Schizophrenia is a clinical construct comprising manifold phenotypes underlying heterogeneous biological underpinnings. The Positive and Negative Syndrome Scale (PANSS) represents the standard tool in the clinical characterization of patients affected by schizophrenia, allowing to detect different clinical profiles within the disorder. Frontal lobes are a key area of brain dysfunction in schizophrenia. We investigated whether different clinical profiles in acute schizophrenia show differences in frontal lobes dysfunction or not.
MethodsWe defined PANSS-derived principal components in a sample of 516 acute patients. These components were used as clustering variables in a finite-mixture model. Frontal lobe impairment, measured with the frontal assessment battery (FAB) score, was adjusted for disease duration and compared across the clinical clusters with ANCOVA. A supervised-learning approach was then implemented to reveal most informative PANSS items.
ResultsA three-cluster solution emerged: a first profile with high-moderate expression for the positive and excitability/hostility component; a second profile scoring high on depression/anxiety and low on other components; a third profile, comprising the majority of the study population (74%), with a heavy affection on the negative-disorganization dimensions. After controlling for disease duration, frontal lobe impairment significantly differed across the aforementioned clusters, with the third cluster being the most affected. Two PANSS items presented the highest predictive value for FAB total score.
ConclusionsAmong negative and disorganization symptoms, “difficulty in abstract thinking” and “lack of spontaneity/flow in conversation” are specifically mapped to higher levels of frontal lobes dysfunction, hinting at similar features with other neurological disorders involving frontal lobes.
Schizophrenia is a severe psychiatric condition that affects approximately 1% of the general population.1 Although considered a single nosographic entity for over a century, research on schizophrenia has more recently performed an effort directed at a psychopathological deconstruction, with the aim to generate insights into its pathophysiological underpinnings and potential novel treatment targets. The clinical features of schizophrenia can be dissected into a range of dimensions, the severity and relative proportions of which can vary across patients and through the course of the illness.2
A number of psychometric tools have been proposed to quantitatively describe schizophrenia phenomenology. The Positive and Negative Syndrome Scale (PANSS)3 is among the most popular rating scales for assessing acute schizophrenia psychopathology.4 The PANSS categorizes the symptoms into three dimensions: positive syndrome, negative syndrome, and general psychopathology. However, different PANSS subscales based on regrouped covarying questionnaire items were attempted with the aim of better describe schizophrenia heterogeneous psychopathology.5 Such attempts are needed considering that the PANSS is the gold standard primary efficacy measure in acute treatments studies of schizophrenia,6 effective treatments for negative/cognitive symptoms are still lacking7 and the correlation of PANSS factors might prevent understanding whether observed improvements in the 5 PANSS factors outline domain-specific treatment effect, or indirect improvement due to primary improvement in correlated PANSS items.8 The majority of previous studies evaluating the PANSS factorial structure convergently reported five-component solutions: (1) negative, (2) cognitive/disorganization, (3) positive, (4) excitement, and (5) depression/anxiety.9–12 Most of the past attempts to improve PANSS informative value adopted the statistical framework of principal component analysis (PCA). Cluster analysis methods are a powerful addition to the repertoire of previously used statistical tools and can be leveraged to break down schizophrenia heterogeneity, identifying subsets of patients based on principal components of psychometric scales. Indeed, an unmet need exists for the definition of intermediate clinical phenotypes along the main dimensions captured by the PANNS,13 advancing diagnostic criteria and delivering treatment plans specific for different subsets of patients.14 In fact, the biological underpinnings of schizophrenia might differ across different clinical phenotypes.
The frontal lobes in particular are widely believed to be a key area of brain dysfunction in schizophrenia, based on functional imaging studies and to some extent on neuro-pathological findings. However, the degree and the type of frontal lobes dysfunction vary across patients.15–17 Negative and disorganized symptoms specifically have been suggested to correlate with frontal lobes impairment and resemble symptoms suffered by frontal lesioned patients, thus giving rise to the ‘frontal lobe’ hypothesis of schizophrenia.18,19 The frontal assessment battery (FAB) scale is a brief battery of six neuropsychological tasks that was specifically designed to assess frontal lobe function at bedside.20 It is becoming increasingly popular for a variety of applications in neurology, most notably the early diagnosis of neurodegenerative dementing diseases such as the behavioral variant of fronto-temporal lobar degeneration.21,22 The FAB was previously benchmarked against the Stroop Color Word Test (SCWT) in patients with schizophrenia and its clinical efficacy as a representative task of executive function was established.23
The aim of this study was to find clinical clusters of patients suffering from acute schizophrenia based on the PANSS principal components and to test whether frontal lobes dysfunction measured by the FAB total score is equal across the so-found clinical clusters. Secondarily, in order to evaluate to what extent the psychopathological profile described by the PANSS can predict the degree of frontal lobe dysfunction recorded by the FAB total score and to pinpoint the single items with the highest predictive value for the FAB total score, a machine learning approach was used and each PANSS item was further investigated for correlation with PANSS total score.
MethodsParticipantsParticipants were recruited at the moment of admittance to the Psychiatry Inpatient Unit of the N. A. Alekseev Psychiatric Hospital No. 1, Moscow, Russia. All patients were adults (aged 18 or over), meeting the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for schizophrenia.24 To ensure that participants’ current episode was acute, duration of current episode must be<2 weeks. Exclusion criteria were intellectual disabilities, substance abuse and dependence, and any comorbid severe somatic or neurological disorder. No patient received electroconvulsive treatment in the three months prior to the study recruitment. The study protocol was approved by the Interdisciplinary Ethics Committee, Moscow (22/07/2017). Written consent was obtained from the subjects who agreed to take part in this study.
AssessmentsThe symptoms of schizophrenia were assessed using the validated Russian version of the PANSS.25 The PANSS is a 30-item questionnaire structured into three subscales: the first two capture the positive and negative syndromes, consisting of seven different items each; the third 16-item subscale grasps the general psychopathology. Each of the items is scored on a 7-point Likert-type scale (1=absent and 7=extreme), representing increasing levels of psychopathology.
Frontal lobes dysfunction was evaluated using the FAB.20 The FAB consists of 6 subtests exploring different functions related to the frontal lobes and correlated with frontal metabolism, as measured with the regional distribution of fludeoxyglucose F 18 in a positron emission tomography study of patients with frontal lobes damage of various causes.26 The 6 subtests of the FAB explore the following: (1) conceptualization and abstract reasoning (similarities test); (2) mental flexibility (verbal fluency test); (3) motor programming and executive control of action (Luria motor sequences); (4) resistance to interference (conflicting instructions); (5) inhibitory control (go–no go test); and (6) environmental autonomy (prehension behavior). Each subtest is scored from 3 (better score) to 0, for a maximum score of 18 (higher scores indicating better performance).
All clinical assessments were carried out by a group of four psychiatrists. Prior to study inception their clinical ratings for the PANSS and FAB questionnaires were tuned to reach an intraclass correlation coefficient (ICC)≥.75. Every three months calibration on patients not included in the study took place to make sure that an ICC≥.75 was preserved throughout the study.
Data analysis and statistical methodsReducing the PANSS questionnaire to its underlying principal dimensions: principal component analysisPCA reduces the dimensionality of a large number of variables into a smaller set of variables (components), while preserving most of the variability, by identifying linear combinations of the measured variables that maximize variance from the measured variables.27 Following previous studies convergently reporting five-component solutions,9–12 five PCA directions were extracted from our patient sample and compared to findings from the previous literature.
Identifying clinical clusters based on the PANSS principal components: model-based cluster analysisCluster analysis is statistical method used to determine underlying structure within a multi-dimensional dataset, e.g. PANSS components, with the aim to identify homogeneous subgroups of cases in a population.28 Individual participants’ scores on the first five principal components were used as clustering variables. Clustering tendency, i.e. inherent grouping structure, was assessed using the Hopkins statistic: a value close to 1 indicates highly clustered data, random data results in values around .5 while uniformly distributed data yield values close to 0.29
Finite-mixture models based on Gaussian distribution were used to explain associations between observed manifest indicator variables (PCA components) and hypothesized underlying unobserved latent variables (clusters). Mixture models are based on the assumption that k latent groups underlie the observed data, which would then represent a population consisting of several subpopulations.30 In Gaussian mixture distribution each subpopulation can be described as different means and covariances and parameters are estimated by maximum likelihood estimation using the expectation-maximization (EM) algorithm.
This model-based cluster analysis is an inferentially based, statistically principled procedure and the task of identifying the unknown number of clusters reduce to a model selection problem, for which objective procedures exist. We used the Bayesian Information Criterion (BIC) to compare multiple models and identify the optimum number of clusters.31 The parameters of the final model are then used to assign each observation to the cluster for which the posterior probability of belonging is highest.
In order to assess cluster stability, the best fit to our sample was tested for reproducibility in 100 datasets obtained with bootstrap resampling. Cluster stability of each cluster in the original dataset was computed as the mean value of its Jaccard coefficient for the most similar cluster across all the bootstrap iterations.32
To characterize the identified clusters, Multivariate Analysis of Variance (MANOVA) was used to compare multivariate sample means. Each principal component was then examined separately using Analysis of Variance (ANOVA) to assess individual contribution. Assumptions of MANOVA and ANOVA, i.e. independence of observations, (multivariate) normality, homogeneity of variances of the residuals (homoscedasticity), and homogeneity of covariance matrices, were checked.
Testing whether frontal lobes dysfunction is equal across the clinical clusters: analysis of covarianceWe used Analysis of Covariance (ANCOVA) to compare mean FAB total score across the clinical clusters after adjusting for the effect of duration of illness (in years) as covariate. Assumptions of ANCOVA, i.e. linearity of regression, homogeneity of error variances, independence of error terms, normality of error terms, and homogeneity of regression slopes, were checked.
Frontal lobes dysfunction predictability from the PANSS questionnaire: conditional variable importance for random forest and Pearson product-moment correlation testPANSS items ability to predict frontal lobes dysfunction as measures by the FAB scale was investigated using unbiased recursive partitioning by regression conditional inference trees and corresponding random forests.33 Random forest was selected as it usually attains excellent performance on structured/tabular data and can learn complex, non-linear decision boundaries while still generalizing well to unseen data. Along with high predictive performance, it provides measures of feature importance, i.e. the predictive value of a feature in the context of the trained model.26 An ensemble of regression conditional inference trees was since this reflects the true impact of each predictor variable more reliably than the original marginal approach, which overestimates spurious correlations.34 Nested cross-validation was used as resampling strategy in this study to obtain robust and unbiased performance estimates.35 In the outer resampling loop, five pairs of training/test tests were produced. In each of these outer training sets the optimum tuning parameter mtry, which controls the number of random variables to be tried at each split, for forests of 1000 trees was selected searching the integers from 5 to 15 during 10-fold cross-validation repeated 3 times through an R-Squared maximization process. Then the so-tuned forests were fitted on each outer training set and their performance was evaluated on the outer test sets. Conditional permutation importance for the predictor variables was then averaged across the five trained models.
Lastly, for each PANSS item a two-tailed Pearson product-moment correlation test with respect to FAB total score was carried out implementing Bonferroni-correction to account for multiple testing.
Code availabilityAll statistical analyses were performed with R Statistical Software,36 version 3.6.1. The following user-written R packages were used: factoextra37 for PCA, mclust38 for finite mixture analyses, fpc39 for assessing cluster stability, stats36 for MANOVA, ANOVA, and ANCOVA, party40 for conditional random forest, mlr41 for nested-cross validation, and ggplot242 for data visualization. All R-code we developed for statistical modeling is available upon request.
ResultsA total of 516 inpatients (261 women, 255 men) were enrolled in the study. The subjects had a mean age of 37.11 (± 15.27) years and a mean duration of illness of 11.34 (± 12.42) years. All subjects were receiving antipsychotics.
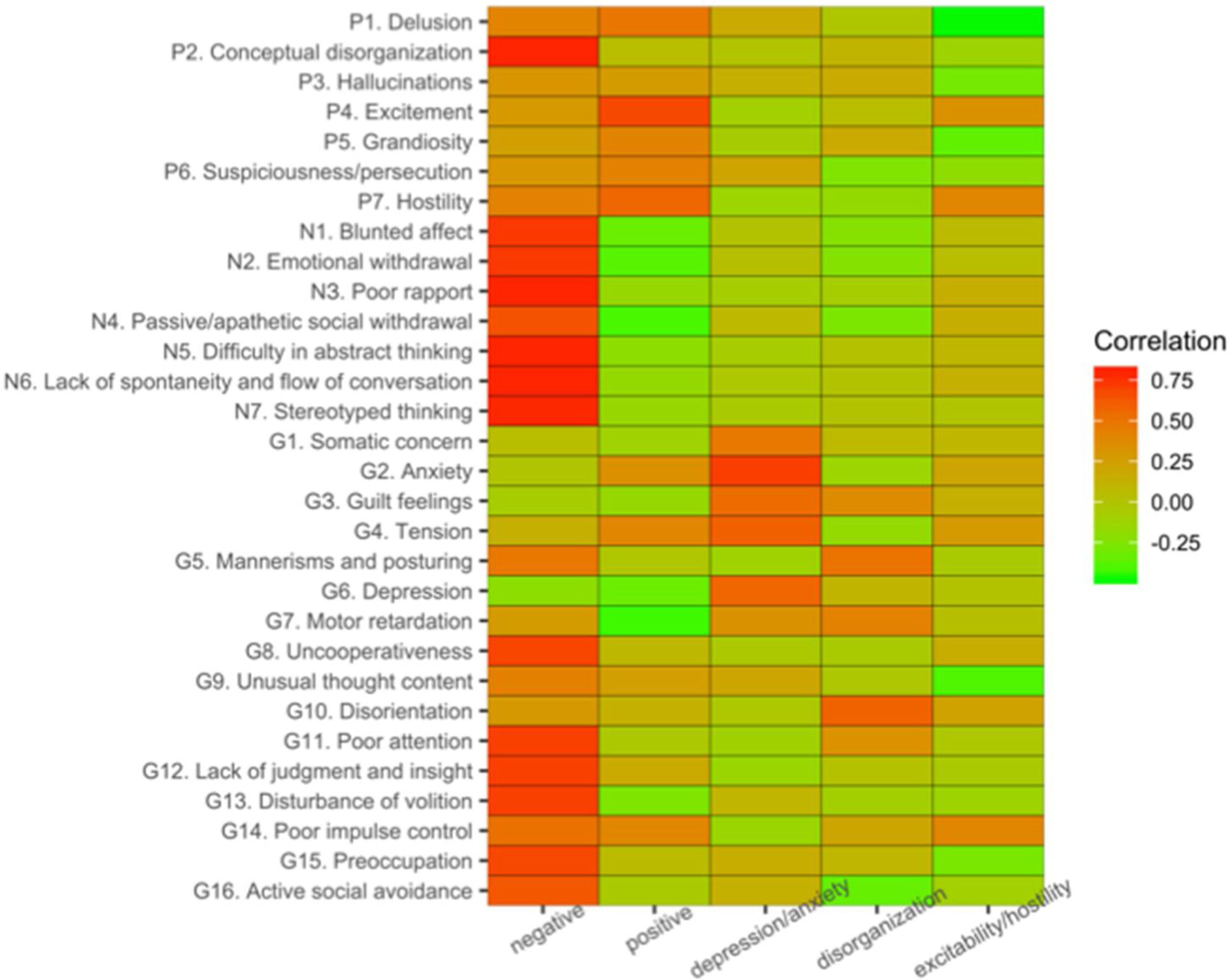
Factor-structure identified with PCAWe computed the five dominant components of variation in our sample using PCA and largely replicated the PCA of previous studies.9,10,12,43 The present PCA decomposition of PANSS items accounted for 57% of the variance across symptom scales. The component most associated with negative symptom items (Fig. 1) captured the largest amount of the total variance (29.2%). Our second component exhibited most associations with various positive symptom items (9.8% explained variance). The three remaining components accounted respectively for 7%, 5.4%, and 4.9% of the variance. The third component was mainly associated with anxiety and depression items, the fourth component with disorganization items, while the fifth component related to excitability and hostility. A description of the PANSS items has been added to the supplementary material (Suppl. Table 1).
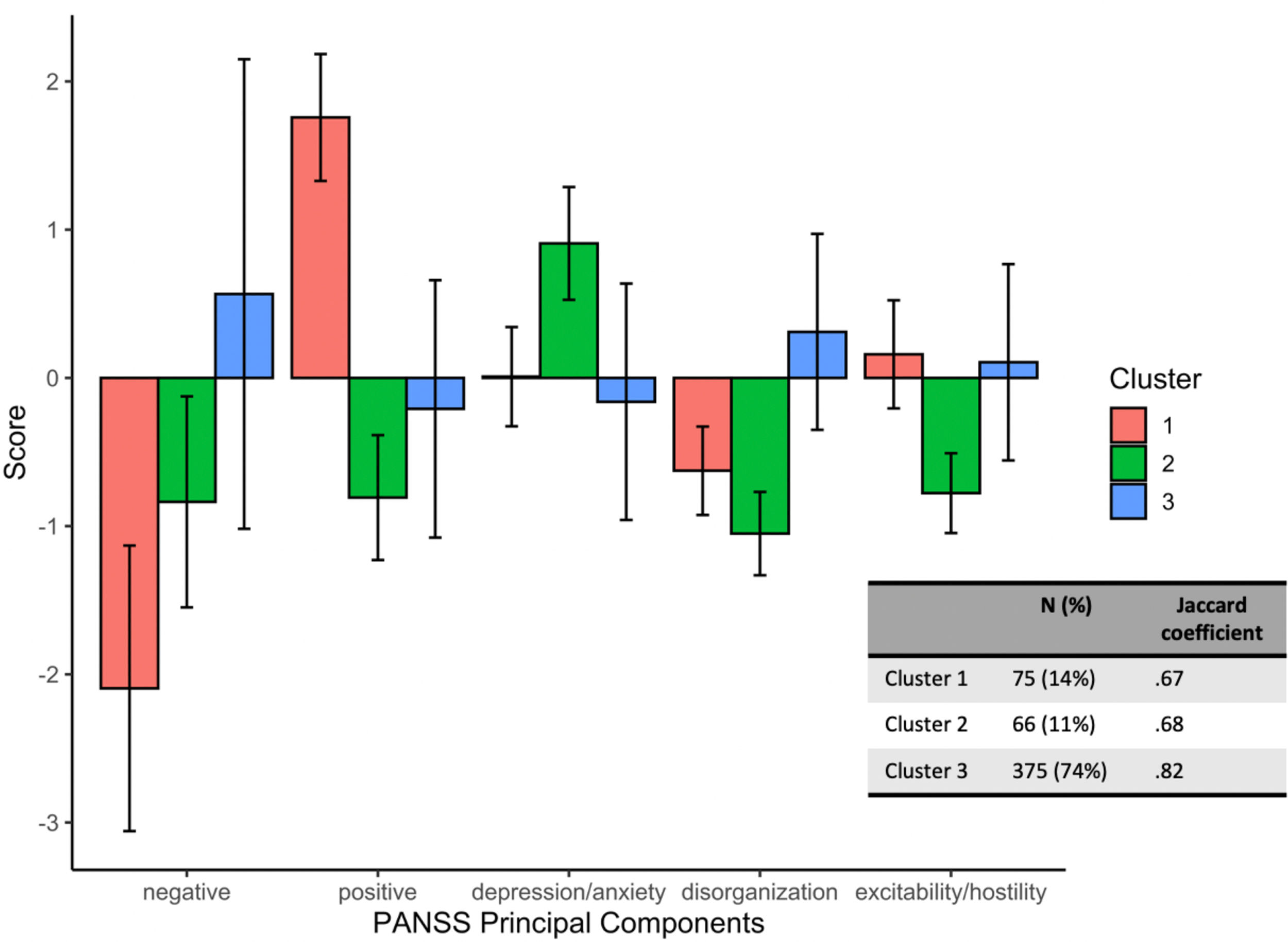
Patient groups from clustering PANSS principal componentsHopkins index of .19 among the 5 components suggested inherent grouping structure. Based on BIC model section, we identified a three-cluster solution as the best fitting clustering model. The mixing proportions were .14 (n=75), .11 (n=66), and .74 (n=375) for Clusters 1, 2, and 3 respectively. The average posterior probabilities for the most likely cluster classification were .6848, .6995 and .9195 for Clusters 1, 2, and 3 respectively (entropy for the three-cluster solution of .78, suggesting moderate separation between the groups). Multivariate normality was observed in each cluster by examining the marginal distributions (univariate normality), and also by examining variance for univariate data and generalized variance for multivariate data. Cluster 3 displayed high stability (Jaccard coefficient=.82), cluster 1 and 2 had acceptable stability (Jaccard coefficient=.67 and .68 respectively) across the 100 clustering iterations. There were not violations of MONOVA and ANOVAs assumptions. Using Pillai's trace, MANOVA showed a significant main effect for cluster group (V=.58, F2,1020=41.88, p<.001); post hoc univariate ANOVAs showed a significant main effect for all five principal components (all p<.001). Patients’ scores on the first five principal components across the three clusters (Fig. 2) revealed that cluster 1 comprised patients high on the positive and excitability/hostility component and low on the negative component. Cluster 2 had patients high on the depression/anxiety component but low on the other components. Patients belonging to cluster 3 had relatively higher scores on the negative and disorganization components but low score on the positive and depression/anxiety component.
PANSS principal components across clusters. Mean values and standard deviation are shown. The first cluster had high-moderate expression for the positive and excitability/hostility component; the second scored high on depression/anxiety and low on all other components; the third cluster has a heavy affection on the negative and disorganization dimension. Jaccard coefficients suggested high stability for cluster 3 across the 100 clustering iterations and acceptable stability for cluster 1 and 2.
ANCOVA assumptions were not violated. There was a significant effect of cluster membership on FAB total score after controlling for the effect of duration of illness (F2,453=16.05, p<.001, partial η2=.18). The covariate, duration of illness, was also significantly related to the participants’ FAB total score (F1,453=34.58, p<.001, r=.32). Tukey post hoc tests revealed that the covariate adjusted mean of Cluster 3 was significantly lower compared to that of Cluster 2 and Cluster 1 (difference=−2.94 (95%CI: −4.44; −1.43), t=−4.57, p<.001, d=−.59 and difference=−2.53 (95%CI: −3.99; −1.07), t=−4.06, p<.001, d=−.51, respectively). However, there was no significant difference between Cluster 2 and Cluster 1 (difference=.40 (95%CI: −1.52; 2.32), t=.49, p=.87, d=−.08). Distribution of FAB items score across clusters is reported in Supplementary Table 2.
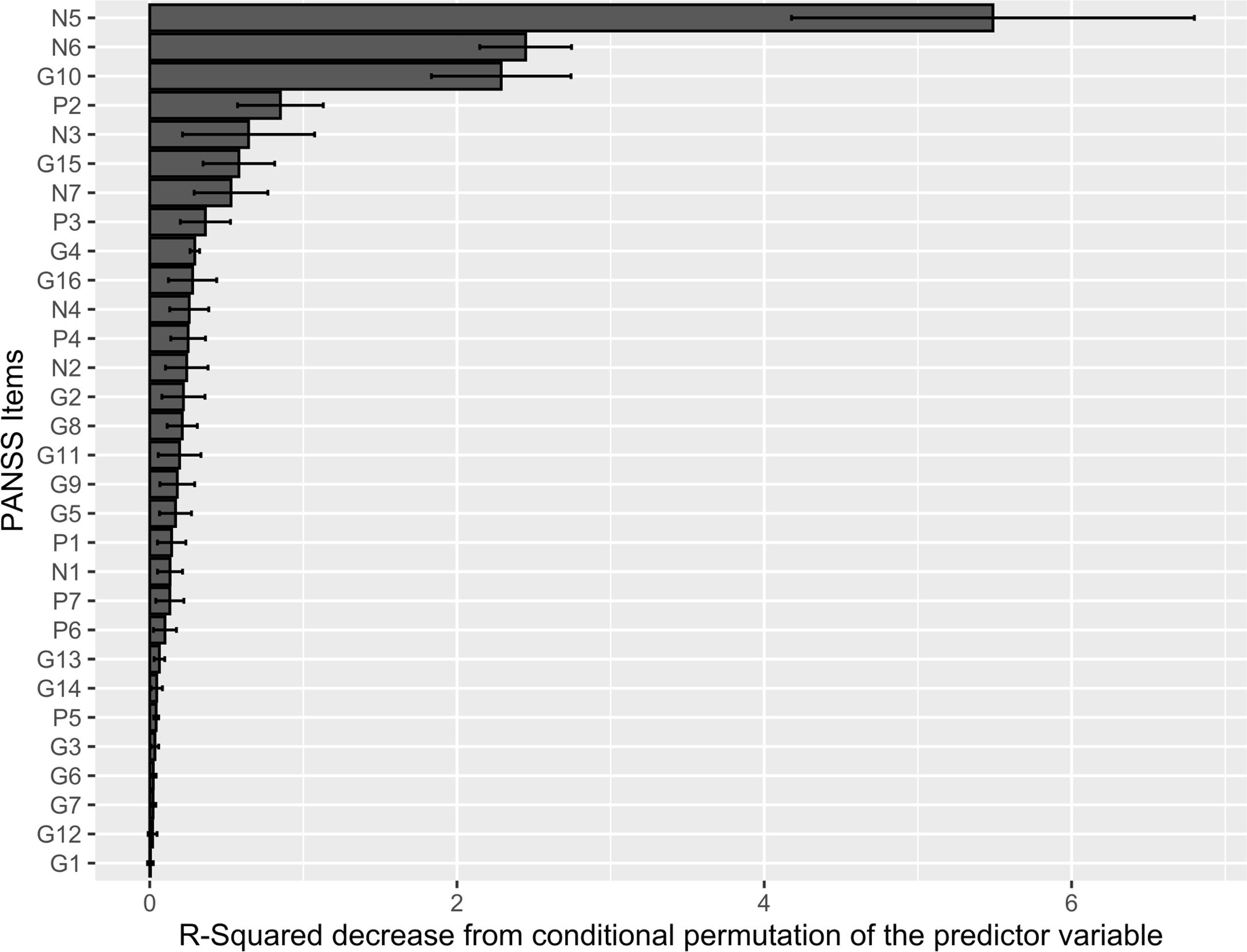
Conditional random forest and Pearson product-moment correlation test: PANSS items most related to frontal lobes dysfunctionThe R-Squared value was stable across the five outer test sets of the nested cross-validation (mean=.41, sd=.04), suggesting that the performance of the learners was fairly consistent across the data splits used for training. The PANSS items with the highest predictive value for FAB total score were “N5 – Difficulty in abstract thinking”, “N6 – Lack of spontaneity & flow of conversation”, and “G10 – Disorientation” (Fig. 3). The Pearson product moment correlation test confirmed “N5 – Difficulty in abstract thinking” and “N6 – Lack of spontaneity & flow of conversation” as PANSS items most (negatively) correlated with FAB total score. Overall, the PANSS items correlating most strongly with the first PCA direction, i.e. the negative dimension, displayed the highest negative correlation with FAB total score (Table 1). Distribution of PANSS items score across clusteres is reported in Supplementary Table 2.
PANSS items conditional permutation importance. The conditional permutation scheme for the computation of variable importance preserves the correlation structure between each examined feature and the other predictor variables. This corrects for spurious correlations. The decrease in the R-squared of the model was computed after the feature's values were permuted, which breaked the relationship between the feature and the outcome. Raw conditional permutation importance values are reported.
Pearson product-moment correlation test. Correlation between each PANSS item and FAB total score was computed.
| PANSS item | Estimate (95% CI) | Statistic (two-sided) | Bonferroni-corrected p |
|---|---|---|---|
| N5 | −.47 (−.54;−.40) | −12.05 | <.001 |
| N6 | −.41(−.48;−.34) | −10.09 | <.001 |
| Р2 | −.38 (−.45;−.30) | −9.14 | <.001 |
| N3 | −.35 (−.43;−.28) | −8.49 | <.001 |
| N7 | −.34 (−.42;−.27) | −8.22 | <.001 |
| G10 | −.34 (−.42;−.26) | −8.18 | <.001 |
| G11 | −.33 (−.41;−.25) | −7.82 | <.001 |
| N2 | −.32 (−.40;−.24) | −7.65 | <.001 |
| N4 | −.31 (−.38;−.22) | −7.17 | <.001 |
| G8 | −.30 (−.38;−.22) | −7.12 | <.001 |
| N1 | −.29 (−.37;−.21) | −6.77 | <.001 |
| G5 | −.26 (−.34;−.18) | −6.12 | <.001 |
| G12 | −.25 (−.33;−.17) | −5.79 | <.001 |
| G13 | −.24 (−.32;−.16) | −5.62 | <.001 |
| G14 | −.24 (−.32;−.15) | −5.48 | <.001 |
| P3 | −.21 (−.29;−.12) | −4.81 | <.001 |
| P7 | −.21 (−.29;−.12) | −4.69 | <.001 |
| P4 | −.20 (−.28;−.12) | −4.60 | <.001 |
| G15 | −.18 (−.26;−.09) | −4.04 | .002 |
| G16 | −.16 (−.24;−.07) | −3.65 | .009 |
| G7 | −.14 (−.22;−.05) | −3.10 | .061 |
| G9 | −.07 (−.16;.02) | −1.61 | >.99 |
| P5 | −.04 (−.13;.04) | −.97 | >.99 |
| P1 | −.04 (−.13;.05) | −.92 | >.99 |
| G1 | .00 (−.09;.08) | −.09 | >.99 |
| P6 | .00 (−.09;.09) | .03 | >.99 |
| G3 | .04 (−.05;.13) | .92 | >.99 |
| G4 | .06 (−.03;.14) | 1.28 | >.99 |
| G6 | .10 (.01;.18) | 2.18 | .883 |
| G2 | .11 (.02;.19) | 2.36 | .553 |
This study aimed to identify clinical subgroups of patients suffering from schizophrenia exacerbation according to PANSS principal components and to assess whether distinct symptom profiles underlie different degrees of frontal lobe impairment. In line with previous PCA decompositions of the PANSS questionnaire, we decided a priori to retain five PCA directions, which explained 57% of the variance in our patient sample. This result mirrored what was found in previous studies: 51%,11 52%,10,12 up to 57%9 of variance explained. Furthermore, inspection of the cumulative variance explained against principal components sorted by eigenvalues showed that components further to the fifth one only explained little amount of original variance relatively to the first five. Our five PCA directions had loadings on the PANSS items which suggested their conceptualization as negative, positive, depression/anxiety, disorganization, and excitability/hostility dimensions, similarly to previous reports. Using a clustering method, three types of distinct, clinically meaningful symptom clusters based on PANSS principal components emerged: a first profile with high-moderate expression for the positive and excitability/hostility component; a second profile scoring high on depression/anxiety and low on all other components; a third profile, comprising the majority of the study population (74%), with a heavy affection on the negative and disorganization dimension. After controlling for disease duration, the degree of frontal lobe impairment significantly differed across the aforementioned clusters, with the third cluster standing out as the worst affected; on the other hand, the first two clusters did not differ significantly from each other. The proportion of total variance in FAB total score explained by cluster membership that was not accounted by disease duration (the covariate) was moderate (partial η2=.18). Disease duration was also significantly associated with FAB total score, with a moderate effect size and a positive direction (r=.32). The PANSS items most predictive of frontal lobe dysfunction at the single individual level were “N5 – Difficulty in abstract thinking”, “N6 – Lack of spontaneity & flow of conversation”, and “G10 – Disorientation”. Using a non-linear learning algorithm, i.e. conditional random forest, the proportion of variance in FAB total score that was explained by the PANSS questionnaire was around 40 per cent (R-squared=.41). To the best of our knowledge, no previous study attempted to predict FAB total score from individual PANSS items using a machine learning framework. So, we could not compare our results in this regard against other reports pursuing the same (or a comparable) aim.
These findings support the view that schizophrenia is a clinically heterogenous disorder and that distinct intermediate phenotypes may stem from different biological underpinnings. In particular, while frontal lobe dysfunction is considered as a core feature in schizophrenia, the patterns of frontal lobe impairment seem to vary across symptom clusters. In our study, the clinical cluster high on negative and disorganization symptoms experienced the heaviest load of frontal lobe dysfunction in comparison to other clusters. This is in line with a meta-analysis of previous studies which established that negative symptoms and disorganization are associated with partially dissociable patterns of executive impairment.18 Alterations within left prefrontal (and superior temporal) regions may also be linked to unremitted primary positive symptoms.44
To the best of our knowledge, this is the first study to compare clinical clusters using the FAB questionnaire as a measure of frontal lobe dysfunction. The specificity of the association between higher levels of frontal lobe dysfunction and a higher burden of negative symptoms and disorganization, as revealed in this study, hints that a specific clinical cluster of schizophrenia patients shares similar features with other neurological disorders involving frontal lobes.
Results coming from this analysis should be balanced against some limitations. Firstly, while all patients were on psychotropic medications, relevant data were not collected so that it was not possible to adjust for administered drugs. In a similar vein, while patients with intellectual disabilities, i.e. intelligence quotient (IQ)<90, were excluded from this study, IQ was not recorded and so could not be controlled for. Secondly, it should be acknowledged that cluster analysis is exploratory in nature; however, great methodological care was taken to assess clustering tendency and stability. Thirdly, albeit largely used in previous studies, PCA constrains hidden dimensions to be orthogonal to each other; this is helpful in determining hidden traits disjoint from each other but may be disputable from the clinical standpoint. Lastly, the FAB scale, albeit a validated tool, is only a proxy for frontal lobes function and does not equal neuroimaging.
In sum, our findings corroborate previous findings suggesting that schizophrenia is a spectrum disorder embracing manifold clinical clusters. Different symptom profiles may underlie disruption at different biological levels. In particular, a high affection on negative symptoms and disorganization seems to be associated with significantly higher frontal lobe impairment in comparison to other symptom profiles. The FAB scale is short bedside test which was originally designed to screen for a dysexecutive syndrome in neurodegenerative diseases. The present study showed that distinct schizophrenia clinical clusters are mapped to significantly different degrees of frontal lobe impairment using the FAB.
Conflict of interestsAS is or has been a consultant to or has received honoraria or grants unrelated to the present work from: Abbott, Abbvie, Angelini, Astra Zeneca, Clinical Data, Boheringer, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier. EV has received grants and served as consultant, advisor or CME speaker unrelated to the present work for the following entities: AB-Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Sage, Sanofi-Aventis, Sunovion, and Takeda. DS was part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. All other authors have no conflicts of interest to declare.
EV thanks the support of the Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. DS was part-funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.