Trazodone was authorized for the treatment of depression in the 1970s. Several additional therapeutic uses have been proposed due to its heterogeneous mechanism. This study aims to determine the use of trazodone in the elderly in Spain.
MethodsA nationwide, longitudinal and descriptive analysis was conducted using data from patients aged >65 years with a first prescription of trazodone during the period 2002–2011. Information on dose, comorbidities and relevant co-medication was gathered from the Spanish Primary Care database BIFAP. Incidence rates of trazodone use per 10,000 person-years were calculated by sex and age.
ResultsA total of 11,766 patients receiving a first prescription of trazodone were included. The incidence rate of trazodone use was 47.2 (95% CI: 46.33–48.04) per 10,000 person-years. An increasing trend in the use of trazodone was observed (5-fold increase in 2011 as compared to 2002). The most common therapeutic indications were: depression (21.41%), Alzheimer/dementia (20.36%), sleep disorders (16.22%), and anxiety disorder (8.91%). The median dose was 100mg/day. The use of trazodone concomitantly with interacting medicines was frequent: anti-hypertensives (53.60%), and CNS depressors (59.32%).
ConclusionsTrazodone use is increasing in elderly patients, and a high proportion of use in non-approved indications was observed. Trazodone is not being used at high doses, but interacting medicines were frequent, and it may pose additional risks for elderly patients.
La trazodona se autorizó para el tratamiento de la depresión en los años 70, y se han propuesto otros usos por su mecanismo de acción heterogéneo. Este estudio tiene como objetivo caracterizar la utilización de trazodona en ancianos en España.
MétodosSe llevó a cabo un análisis longitudinal en pacientes>65 años con una primera prescripción de trazodona durante el periodo 2002-2011. Se obtuvo información a partir de datos de BIFAP sobre la dosis, indicaciones, comorbilidades y medicación concomitante. Se calcularon las tasas de uso por 10.000 personas-año por grupos de edad (66-75; >75) y sexo. Se llevó a cabo un análisis descriptivo de las principales indicaciones y la comedicación.
ResultadosSe identificaron 11.766 pacientes con una primera prescripción de trazodona en el periodo de estudio. La tasa de incidencia de utilización fue de 47,2 (IC95%: 46,33-48,04) por 10.000 personas-año. El uso se quintuplicó en 2011 respecto a 2002. Las indicaciones terapéuticas más frecuentes fueron: depresión (21,41%), enfermedad de Alzheimer/demencia (20,36%), trastornos del sueño (16,22%), trastorno de ansiedad (8,91%). La mediana de la dosis fue 100mg/día. El uso de medicación concomitante que podría interaccionar fue frecuente: antihipertensivos (53,60%) y depresores del sistema nervioso central (59,32%).
ConclusionesLa utilización de trazodona ha aumentado en pacientes ancianos y se ha registrado una gran proporción de uso en indicaciones no autorizadas. El empleo de trazodona a dosis altas es infrecuente, sin embargo una gran proporción de pacientes estaban siendo tratados con medicamentos que interaccionan con trazodona y que podrían aumentar el riesgo en ancianos.
Trazodone was originally licensed in Europe and U.S. for the treatment of depression1 and is available since the 70s. Whereas its neuropharmacology is not fully understood, trazodone has been defined as a multifunctional drug. Its activity inhibiting the serotonin reuptake and antagonism on the 5HT2 receptors and alpha-1 adrenergic receptors is well recognized. In addition, a moderate antihistaminic activity and a low anticholinergic and dopamine activity have been described.2 Rare adverse reactions like hepatotoxicity or arrhythmia have been reported.3 However some of the most common adverse reactions of trazodone seem to be dose-dependent and include orthostatic hypotension, dizziness, somnolence and syncope.4,5 Furthermore, trazodone adverse effects can be more common in the elderly compared with younger groups due to its higher distribution volume, higher levels of comorbidity, age related physiological changes, and polypharmacy.4,6,7
The trends of trazodone utilization in current clinical practice in Spain have not been studied. Information about the clinical profile of the treated elderly patients is scarce. In this context, this study examines the trends of utilization of trazodone and analyzes the pharmacological pattern of trazodone utilization, including the study of relevant co-medication which may increase the risk for adverse reactions in the elderly.
MethodsSettingA descriptive study was conducted in BIFAP (Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria), an electronic health care database from Spain. BIFAP is a longitudinal population-based database managed by the Spanish Agency for Medicines and Medical Devices collecting computerized medical records from 4871 general practitioners (GPs) throughout Spain from 2001 onwards. This database includes anonymized information on 7,603,394 patients, accounting for 38,649,688 person-years of follow up.8
The dataset is comparable to the Spanish population with respect to its age and sex distribution. Data recorded in BIFAP include demographic information, prescription details, clinical events, specialist referrals and laboratory test data.9
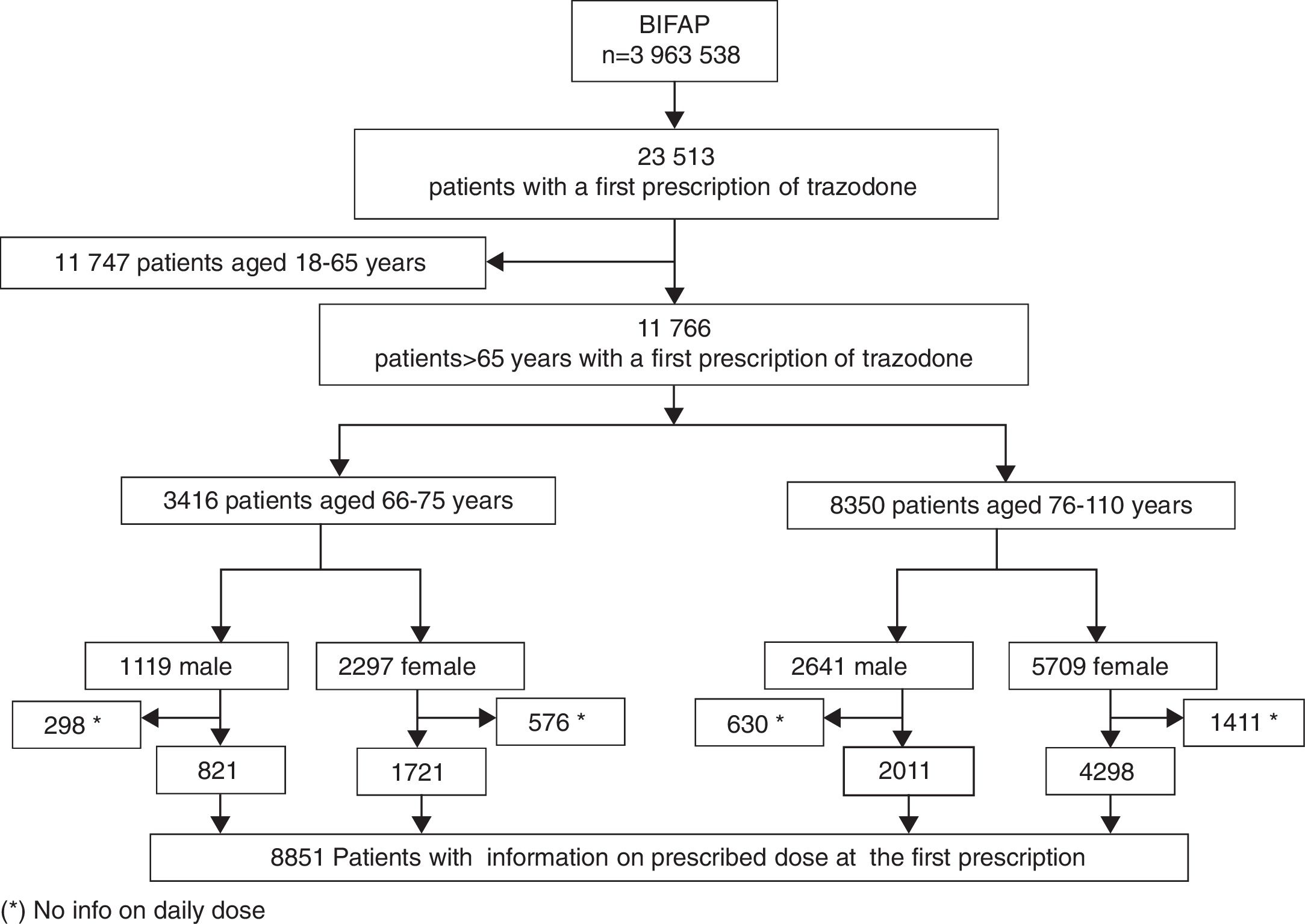
Study populationFrom the BIFAP database, we included patients aged >65 years old with at least one year of registration with a GP, and who received at least one trazodone prescription, between 1 January 2002 and 31 December 2011. Date of first prescription served as the index date.
Data on diagnoses and medicationThe indication was assigned according to the International Classification of Primary Care (ICPC-2) code linked to the first prescriptions of trazodone for each patient (codes available upon request to the authors).
For those patients whose prescription was not linked to codes related to depressive disorders, an additional search was performed any time before the index date to assess previous diagnosis of depressive disorders following the strategy used in previous studies.10
Information on the prescription was collected from the trazodone prescriptions including date, prescribed dose, frequency of administration, and number of packages prescribed. Information from the prescriptions of potential interacting drugs according the trazodone summary of product characteristics (including antihypertensives, antihistamines, antipsychotics, selective serotonin reuptake inhibitors – SSRIs, anxiolytics, hypnotics/sedatives, CYP3A4 inhibitors) was also gathered.4,5
Exposure to drugs was classified into different categories according to the end of the corresponding drug supply and the index date as follows: current use (the index date or within 30 days prior to the index date), recent use (between 31 and 60 days before the index date) and past use (between 61 and 180 days before the index date).
Statistical methodsAnnual incidence rates of trazodone prescribing were calculated by dividing the number of patients which received a first ever prescription of trazodone by the number of person-years (p-y) in every calendar year of the study period per 10,000 patient-years (p-y). The 95% confidence intervals (95% CI) for the incidence rates were calculated using Poisson distribution. Quantitative variables with normal distribution were expressed using the mean and standard deviation (SD), otherwise medians and percentiles 25 (p25) and 75 (p75) were used. Differences between categorical variables were analyzed by chi-square test (χ2) or Fisher's exact test, as appropriate. All statistical analyses were performed using the Stata 12.1 (Stata Corp., College Station, TX) statistical software.
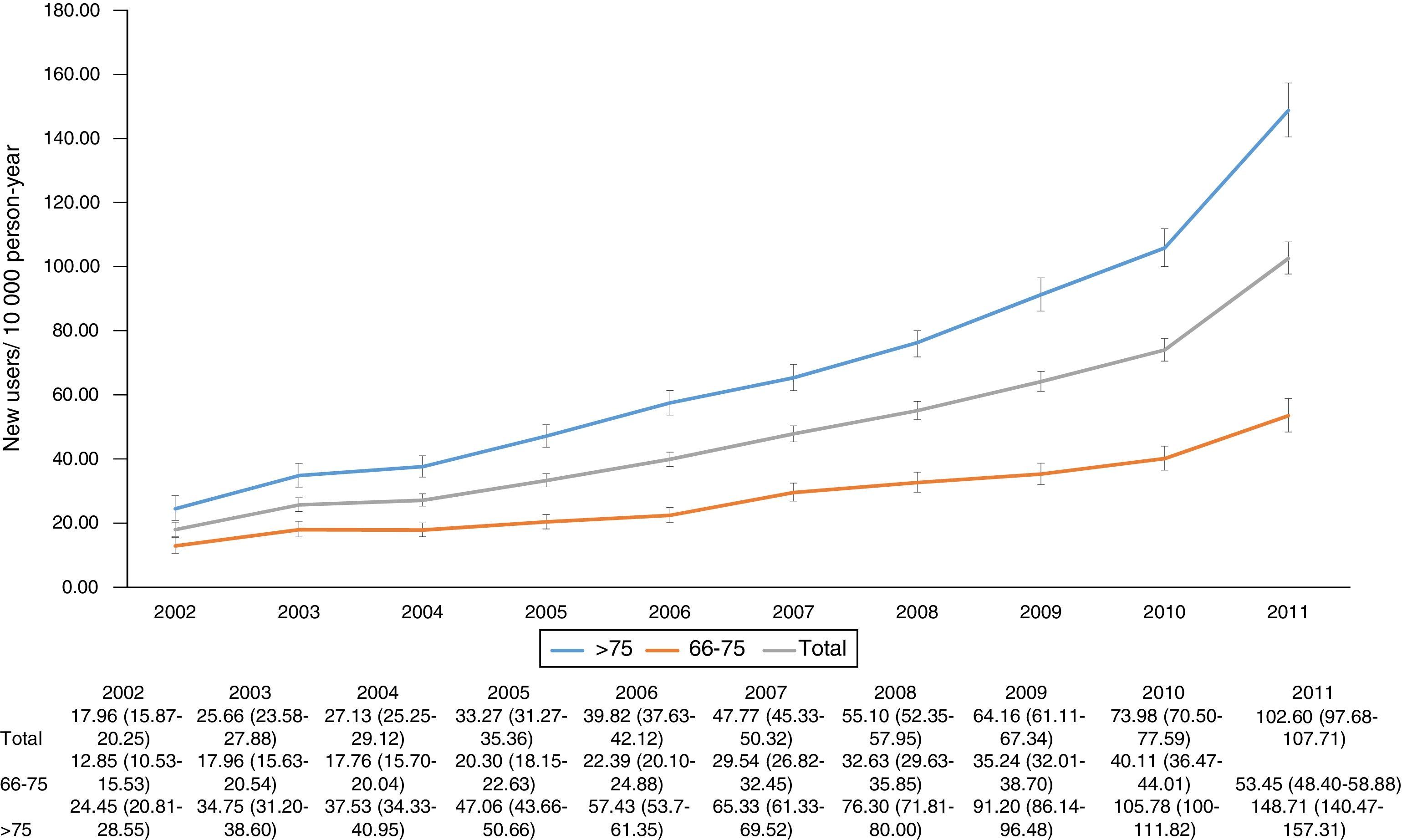
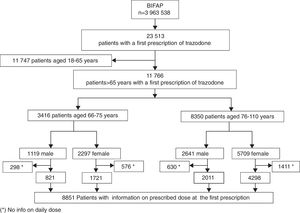
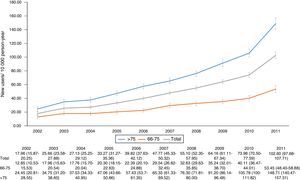
ResultsA total of 11,766 patients >65 years old with a prescription of trazodone were identified during the study period. The median age was 80 years (p25=74, p75=85) and 8006 (68.04%) patients were female. The most frequent users of trazodone were patients aged more than 75 years old (8350; 71%) (Fig. 1). The overall incidence rate of trazodone prescribing for the whole period (2002–2011) was 47.2 (95% CI: 46.33–48.04) per 10,000p-y. Patients aged >75 years registered higher incidence rate of prescription than patients aged 66–75 years (67.71; 95% CI: 66.26–69.18 and 27.10; 95% CI: 26.20–28.02 per 10,000p-y respectively). Incidence rate was higher in women than in men: 54.96 (95% CI: 53.08–55.47) and 36.92 (95% CI: 35.75–38.12) per 10,000p-y respectively. The incidence rate of prescription increased across the study period from 17.96 (95% CI: 15.87–20.25) in 2002 to 102.60 (95% CI: 97.68–107.71) in 2011 (5 fold of increase) (Fig. 2).
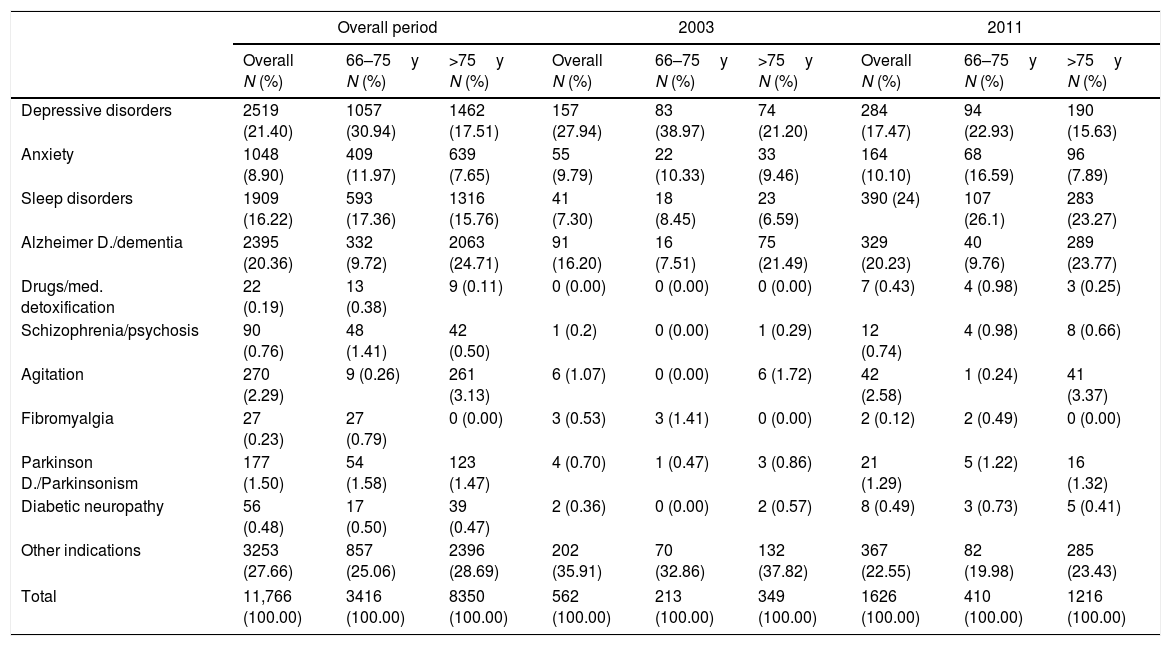
In the whole study period trazodone was mainly prescribed for depressive disorders (2519 patients; 21.40%), followed by Alzheimer/dementia (2395 patients; 2036%), sleep disorders (1909 patients; 16.22%) and anxiety (1048 patients; 8.90%) (Table 1). The most frequent therapeutic indication linked to trazodone was depressive disorders in patients aged 66–75 years (1057 patients; 30.94%) and Alzheimer's disease or dementia in patients older than 75 years (2063 patients: 24.71%). In 2011, sleep disorders become the most frequent therapeutic indication linked to trazodone prescriptions followed by Alzheimer's disease and dementia (Table 1).
Indication of trazodone prescriptions, in the overall period and the years 2003 and 2011.
| Overall period | 2003 | 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall N (%) | 66–75y N (%) | >75y N (%) | Overall N (%) | 66–75y N (%) | >75y N (%) | Overall N (%) | 66–75y N (%) | >75y N (%) | |
| Depressive disorders | 2519 (21.40) | 1057 (30.94) | 1462 (17.51) | 157 (27.94) | 83 (38.97) | 74 (21.20) | 284 (17.47) | 94 (22.93) | 190 (15.63) |
| Anxiety | 1048 (8.90) | 409 (11.97) | 639 (7.65) | 55 (9.79) | 22 (10.33) | 33 (9.46) | 164 (10.10) | 68 (16.59) | 96 (7.89) |
| Sleep disorders | 1909 (16.22) | 593 (17.36) | 1316 (15.76) | 41 (7.30) | 18 (8.45) | 23 (6.59) | 390 (24) | 107 (26.1) | 283 (23.27) |
| Alzheimer D./dementia | 2395 (20.36) | 332 (9.72) | 2063 (24.71) | 91 (16.20) | 16 (7.51) | 75 (21.49) | 329 (20.23) | 40 (9.76) | 289 (23.77) |
| Drugs/med. detoxification | 22 (0.19) | 13 (0.38) | 9 (0.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 7 (0.43) | 4 (0.98) | 3 (0.25) |
| Schizophrenia/psychosis | 90 (0.76) | 48 (1.41) | 42 (0.50) | 1 (0.2) | 0 (0.00) | 1 (0.29) | 12 (0.74) | 4 (0.98) | 8 (0.66) |
| Agitation | 270 (2.29) | 9 (0.26) | 261 (3.13) | 6 (1.07) | 0 (0.00) | 6 (1.72) | 42 (2.58) | 1 (0.24) | 41 (3.37) |
| Fibromyalgia | 27 (0.23) | 27 (0.79) | 0 (0.00) | 3 (0.53) | 3 (1.41) | 0 (0.00) | 2 (0.12) | 2 (0.49) | 0 (0.00) |
| Parkinson D./Parkinsonism | 177 (1.50) | 54 (1.58) | 123 (1.47) | 4 (0.70) | 1 (0.47) | 3 (0.86) | 21 (1.29) | 5 (1.22) | 16 (1.32) |
| Diabetic neuropathy | 56 (0.48) | 17 (0.50) | 39 (0.47) | 2 (0.36) | 0 (0.00) | 2 (0.57) | 8 (0.49) | 3 (0.73) | 5 (0.41) |
| Other indications | 3253 (27.66) | 857 (25.06) | 2396 (28.69) | 202 (35.91) | 70 (32.86) | 132 (37.82) | 367 (22.55) | 82 (19.98) | 285 (23.43) |
| Total | 11,766 (100.00) | 3416 (100.00) | 8350 (100.00) | 562 (100.00) | 213 (100.00) | 349 (100.00) | 1626 (100.00) | 410 (100.00) | 1216 (100.00) |
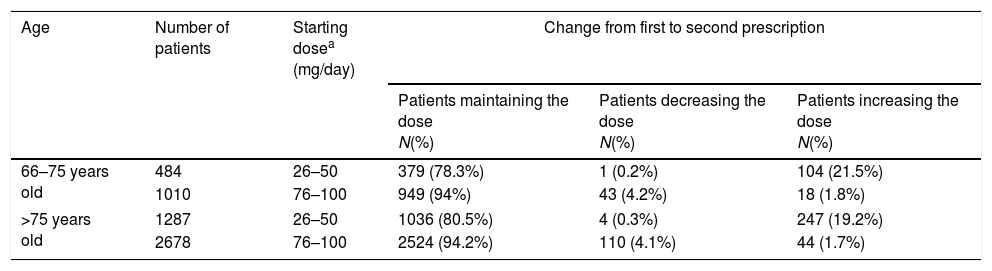
Overall, 8851 patients (75.23%) had information on the prescribed daily dose (Fig. 1). The median prescribed daily dose of trazodone at the first prescription was 100mg/day (p25=50, p75=100). No statistically significant differences in the prescribed daily dose were found by gender (p: 0.60; χ2: 5.48) or by age group (p: 0.15; χ2: 10.7283). The use of high doses (>100mg/day) was infrequent (381 patients; 4.30%). Additionally 6195 of these patients have additional information on daily dose at the second prescription. For most patients (4888 patients; 78.90%), the prescribed daily dose at the second prescription did not vary from the daily dose used in the first prescription. Changes in daily dose from the first to the second prescription are shown in Table 2.
Changes in dose from first to second prescription in the most frequently prescribed posology regimens.
| Age | Number of patients | Starting dosea (mg/day) | Change from first to second prescription | ||
|---|---|---|---|---|---|
| Patients maintaining the dose N(%) | Patients decreasing the dose N(%) | Patients increasing the dose N(%) | |||
| 66–75 years old | 484 | 26–50 | 379 (78.3%) | 1 (0.2%) | 104 (21.5%) |
| 1010 | 76–100 | 949 (94%) | 43 (4.2%) | 18 (1.8%) | |
| >75 years old | 1287 | 26–50 | 1036 (80.5%) | 4 (0.3%) | 247 (19.2%) |
| 2678 | 76–100 | 2524 (94.2%) | 110 (4.1%) | 44 (1.7%) | |
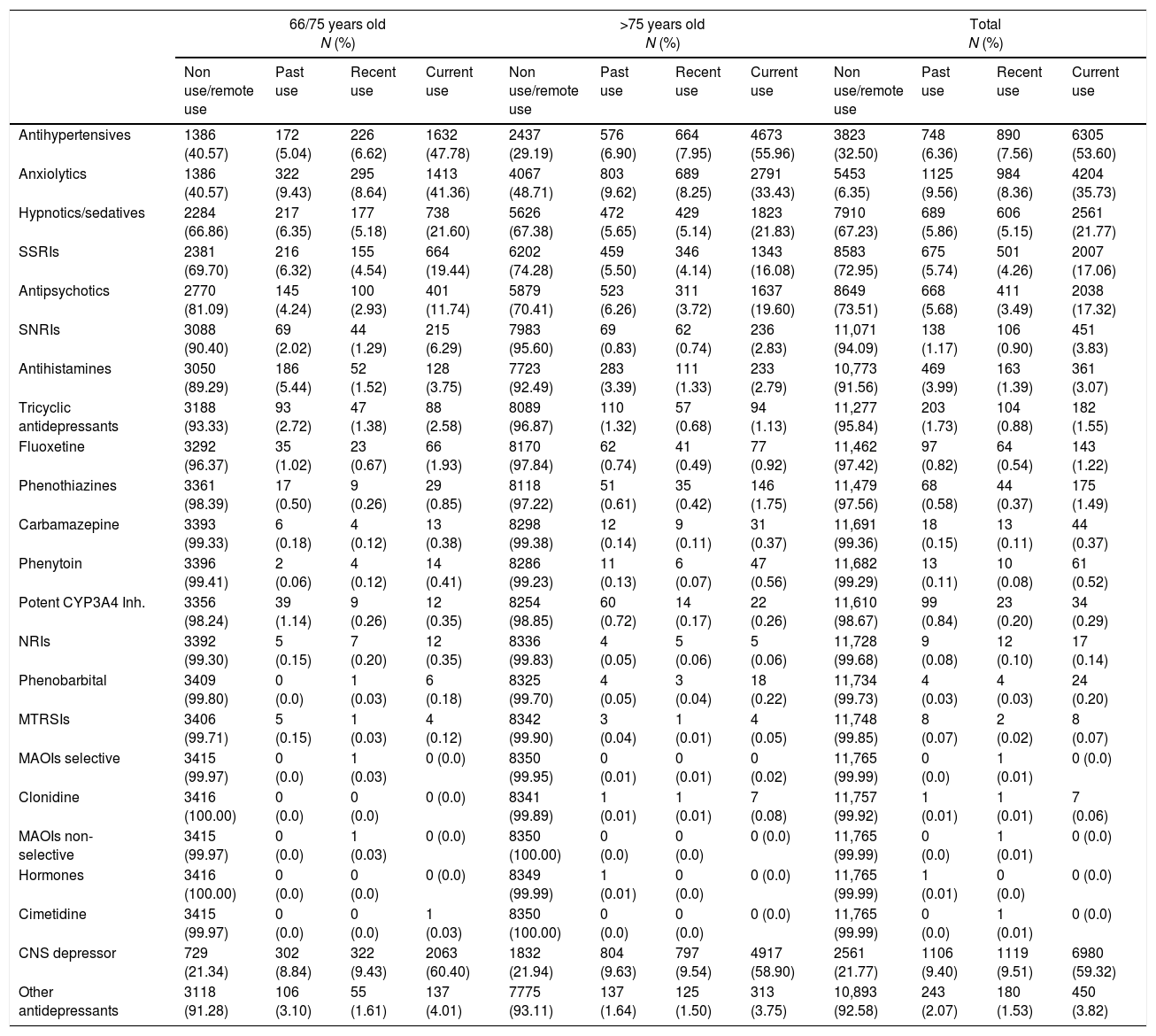
6305 (53.60%) patients were treated concomitantly with antihypertensive drugs and 6980 (59.32%) with CNS depressors. Current use of each potential interacting medicine groups ranged from 3.75 to 47.78% (Table 3). Statistically significant differences on the use of high doses (>100mg) were found in patients treated with trazodone in combination with CNS depressors (5.03%) in comparison with the remainder patients (3.30%; p<0.001; χ2:17.50).
Number and proportion of patients treated with trazodone and potential interacting drugs as stated in the trazodone summary of product characteristics.
| 66/75 years old N (%) | >75 years old N (%) | Total N (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non use/remote use | Past use | Recent use | Current use | Non use/remote use | Past use | Recent use | Current use | Non use/remote use | Past use | Recent use | Current use | |
| Antihypertensives | 1386 (40.57) | 172 (5.04) | 226 (6.62) | 1632 (47.78) | 2437 (29.19) | 576 (6.90) | 664 (7.95) | 4673 (55.96) | 3823 (32.50) | 748 (6.36) | 890 (7.56) | 6305 (53.60) |
| Anxiolytics | 1386 (40.57) | 322 (9.43) | 295 (8.64) | 1413 (41.36) | 4067 (48.71) | 803 (9.62) | 689 (8.25) | 2791 (33.43) | 5453 (6.35) | 1125 (9.56) | 984 (8.36) | 4204 (35.73) |
| Hypnotics/sedatives | 2284 (66.86) | 217 (6.35) | 177 (5.18) | 738 (21.60) | 5626 (67.38) | 472 (5.65) | 429 (5.14) | 1823 (21.83) | 7910 (67.23) | 689 (5.86) | 606 (5.15) | 2561 (21.77) |
| SSRIs | 2381 (69.70) | 216 (6.32) | 155 (4.54) | 664 (19.44) | 6202 (74.28) | 459 (5.50) | 346 (4.14) | 1343 (16.08) | 8583 (72.95) | 675 (5.74) | 501 (4.26) | 2007 (17.06) |
| Antipsychotics | 2770 (81.09) | 145 (4.24) | 100 (2.93) | 401 (11.74) | 5879 (70.41) | 523 (6.26) | 311 (3.72) | 1637 (19.60) | 8649 (73.51) | 668 (5.68) | 411 (3.49) | 2038 (17.32) |
| SNRIs | 3088 (90.40) | 69 (2.02) | 44 (1.29) | 215 (6.29) | 7983 (95.60) | 69 (0.83) | 62 (0.74) | 236 (2.83) | 11,071 (94.09) | 138 (1.17) | 106 (0.90) | 451 (3.83) |
| Antihistamines | 3050 (89.29) | 186 (5.44) | 52 (1.52) | 128 (3.75) | 7723 (92.49) | 283 (3.39) | 111 (1.33) | 233 (2.79) | 10,773 (91.56) | 469 (3.99) | 163 (1.39) | 361 (3.07) |
| Tricyclic antidepressants | 3188 (93.33) | 93 (2.72) | 47 (1.38) | 88 (2.58) | 8089 (96.87) | 110 (1.32) | 57 (0.68) | 94 (1.13) | 11,277 (95.84) | 203 (1.73) | 104 (0.88) | 182 (1.55) |
| Fluoxetine | 3292 (96.37) | 35 (1.02) | 23 (0.67) | 66 (1.93) | 8170 (97.84) | 62 (0.74) | 41 (0.49) | 77 (0.92) | 11,462 (97.42) | 97 (0.82) | 64 (0.54) | 143 (1.22) |
| Phenothiazines | 3361 (98.39) | 17 (0.50) | 9 (0.26) | 29 (0.85) | 8118 (97.22) | 51 (0.61) | 35 (0.42) | 146 (1.75) | 11,479 (97.56) | 68 (0.58) | 44 (0.37) | 175 (1.49) |
| Carbamazepine | 3393 (99.33) | 6 (0.18) | 4 (0.12) | 13 (0.38) | 8298 (99.38) | 12 (0.14) | 9 (0.11) | 31 (0.37) | 11,691 (99.36) | 18 (0.15) | 13 (0.11) | 44 (0.37) |
| Phenytoin | 3396 (99.41) | 2 (0.06) | 4 (0.12) | 14 (0.41) | 8286 (99.23) | 11 (0.13) | 6 (0.07) | 47 (0.56) | 11,682 (99.29) | 13 (0.11) | 10 (0.08) | 61 (0.52) |
| Potent CYP3A4 Inh. | 3356 (98.24) | 39 (1.14) | 9 (0.26) | 12 (0.35) | 8254 (98.85) | 60 (0.72) | 14 (0.17) | 22 (0.26) | 11,610 (98.67) | 99 (0.84) | 23 (0.20) | 34 (0.29) |
| NRIs | 3392 (99.30) | 5 (0.15) | 7 (0.20) | 12 (0.35) | 8336 (99.83) | 4 (0.05) | 5 (0.06) | 5 (0.06) | 11,728 (99.68) | 9 (0.08) | 12 (0.10) | 17 (0.14) |
| Phenobarbital | 3409 (99.80) | 0 (0.0) | 1 (0.03) | 6 (0.18) | 8325 (99.70) | 4 (0.05) | 3 (0.04) | 18 (0.22) | 11,734 (99.73) | 4 (0.03) | 4 (0.03) | 24 (0.20) |
| MTRSIs | 3406 (99.71) | 5 (0.15) | 1 (0.03) | 4 (0.12) | 8342 (99.90) | 3 (0.04) | 1 (0.01) | 4 (0.05) | 11,748 (99.85) | 8 (0.07) | 2 (0.02) | 8 (0.07) |
| MAOIs selective | 3415 (99.97) | 0 (0.0) | 1 (0.03) | 0 (0.0) | 8350 (99.95) | 0 (0.01) | 0 (0.01) | 0 (0.02) | 11,765 (99.99) | 0 (0.0) | 1 (0.01) | 0 (0.0) |
| Clonidine | 3416 (100.00) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8341 (99.89) | 1 (0.01) | 1 (0.01) | 7 (0.08) | 11,757 (99.92) | 1 (0.01) | 1 (0.01) | 7 (0.06) |
| MAOIs non-selective | 3415 (99.97) | 0 (0.0) | 1 (0.03) | 0 (0.0) | 8350 (100.00) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11,765 (99.99) | 0 (0.0) | 1 (0.01) | 0 (0.0) |
| Hormones | 3416 (100.00) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8349 (99.99) | 1 (0.01) | 0 (0.0) | 0 (0.0) | 11,765 (99.99) | 1 (0.01) | 0 (0.0) | 0 (0.0) |
| Cimetidine | 3415 (99.97) | 0 (0.0) | 0 (0.0) | 1 (0.03) | 8350 (100.00) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11,765 (99.99) | 0 (0.0) | 1 (0.01) | 0 (0.0) |
| CNS depressor | 729 (21.34) | 302 (8.84) | 322 (9.43) | 2063 (60.40) | 1832 (21.94) | 804 (9.63) | 797 (9.54) | 4917 (58.90) | 2561 (21.77) | 1106 (9.40) | 1119 (9.51) | 6980 (59.32) |
| Other antidepressants | 3118 (91.28) | 106 (3.10) | 55 (1.61) | 137 (4.01) | 7775 (93.11) | 137 (1.64) | 125 (1.50) | 313 (3.75) | 10,893 (92.58) | 243 (2.07) | 180 (1.53) | 450 (3.82) |
Inh: inhibitors; MAOIs: monoamine oxidase inhibitors; MTRSI: MT receptors selective inhibitors; NRI: norepinephrine reuptake inhibitors; SNRIs: serotonin and norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; CNS depressors (antipsychotics, anxiolytics, hypnotics/sedatives or antihistamines).
In this large nationwide population-based study of patients older than 65 years old who received a first prescription of trazodone, we found an increased trend of prescribing. Results suggested trazodone repositioning toward the treatment of sleep disorders and the management of Alzheimer's disease and dementia associated symptoms.
In our study, one in four prescriptions of trazodone in 2011 was used for the treatment of sleep disorders. A study found that trazodone was used as a hypnotic agent by roughly 1% of U.S. adults.11 Since trazodone has a sedative effect, it could be hypothesized that it can be useful in the treatment of sleep disturbances. However, there is a lack of objective evidence to support this off label use. To our knowledge only one small double blinded randomized clinical trial (n=12) has studied the efficacy of trazodone in primary insomnia for three months suggesting that acute trazodone administration (100mg) at night is an effective sleep promoting agent in psychophysiological insomnia.12 In other small clinical trial (n=30), trazodone at 50mg improved sleep efficiency (the percentage of time in bed spent sleeping) in Alzheimer disease patients, but had no effect on the time spent awake after falling asleep, or on the number of times participants woke up at night.13
Moreover, in our study we found that Alzheimer's disease, dementias and senile behavior (irritability, anxiety and verbal aggression) were the most frequent therapeutic indications linked to trazodone in patients aged more than 75 years. Although the sedative effect of trazodone may explain the use found in the management of agitated behaviors, few randomized controlled trials have studied trazodone in agitation and psychosis in dementia.1 One study compared to placebo did not find any significant difference in the Cohen-Mansfield Agitation Inventory (CMAI) total scores and two clinical trials comparing trazodone vs. haloperidol also failed to detect any difference in change in CMAI total scores.14 Trazodone has been studied in a small open label trial for the treatment of sleep disorders in Alzheimer's disease and other dementias in the elderly,15 thus the utilization of trazodone to treat empirically several dementia related symptoms – including insomnia – may explain the wide use found for this population in the study. A recent study of trazodone utilization performed in Canada has observed a similar trend of trazodone utilization which has increased in elderly patients with dementia.16
Additional off-label therapeutic indications representing less than 2% of the total prescriptions of trazodone included fibromyalgia, drug detoxification, or Parkinson Disease. These conditions curse with a number of different symptoms including comorbid insomnia for which trazodone may be used empirically. Although trazodone has been tested for the management of sleep disturbances after alcohol detoxification, its use was not recommended according to the results of randomized clinical trial.17 In an open label study for the treatment of fibromyalgia using high doses of trazodone, an improvement in sleep quality was found, however, tachycardia was reported in 21% of the patients.18 An extension of that open-label study found improvements in fibromyalgia symptoms when trazodone was combined with pregabalin.19 Depression and insomnia are common in patients diagnosed of Parkinson disease; however physicians should take special caution since trazodone can accelerate the metabolism of levodopa.4 On the other hand it has been hypothesized that trazodone may improve motor phases in Parkinson Disease by increasing the dopaminergic activity due to the antagonism of 5-HT2c receptors, however in a small clinical trial performed in 20 patients with Parkinson Disease no improvement in motor function has been found.20
Trazodone has a slower clearance in the elderly in comparison to young adults which pose a significant potential for adverse effects. Accordingly, the recommended initial dose for trazodone as antidepressant in elderly is 50–100mg/d, in comparison to young adults in which the initial recommended daily dose could reach 150mg/d.4,5 In our study few elderly patients started trazodone at doses over 100mg/d, or exceed that dose at the second prescription.
Polypharmacy is frequent in the elderly,6 and some concomitant medications could interact with trazodone to increase the risk of adverse reactions. In our study half of the patients were simultaneously treated with antihypertensive drugs, which can interact with trazodone causing orthostatic hypotension. Similarly the risk of neuropsychiatric adverse reactions of trazodone (somnolence, syncope and orthostatic hypotension) is higher in users of psychotropic drugs or CNS depressors – which may increase the risk of sedative effects associated to trazodone and therefore the use of lower doses it is advised. It has also been suggested that trazodone could be useful for SSRI-associated insomnia.21 Approximately 18% of the patients in the study were receiving trazodone in combination with SSRIs. However, this combination is not recommended because of the increased risk of serotonin syndrome/neuroleptic malignant syndrome,4,5 Potential limitations of our study merit consideration. First, health care databases studies may suffer from incomplete data recording for certain variables compared studies based on primary data. In turn, since the “off label” prescription of medicines may have legal implications retrospective studies based on clinical records are usually preferred over primary data studies (in order to avoid the Hawthorne effect bias). Second, there could be a certain degree of misclassification of the therapeutic indication linked by the doctors to trazodone which may not reflect the real diagnosis of the prescriptions, thus classification bias cannot be ruled out. Finally, there is a lack of information on prescriptions made in the field of specialized care/hospital, prescriptions made outside the Spanish National Health System (NHS) and use of the medicine without a prescription. However, trazodone is widely used in primary care and the acquisition of this medicine without a prescription is relatively uncommon.
This study showed that trazodone is an example of drug repositioning which is a process of developing new indications for existing drugs.22 Despite the existence of trazodone for nearly 40 years in the market, there is a lack of well designed studies in the off label indications where its use has become popular. Neither possible beneficiaries in these indications nor the risk established are well characterized, thus well designed double blinded clinical trials are needed to assess the safety and efficacy in off-label indications. Physicians should be aware of the risks when they manage drugs in off label indications. The increase on the prescribing trend of trazodone in the elderly along with the high prescribing of interacting co-medication could jeopardize these patients.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to acknowledge the excellent collaboration of general practitioners taking part in BIFAP.