People with mental health disorders are at increased risk for coronavirus disease 2019 (COVID-19) and its severe complications, including a high mortality rates, compared to the general population.1–5 In addition, the high prevalence of medical comorbidities in this population6 make the COVID-19 treatment more challenging and potentially less effective.7,8
In this sense, the use of clozapine, an atypical antipsychotic medication indicated for treatment-refractory schizophrenia9 has been associated to an increased susceptibility to COVID-19 infection compared with other antipsychotic medications.10 Moreover, prescribing clozapine requires close monitoring for serious adverse events, including agranulocytosis, myocarditis, aspiration pneumonia, and clozapine toxicity, as seizures, ileus and delirium,11,12 which could be higher in the setting of COVID-19, as clozapine plasma concentrations increase during acute systemic infection because of the CYP1A2 enzymes’ inhibition by cytokines,13 and the reduction or ceasing tobacco smoking in patients during acute illness.14
Owing to the risk of clozapine-associated severe neutropenia, absolute neutrophil count monitoring programs are a prerequisite for clozapine dispensation.11,12 Clozapine neutropenia appears usually in the first 18 weeks of treatment with progression to life-threatening agranulocytosis in a minority of patients (0.8%).11,12 However, during COVID-19 pandemic, some case series have reported a reduction in neutrophil count and severe neutropenia in patients on clozapine treatment, resulting in clozapine withdrawal.15–19 and raising the concern of clozapine toxicity in the setting of COVID-19.20,21 Nevertheless, these studies were case series and did not have a group control of patients without clozapine treatment to evaluate the effects of the medication or the infection on the neutrophil counts.
Therefore, the aims of study was to compare the neutrophil dynamics during COVID-19 between patients with mental illness according to the clozapine use.
This observational study was conducted at the Emili Mira Healthcare Center, a public acute and long-term psychiatric care center in Barcelona (Spain) with 300 places and dependent on a large tertiary teaching hospital.
People with mental illness on the basis of ICD-10, who had a positive determination of coronavirus SARS-CoV-2 real time reverse-transcription-polymerase chain reaction (cobas® SARS-CoV-2 Test Roche laboratories) in nasopharyngeal samples were included in the study. Patients with lack of data on hematological parameters during COVID-19 or suffering from hematological malignancies or immunodeficient states (excluding diabetes mellitus) or less than six months on stable psychiatric treatment were excluded from the study. Patients were identified from the database of the patients admitted in the center from March 2020 to February 2021.
For the purpose of the study, socio-demographic and clinical characteristics were extracted from the patients’ medical records and categorized to maintain anonymity where necessary.
The total leukocytes count, absolute neutrophil count and lymphocyte count were the registered hematological parameters. Baseline counts were considered those taken at least 30 days before a positive SARS-CoV-2 test; COVID-19 counts, those taken between the day of the positive SARS-CoV-2 test and seven days later; and post-COVID-19 count, those counts taken between seven and fourteen days before of positive SARS-CoV-2 test. Psychiatric polypharmacy was defined as the prescription of two or more psychiatric medications concurrently to a patient.
The main outcome of the study was the change of the neutrophil count during COVID-19, measured as the total difference between the baseline and COVID-19 count. The secondary objectives were the incidence of neutropenia, measured as a neutrophil count <1500cells/mcL and the change of neutrophil count during COVID-19 according to the use of clozapine treatment.
Descriptive statistics were expressed as median and interquartile range for quantitative variables and absolute frequencies and percentages for qualitative variables. Wilcoxon test was used to compare quantitative variables. Analysis were made using SPSS software, version 17.0.0 (Chicago, IL).
The study complied with the ethical statements in the Declaration of Helsinki (64th General Assembly, Fortaleza, Brazil, October 2013) and was approved by the local Institutional Review Board.
There were 69 patients admitted in the center who had COVID-19 during the study period. Thirty-eight patients were excluded for lack of hematological parameters. Finally, a total of 31 patients were included in the study.
Clinical characteristics of the patients are shown in Table 1. All patients had schizophrenia spectrum disorders, psychiatric polypharmacy and were on second-generation antipsychotic treatment. The main medical comorbidities were: obesity in 17 (54.8%) patients, hypertension in 11 (26.8%), chronic respiratory diseases in 8 (25.8%), diabetes in 6 (19.3%), chronic heart diseases in 3 (9.6%), and chronic kidney diseases in 3 (9.6%).
Clinical characteristics of 31 patients with mental illness and COVID-19 included in the study.
| n | 31 |
| Age1 | 55 (50–77) |
| Men | 22 (70.9%) |
| Psychiatric diagnostic categories | |
| Schizophrenia disorder | 23 (74.2%) |
| Deficit intellectual | 6 (19.4%) |
| Bipolar disorder | 2 (6.4%) |
| COVID-19 severity | |
| Mild | 24 (77.4%) |
| Moderate | 7 (22.6%) |
| Antipsychotic treatment | 31 (100%) |
| >2 antipsychotic drugs | 20 (64.5%) |
| Clozapine treatment | 16 (51.6%) |
| Mood stabilizers treatment | 8 (25.8%) |
| Antidepressants treatment | 8 (25.8%) |
| Benzodiacepines treatment | 19 (61.3%) |
| Leukocyts1(cell/mcL) | 7000 (4098–8120) |
| Neutrophils1(cell/mcL) | 4480 (2224–5160) |
| Lymphocits1(cell/mcL) | 2080 (1112–2375) |
Data are presented as Number. (%) unless otherwise indicated. 1: Data presented as mean±standard deviation. 2: Data presented as median and interquartile range.
Three quarter of patients had mild COVID-19 and two patients died.
Half of patients were on clozapine treatment with a median clozapine doses of 350 (IQR: 175–400) mg/day and baseline plasma concentration of 450 (IQR: 370–490) ng/mL. Median plasma concentrations at COVID-19 (available for 12 patients) were 604 (400–870) ng/mL and statistically significant (p 0.04) compared to baseline levels. Five patients had clozapine levels >600ng/mL requiring dose reduction.
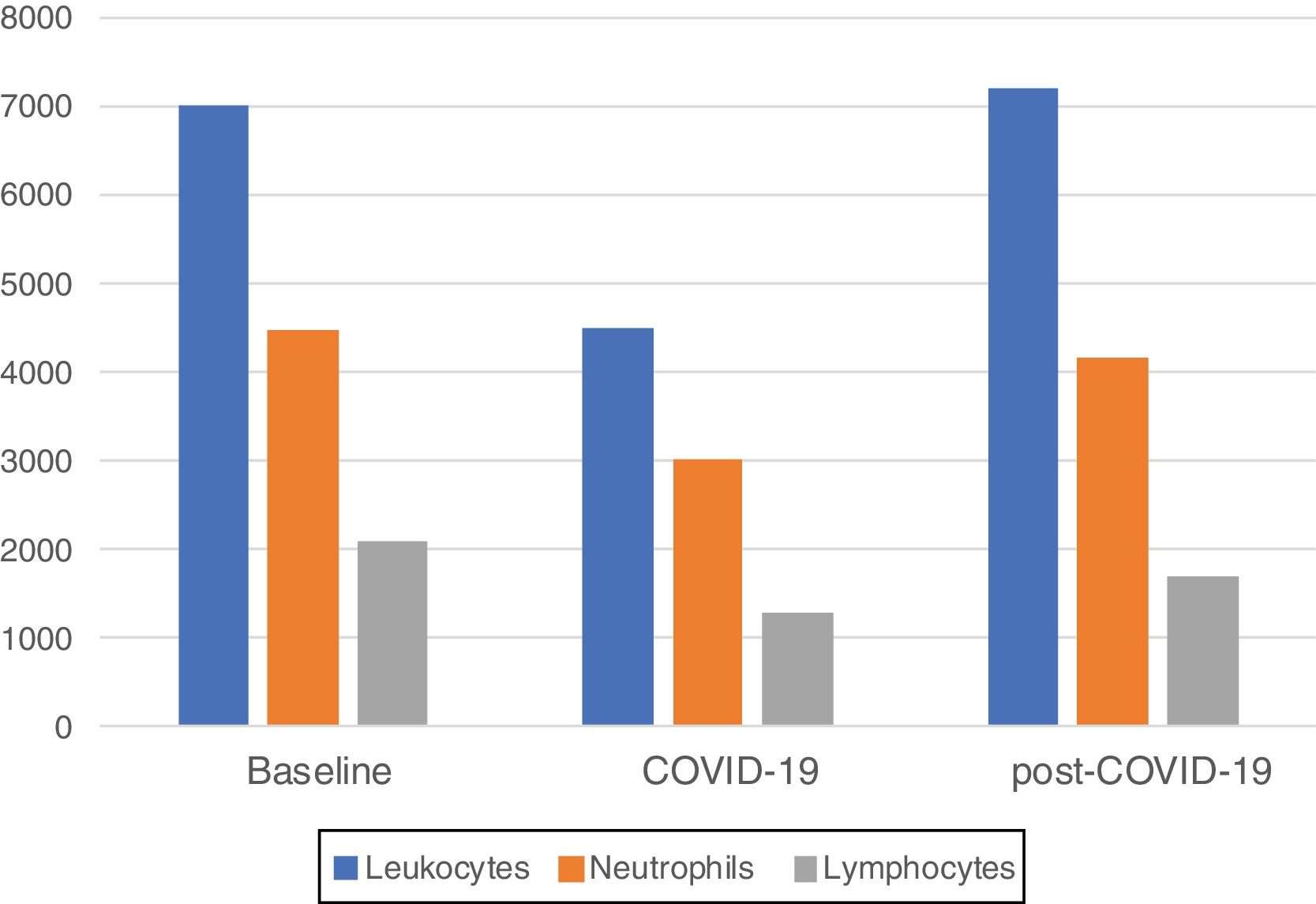
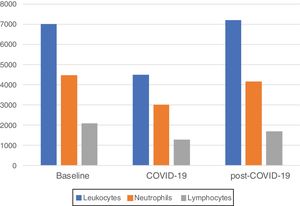
Median blood parameters by time period are shown in Fig. 1. A significant decrease of total leukocytes (4510cells/mcL), absolute neutrophils (3000cells/mcL) and lymphocytes (1280cells/mcL) were observed at COVID-19 compared to baseline (p<0.01 for all comparisons). A significant increase of total leukocytes (7200cells/mcL, p<0.01), absolute neutrophils (4150cells/mcL, p<0.01) and lymphocytes (1705cells/mcL, p<0.03) at post-COVID-19 were observed compared to COVID-19 counts. There was not statistically significant differences between total leukocytes (p 0.85), absolute neutrophil (p 0.81) counts between baseline and post-COVID-19 counts, while lymphocyte was significant (p 0.05).
Median change of neutrophil count at COVID-19 was -560cells/mcL (IQR: −1510, −1160) representing a median percentage of −27.6% (IQR: −47%, −2%). There was not differences in median neutrophil change according to clozapine use; −680 (IQR: −1502, −145) cells/mcL for the clozapine group and −550 (IQR: −1805, −405) cells/mcL for the non-clozapine group (p 0.51).
Clinical characteristics of the three patients with neutropenia are shown in Table 2. Two patients received clozapine treatment and had previous neutropenia during clozapine titration. Clozapine treatment was temporally discontinued in one of them for decreasing neutrophil count to 100cells/mcL. After withdrawal of new medication introduced for the COVID-19 treatment, neutropenia was resolved.
Clinical characteristics of the three patients with neutropenia.
| Patient | 1 | 2 | 3 |
|---|---|---|---|
| Age (years) | 57 | 55 | 68 |
| Sex | Woman | Woman | Man |
| Psychiatric diagnostic | Schizophrenia | Schizophrenia | Schizophrenia |
| Clozapine use | Yes | Yes | No |
| Baseline levels (ng/mL) | 454 | 378 | – |
| COVID-19 levels (ng/mL) | 850 | 587 | – |
| Severity COVID-19 | Mild | Moderate | Mild |
| Neutrophil count(cell/mcL) | |||
| Baseline | 2390 | 2130 | 4500 |
| COVID-19 | 970 | 100 | 1300 |
| Post-COVID-19 | 1850 | 3150 | 5380 |
| Cause | Hydroxychloroquine | Metamizol | Ibuprophene |
The results observed in this study showed that during COVID-19 there was a transient drop fall in the neutrophil count in patients with mental illness, regardless of the type of second-generation antipsychotic treatment they received. In addition, cases of neutropenia were related to the introduction of new medications for the COVID-19 treatment.
Viral infections, including those caused by Epstein–Barr virus, cytomegalovirus, hepatitis A and B viruses, parvovirus, Influenzavirus species, measles and HIV, are a common cause of neutropenia, due either to bone marrow suppression or to peripheral destruction.22,23 In the case of COVID-19, lymphopenia followed by thrombopenia are the most usual hematological findings.24–28 However, alterations in neutrophils have also been reported and associated to the severity and progression of the disease; while a small fall of neutrophil counts has been observed in mild cases,28 an increased in neutrophil counts together a decrease in lymphocyte count has been considered a risk factor for Acute Respiratory Distress Syndrome during the disease course.24–28
Thus, the transitory neutrophil count drop observed in this study, which included mostly non-severe COVID-19 cases, is according to data observed in mild COVID-19 cases.28 This finding have several major implications. First, clozapine should generally be continued as the neutrophil count decrease seems to be a non-pathological event during mild COVID-19.28 However, close plasma concentration monitoring is advisable, as clozapine plasma concentrations increased during COVID-19 in this study, resulting in dose readjustment in five patients. The CYP1A2 enzymes’ inhibition by cytokines released during an infection and the reduction or ceasing tobacco smoking in patients during acute illness are well established risk factors that can increase clozapine plasma concentrations during COVID-19.13,14
Second, patients who experience neutropenia in the course of COVID-19 should be investigated and monitored as per routine clinical practice,29 as it cannot be assumed to be secondary to the viral infection or clozapine treatment. Nevertheless, other causes of drug-induced neutropenia30 should be evaluated, particularly medications used for the treatment of infection. In addition, it is interesting to note that the two patients with neutropenia had the antecedent of previous neutropenia during clozapine titration and a low baseline neutrophil, which could be used as a marker of future risk of neutropenia
Finally, it is important to evaluate the risk of drug-drug interactions of COVID-19 treatments to prevent the risk of clozapine toxicity; as some antiretrovirals, as lopinavir and darunavir, which were widely used during the first months of the pandemic without clinical efficacy against coronavirus SARS-CoV-2, are inhibitors of the cytochrome P45031.
The study had some limitations, namely the observational design and the small number of patients included. However, patient's clinical characteristics were well defined, including a stable antipsychotic treatment. These results should be confirmed in larger samples.
In conclusion, this study showed the relevance of a comprehensive clinical assessment of suspected COVID-19 infection in patients with mental illness, including evaluation for features of pneumonia, full blood count and risk of side effects of newly introduced treatments. In addition, clozapine treatment should be continued at least in mild cases, however, consideration should be given to dose reduction.