd)] | 9.3 (7.1) |
| GAF [mean (sd)] | 50.5 (14.7) |
| PSP total score [mean (sd)] | 49.1 (16.2) |
sd: standard deviation.
Not working included: temporary disabled for other health conditions than mental disorder, temporary disabled for schizophrenia, retired, and unemployed. CGI-SCH: Clinical Global Impression, Schizophrenia. PANSS: Positive and Negative Syndrome Scale. HDRS: Hamilton Depression Rating Scale. GAF: Global Assessment of Functioning. PSP: Personal and Social Performance scale.
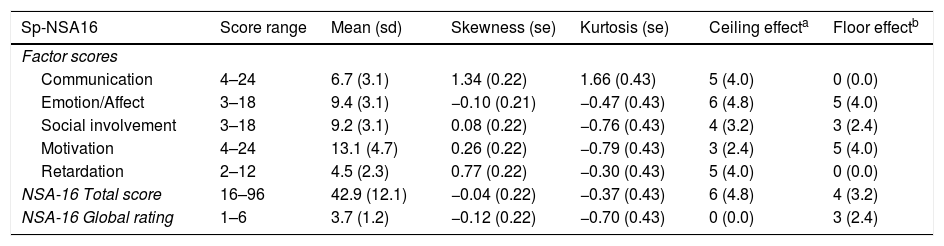
The mean scores and the psychometric characteristics of the total Sp-NSA-16 and its factors are shown in Table 2. For all global measures and factors, the scores show symmetrical and mesokurtic distribution, except the communication factor, which shows slightly right-skewed and leptokurtic distribution. There were no ceiling or floor effects in any of them.
Distribution characteristics of Sp-NSA-16 scores.
| Sp-NSA16 | Score range | Mean (sd) | Skewness (se) | Kurtosis (se) | Ceiling effecta | Floor effectb |
|---|---|---|---|---|---|---|
| Factor scores | ||||||
| Communication | 4–24 | 6.7 (3.1) | 1.34 (0.22) | 1.66 (0.43) | 5 (4.0) | 0 (0.0) |
| Emotion/Affect | 3–18 | 9.4 (3.1) | −0.10 (0.21) | −0.47 (0.43) | 6 (4.8) | 5 (4.0) |
| Social involvement | 3–18 | 9.2 (3.1) | 0.08 (0.22) | −0.76 (0.43) | 4 (3.2) | 3 (2.4) |
| Motivation | 4–24 | 13.1 (4.7) | 0.26 (0.22) | −0.79 (0.43) | 3 (2.4) | 5 (4.0) |
| Retardation | 2–12 | 4.5 (2.3) | 0.77 (0.22) | −0.30 (0.43) | 5 (4.0) | 0 (0.0) |
| NSA-16 Total score | 16–96 | 42.9 (12.1) | −0.04 (0.22) | −0.37 (0.43) | 6 (4.8) | 4 (3.2) |
| NSA-16 Global rating | 1–6 | 3.7 (1.2) | −0.12 (0.22) | −0.70 (0.43) | 0 (0.0) | 3 (2.4) |
sd: standard deviation; se: standard error.
The Sp-NSA-16 scale had good internal consistency (Cronbach's alpha of 0.86). Cronbach's alphas for the Sp-NSA-16 factors were good with the exception of the social involvement factor (Cronbach's alpha: communication=0.70; emotion/affect=0.70; social involvement=0.44; motivation=0.78; retardation=0.65).
Convergent validityThe Pearson correlation coefficient between the Sp-NSA-16 total score and the total score on the PANSS negative scale was 0.84 (p<0.0001). When we used the PANSS negative factor described by Marder, the coefficient was 0.84 (p<0.0001). With the CGI-negative symptoms, the Pearson coefficient was 0.91 (p<0.0001). When we consider the global assessment provided by item 17 of the NSA-16 (Global Negative Symptom Rating – Severity), the Pearson correlation coefficients between this score and scores on the PANSS negative scale, PANSS Marder Negative Factor, and CGI-negative symptoms were 0.81, 0.82, and 0.94, respectively.
Divergent validityPearson correlation coefficients between the Sp-NSA-16 total score and total scores on the PANSS positive scale and the Hamilton Depression Rating Scale were low in magnitude (r=0.17, p=0.061, and r=0.32, p<0.0001, respectively). Similar results were obtained when we consider the Global Negative Symptom Rating – Severity provided by item 17 of the Sp-NSA-16 (r=0.10, p=0.254, and r=0.34, p<0.0001, respectively). Finally, Pearson correlation coefficients between the scores on the NSA-16 and the PSP total score were high (Sp-NSA-16 total score: r=0.73, p<0.0001; Sp-NSA-16 global negative symptom severity: r=0.75, p<0.0001, respectively).
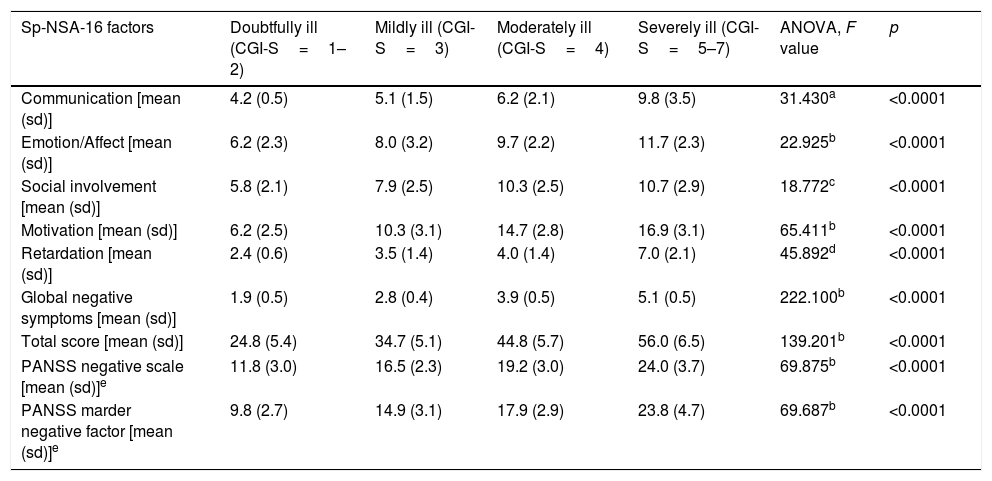
Discriminant validityThe Sp-NSA-16 and its factors were able to discriminate between the different levels of negative symptom severity according to CGI-SCH negative symptom severity scores (see Table 3). We found similar results for the PANSS negative scale and PANSS Marder Negative Factor (see Table 3). The area under the ROC curve was 0.97 (95% CI=0.94–1.00), indicating good accuracy of the test. A cut-off point of 31 provided excellent sensitivity (94.3%) and good specificity (83.3%) for separating patients without negative symptoms and patients with negative symptoms according to the CGI negative symptom scores (1 and 2 versus ≥3) (see Fig. 1).
Sp-NSA-16 and PANSS negative scale and factor scores according to severity of the negative symptoms as determined by CGI negative symptoms severity scores.
| Sp-NSA-16 factors | Doubtfully ill (CGI-S=1–2) | Mildly ill (CGI-S=3) | Moderately ill (CGI-S=4) | Severely ill (CGI-S=5–7) | ANOVA, F value | p |
|---|---|---|---|---|---|---|
| Communication [mean (sd)] | 4.2 (0.5) | 5.1 (1.5) | 6.2 (2.1) | 9.8 (3.5) | 31.430a | <0.0001 |
| Emotion/Affect [mean (sd)] | 6.2 (2.3) | 8.0 (3.2) | 9.7 (2.2) | 11.7 (2.3) | 22.925b | <0.0001 |
| Social involvement [mean (sd)] | 5.8 (2.1) | 7.9 (2.5) | 10.3 (2.5) | 10.7 (2.9) | 18.772c | <0.0001 |
| Motivation [mean (sd)] | 6.2 (2.5) | 10.3 (3.1) | 14.7 (2.8) | 16.9 (3.1) | 65.411b | <0.0001 |
| Retardation [mean (sd)] | 2.4 (0.6) | 3.5 (1.4) | 4.0 (1.4) | 7.0 (2.1) | 45.892d | <0.0001 |
| Global negative symptoms [mean (sd)] | 1.9 (0.5) | 2.8 (0.4) | 3.9 (0.5) | 5.1 (0.5) | 222.100b | <0.0001 |
| Total score [mean (sd)] | 24.8 (5.4) | 34.7 (5.1) | 44.8 (5.7) | 56.0 (6.5) | 139.201b | <0.0001 |
| PANSS negative scale [mean (sd)]e | 11.8 (3.0) | 16.5 (2.3) | 19.2 (3.0) | 24.0 (3.7) | 69.875b | <0.0001 |
| PANSS marder negative factor [mean (sd)]e | 9.8 (2.7) | 14.9 (3.1) | 17.9 (2.9) | 23.8 (4.7) | 69.687b | <0.0001 |
sd: standard deviation. CGI: Clinical Global Impression.
Duncan test showed that severely ill patients were different from those doubtfully, mildly and moderately ill, and moderately ill patients were different from those doubtfully ill.
Duncan test showed that severely and moderately ill patients were different from those mildly and doubtfully ill, and mildly ill patients were different from those doubtfully ill.
It is essential for clinicians to have effective instruments for measuring the severity of negative symptoms and changes in these symptoms over time. This study validated the Spanish version of the NSA-16 in a sample of patients with schizophrenia. The mean age (40.7, SD=10.4) of the sample was similar to the study that validated the original version of the NSA-16 (40.0, SD=11.0).18
The internal consistency of the overall scale was adequate and similar to previous studies.18,30 Regarding convergent validity, as expected, there were highly significant correlations between the NSA-16 and the PANSS negative scale, Marder factor, and CGI-negative symptoms, indicating strong convergent validity. Similar results have been reported for the original NSA-16.31 On the contrary, divergent validity was supported by lower correlations with instruments assessing other psychopathological aspects, i.e. positive and depressive symptoms. It is worth noting the moderate correlation found between scores for negative symptoms and functioning in keeping with the results of Velligan et al. (2009). This may be related to the widely reported negative influence of negative symptoms on real-world functioning.6,32 In fact, it has been observed that improvements in negative symptoms correlate with improvements in a global measure of functional outcome and functional capacity. However, the relationship between the two is complex.33 On the one hand, it has been pointed out that the main limitation of the NSA is its high reliance on functioning or behaviours even for experiential symptoms such as reduced social drive whose severity is measured by type and frequency of social interactions.34 This could explain the complexity of their relationship. On the other hand, this scale also includes a rating of reduced emotional range encompassing both anhedonia and lack of negative emotional experiences (such as anxiety, sadness, or anger). Thus, individuals who have healthy emotional functioning but experienced no negative emotional events during the observation period may score high, increasing their negative symptom severity.35 Finally, the Sp-NSA-16 and its factors were able to discriminate between different levels of negative symptom severity according to CGI-SCH negative symptom scores. Our results show that the NSA total score increases by ten for each increased level on the CGI-SCH, and the same happens in the NSA global negative symptoms, which increases by one.
One potential limitation of this study is that the inter-rater reliability was not estimated, as the assessments were conducted by the same rater, so it would be appropriate to investigate these relationships among instruments for assessments conducted by different raters. Another limitation was related to the generalizability of our results, since all patients were outpatients from the same region of Spain (Asturias), and there was a lack of patients with extremely severe negative symptoms, such as institutionalised individuals. The main strength of this study relies on the non-restrictive inclusion and exclusion criteria, so our patients are very similar to those seen in daily clinical practice.
In conclusion, we were able to demonstrate that the Spanish version of the NSA-16 (Sp-NSA-16) is an accurate and valid instrument for measuring negative symptoms in Spanish patients with schizophrenia. Furthermore, it seems to be appropriate for use in clinical trials and routine clinical practice as a means of measuring and monitoring changes in negative symptoms in these subjects. Moreover, the NSA-16 is easier to apply than other new scales such us the Clinical Assessment Interview for Negative Symptoms (CAINS)36 or the Brief Negative Symptom Scale (BNSS)37 since it includes the questions that should be asked to the patients and it is not necessary to make so many inferences (especially in regard to the observable signs). Moreover, nurses or non-psychiatrists doctors could obtain more reliable information assessing psychotic patients using this scale than others which require deeper training and knowledge, like CAINS or BNSS.
This work was partly supported by the CIBERSAM (Ref. BICIBERSAM, September 2012/C410163) and Instituto de Salud Carlos III (Ref. PI13/02263). We thank Sharon Grevet, CT, University of Pennsylvania, for her English assistance. She was sponsored by a research grant. She has no financial or other relationship relevant to the subject of this article.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Garcia-Alvarez L, Garcia-Portilla MP, Saiz PA, Fonseca-Pedrero E, Bobes-Bascaran MT, Gomar J, et al. Validación española de la escala de evaluación de los síntomas negativos-16 (NSA-16) en pacientes con esquizofrenia. Rev Psiquiatr Salud Ment (Barc.). 2018;11:169–175.