Sex differences in first episode of psychosis (FEP) have been widely studied. However, the existence of controversial results may be attributable to not considering relevant factors such as substance use. Cannabis use is associated with an earlier age of onset of psychosis and rates of cannabis use are consistently higher among men. The main objective of this study was to analyze and describe sex differences while considering the presence of substance use and its potential role when predicting age at onset of psychosis.
Material and methodsA cross-sectional study of 223 non-affective FEP patients was performed. Participants were divided into “current substance users”, defined as those who reported having used a substance in the past 30 days, and those who did not as “not current substance users”. Descriptive analyses, general linear modeling and multiple regression modeling were used.
ResultsIn the current substance group, women were older, with an older age of onset, a better premorbid adjustment and a higher cognitive reserve while presenting less clinical severity, a better functioning and a better verbal memory performance in comparison with men. In males, but not in females, lifetime of cannabis use and accumulative lifetime substance use was associated with age of onset.
ConclusionsClinical presentation of FEP varies by sex and especially when considering substance use. Our results suggest that early interventions need to be tailored to the different clinical needs of males and females and according to substance consumption in FEP.
Sex differences in first episode psychosis (FEP) have been widely studied.1 Some of these differences, such as earlier age at onset, worse premorbid adjustment and worse psychosocial functioning for men than women, are well-established.2,3 Other results are inconclusive or less well-established. For example, while many studies have found that males showed more negative symptoms4,5 and worse cognitive performance,6 other studies found no significant clinical differences.7,8 To date, the observed sex differences in established, chronic or enduring psychotic disorders may be attributable to not having taken substance use into account when comparing men to women.9
In patients presenting a FEP, substance abuse is highly prevalent10 and is higher in FEP males than females.9,11–13 The literature underlines the high prevalence of substance use and its relationship with poor prognosis and more serious clinical presentation.10,15 In fact, a more severe psychopathology characterized by negative symptoms is associated with substance use disorders14,15 and is more prevalent in male than female FEP patients.16 Nevertheless, a previous study by González-Rodríguez et al. (2014) about gender differences in psychopathological symptoms in emerging psychosis found that men were more likely to present negative symptoms than women. However, when age, antipsychotic, antidepressant and cannabis use were included as covariates they found that differences in psychopathological symptoms could not be confirmed between groups.17 Therefore, cannabis use could be a factor influencing the clinical heterogeneity of psychosis. Cannabis use has been also associated with an early onset of psychosis.18,19 However, Arranz et al. (2015)11 showed that substance use was related to an earlier onset of psychosis only in men, not in women. These results seem to indicate that sex plays a crucial role.
Cannabis use has been also associated with clinical presentation and trajectory following a FEP. However, empirical research shows controversial results. Some studies reported a significant association between cannabis use, psychotic symptoms and daily functioning20,21 while other studies reported no significant associations.22,23 Regarding neurocognitive functioning, a recent meta-analysis indicates that cannabis use is not generally associated with neurocognitive functioning in FEP patients.24 It seems that there were no differences in the evolution between patients who were cannabis users at the onset of the disease and those who were not,26 but stopping use after the first psychotic episode contributes to a clear improvement in outcome.22,25,26
Therefore, it seems that there is evidence of a complex interrelationship between sex, substance use and clinical presentation. A better understanding of how these factors may impact on clinical presentation is key to achieving the goal of implementing early and more precise personalized treatments.
Aims of the studyThe main objective of this study was to analyze sex differences in sociodemographic characteristics, premorbid adjustment, clinical presentation, psychosocial functioning and neurocognitive performance between male and female patients with a first episode non-affective psychosis in a stable clinical phase, while considering the presence of substance use. Moreover, the secondary objective was to describe sex differences on the potential role of substance use when predicting age at onset of psychosis.
We hypothesized that FEP male will present an earlier age at onset, worse premorbid adjustment, higher negative symptoms and worse psychosocial functioning than women. Cannabis users will present more psychotic symptoms and poorer cognitive functioning than non-users. Based on previous results, we expected to observe that substance use will be related to an earlier onset of psychosis only in men.
Material and methodsSampleThe sample of this study has been recruited though the “2EPs Project”. It is a multicenter, coordinated, naturalistic, and longitudinal follow-up study of three years’ duration. “2EPs” included Spanish patients with a diagnosis of schizophrenia or schizophreniform disorder with a recent FEP. Recruitment was conducted from October 2012 to December 2015. All 15 participating centers invited those patients with a suitable profile for the study. All the information about the methodology of the “2EPs Project” can be found elsewhere.27
The inclusion criteria were: (1) aged between 16 and 40 years at the first evaluation, (2) met diagnostic criteria according to DSM-IV for schizophrenia or schizophreniform disorder, (3) ability to speak Spanish correctly, (4) signed informed consent, (5) have presented a FEP in the last 5 years and are currently in remission according to the Andreasen criteria.28 Remission is achieved when the patient's Positive and Negative Symptom Scale (PANSS) score is 3 or less (mild severity) for 8 items. Severity symptoms must be maintained for a minimum of 6 months without having relapsed after the episode. The exclusion criteria were: (1) having experienced a brain trauma with loss of consciousness, (2) an Intelligent Quotient (IQ) lower than 70 and with significant difficulties or malfunctioning with adaptive processes, and (3) somatic pathology with mental affectation.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. Ethics committees of all participating centers approved the current study. Each subject agreed to participate and signed the informed consent before their inclusion.
AssessmentsAt baseline, patients performed a complete evaluation that included: structured interviews, clinical scales, premorbid adjustment scales, pharmacological and psychological treatment records. Scales were administered by trained and expert clinical staff except for self-administered scales. For the current study, only baseline data was used.
Sociodemographic, clinical and substance use assessmentData on sex, age and age at onset of illness were collected along with the duration of untreated psychosis (DUP). Parental socioeconomic status (SES) was determined using Hollingshead's Two-Factor Index of Social Position.29 The diagnosis was confirmed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM) (SCID-I and II)30 or the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS)31 according to DSM-IV criteria. A psychopathological assessment was carried out with the Spanish versions of the following scales: the Clinical Global Impression–Severity Scale (CGI-S)32 which evaluates severity and psychopathology in the past seven days, maniac and depressive symptom severity were assessed using Young Mania Rating Scale (YMRS)33 and the Montgomery-Asberg Depression Rating Scale (MADRS),34 respectively, and positive, negative, and general symptoms were assessed by the Positive and Negative Syndrome Scale (PANSS).35 On each scale, the items were summed to obtain a total score. Higher scores indicate greater severity.
We have also used the PANSS-Marder Factor Scores36 as it has more restrictive criteria to assess positive and negative symptomatology. The sum of the following items of the PANSS were used to calculate the Positive Symptom Factor (PSF): delusions (P1), hallucinatory behavior (P3), grandiosity (P5), suspiciousness/persecution (P6), stereotyped thinking (N7), somatic concerns (G1), unusual thought content (G9) and lack of judgment and insight (G12), and for Negative Symptom Factor (NSF): blunted affect (N1), emotional withdrawal (N2), poor rapport (N3), passive/apathetic social withdrawal (N4), lack of spontaneity and conversation flow (N6), motor retardation (G7) and active social avoidance (G16). This structure has proved to be beneficial to obtain more specific information.37
Antipsychotic mean doses were collected and converted to chlorpromazine equivalents (CPZ) based on international consensus.38
Drug abuse was assessed using the adaptation of the multidimensional assessment tool European Addiction Severity Index (EuropAsi).39 A systematic register was made to collect current and lifetime drug misuse habits. Due to the potential effect of lifetime exposure to substance use on the FEP prognosis, participants were divided into “current substance users”, defined as those who reported having used a substance in the past 30 days, and those who did not as “not current substance users”. “Lifetime users”, defined as those that had used a substance in their lifetime but not during the last 30 days, and “Never users”, individuals that had never used a substance were also identified. For the purpose of this study, we have focused on the use of “hard and illicit drugs” such as cocaine, hallucinogens, heroin, sedative, stimulant and methadone,40 and also on cannabis due to the relationship between cannabis use and psychosis.41
Functional assessment and quality of lifeThe overall functional outcome was assessed by the Functioning Assessment Short Test (FAST)42,43 and the Global Assessment of Functioning Scale (GAF)44 or the Children's Global Assessment Scale (C-GAS).45 The FAST scale comprises twenty-four items, each item rated from 0 (no difficulty) to 3 (severe difficulty). Higher scores represent higher disability. GAF or C-GAS scales evaluate psychological, social, and occupational functioning. It consists of one item and the score ranges across a continuum from health to disease. Higher scores correspond to better functioning.
Quality of life was assessed by the Quality of Life Scale (QLS),46 which assesses daily activities, social and interpersonal relationships, cognition functions, affectivity, and working or studying issues. Higher scores indicate better functioning in that category.
Premorbid adjustment and cognitive reservePremorbid adjustment, namely levels of functioning before the onset of psychosis, was assessed with The Premorbid Adjustment Scale (PAS).47 Sociability, school adaptation, socio-affective and sexual relationships, school performance, occupation, and school behavior before the onset of psychosis are assessed. The scale considers different life stages: childhood, early adolescence, late adolescence, and adulthood. Only childhood and early adolescence life periods have been taken into account since they were the two time periods for which the answers of all the participants were available. Higher scores indicate worse premorbid adjustment.
The following evaluation was carried out to measure cognitive reserve (CR) at baseline: (1) The estimated premorbid IQ was calculated with the vocabulary subtest of the Wechsler Adult Intelligence Scale (WAIS-III).48 (2) Education was assessed taking into account the number of years of obligatory education that subjects had completed as well as lifetime school performance, (3) Lifetime participation in leisure, social and physical activities was assessed by the PAS scale (scholastic performance) and the FAST scale, which allowed us to assess specific life-domains such as interpersonal relationships and leisure time. When patients were assessed, they had already experienced a FEP. For that reason, we could only estimate premorbid variables.
Neuropsychological assessmentThe neuropsychological battery assembled for this study was designed to assess different cognitive domains through standardized neuropsychological tests that have proven sensitivity and specificity. The “PEPs study”, a previous project, has already used this battery prepared specially for this population.49,50
The neuropsychological battery measured the following cognitive domains: (1) Sustained attention was evaluated with the Continuous Performance Test–II (CPT-II),51 version 5, (2) Verbal learning and memory were assessed with the Verbal Learning Test Spain Complutense for adults (TAVEC),52 (3) Processing speed was measured by the Trail Making Test part A53 and the Digit-Symbol-Coding subtest of the Wechsler Adult Intelligence Scale (WAIS-III),49 (4) Working memory was evaluated using the Digit Span Subtest and the Letter-Number Sequencing Subtest of the WAIS-III, (5) The executive functions were tested using the Tower of London test,54 (6) verbal fluency was assessed with the phonetic verbal fluency: FAS,55 (7) Social cognition was measured by the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT).56
For the purposes of the current study, a Principal Components Analysis (PCA) was performed to avoid redundant information of separate cognitive variables and to reduce measures into a few principal domains (see Supplementary Table 1). The neurocognitive assessment was represented by five factor scores (verbal memory, sustained attention, processing speed, working memory and executive function). A PCA was also performed to create a “Cognitive reserve score” for each subject with the three main proxies. Higher scores correspond to better performance.
Statistical analysisDescriptive analyses were conducted using chi-square for categorical variables and Student's test for continuous variables. Demographic, clinical and neuropsychological differences between groups were examined using unpaired t-tests and chi-square. A general linear modeling was used to test differences between substance use (lifetime/current vs never/not current), sex (male and female) as well as the potential interaction between group and sex. Bonferroni corrections were employed for post-hoc comparisons between groups.
In addition, multiple regression using a backward elimination method was performed to assess the variables predicting age at onset of psychosis (dependent variable). In order to replicate previous results of Arranz et al. (2015), the same variables were entered in the model (sex, lifetime use of each substance, and number of substances). Because the main outcome of the study was to analyze sex differences, analyses were stratified by sex.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS v25). All statistical tests were two-tailed, with an alpha level of significance set at p<0.05.
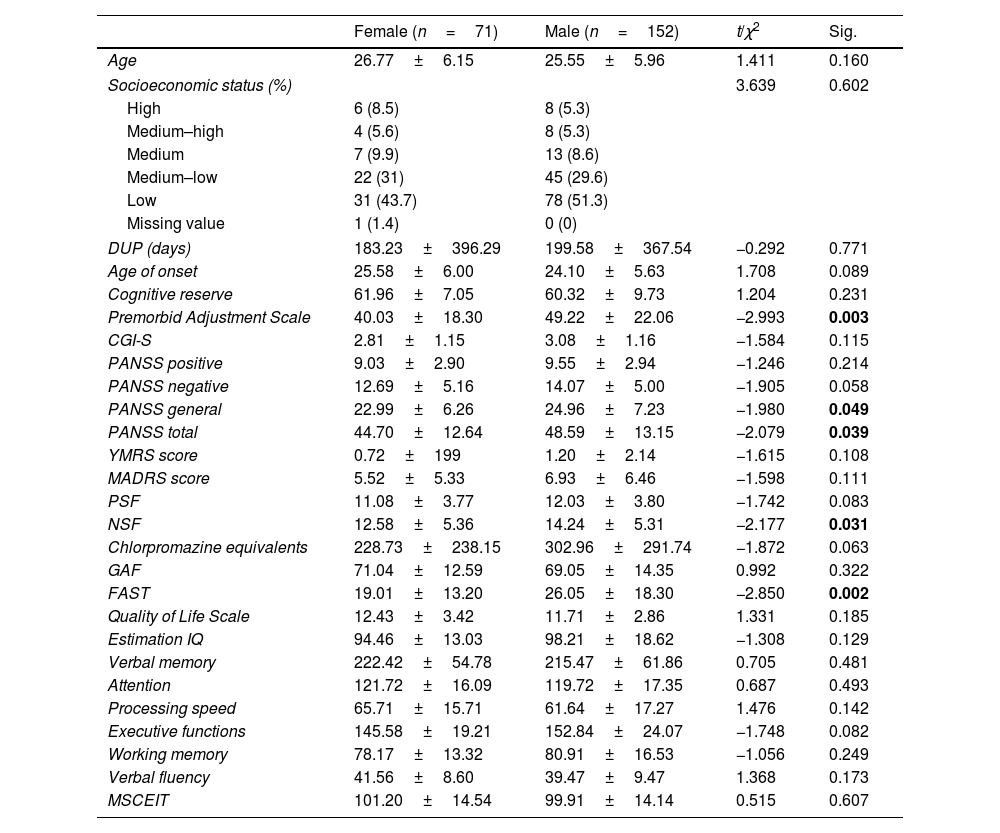
ResultsSociodemographic, clinical, functional and cognitive characteristics of the sample and sex differencesOf the 223 FEP patients participating in the study, 31.8% (n=71) were females and 68.2% (n=152) were males. Mean age of onset was 25.58±6.00 years for female and 24.10±5.63 for male. The mean DUP time was 196.95±375 days (28 weeks approximately). A summary of the sex-stratified sociodemographic, clinical, functional and cognitive characteristics of the sample is shown in Table 1. Females showed a lower severity of general and total symptoms according to the PANSS (p=0.049 and p=0.039), better premorbid adjustment (p=0.003) and greater functionality measured by the FAST scale (p=0.002) but not by GAF (p=0.322). Women also showed fewer negative symptoms than men measured by NSF (p=0.031) while there was only a tendency towards significance in negative symptoms measured by PANSS scale (p=0.058). There were no differences between sex groups in terms of age, SES, age of onset, positive, manic and depressive symptoms, cognitive performance and chlorpromazine equivalents.
Sex differences in sociodemographic, clinical, functional and cognitive characteristics.
| Female (n=71) | Male (n=152) | t/χ2 | Sig. | |
|---|---|---|---|---|
| Age | 26.77±6.15 | 25.55±5.96 | 1.411 | 0.160 |
| Socioeconomic status (%) | 3.639 | 0.602 | ||
| High | 6 (8.5) | 8 (5.3) | ||
| Medium–high | 4 (5.6) | 8 (5.3) | ||
| Medium | 7 (9.9) | 13 (8.6) | ||
| Medium–low | 22 (31) | 45 (29.6) | ||
| Low | 31 (43.7) | 78 (51.3) | ||
| Missing value | 1 (1.4) | 0 (0) | ||
| DUP (days) | 183.23±396.29 | 199.58±367.54 | −0.292 | 0.771 |
| Age of onset | 25.58±6.00 | 24.10±5.63 | 1.708 | 0.089 |
| Cognitive reserve | 61.96±7.05 | 60.32±9.73 | 1.204 | 0.231 |
| Premorbid Adjustment Scale | 40.03±18.30 | 49.22±22.06 | −2.993 | 0.003 |
| CGI-S | 2.81±1.15 | 3.08±1.16 | −1.584 | 0.115 |
| PANSS positive | 9.03±2.90 | 9.55±2.94 | −1.246 | 0.214 |
| PANSS negative | 12.69±5.16 | 14.07±5.00 | −1.905 | 0.058 |
| PANSS general | 22.99±6.26 | 24.96±7.23 | −1.980 | 0.049 |
| PANSS total | 44.70±12.64 | 48.59±13.15 | −2.079 | 0.039 |
| YMRS score | 0.72±199 | 1.20±2.14 | −1.615 | 0.108 |
| MADRS score | 5.52±5.33 | 6.93±6.46 | −1.598 | 0.111 |
| PSF | 11.08±3.77 | 12.03±3.80 | −1.742 | 0.083 |
| NSF | 12.58±5.36 | 14.24±5.31 | −2.177 | 0.031 |
| Chlorpromazine equivalents | 228.73±238.15 | 302.96±291.74 | −1.872 | 0.063 |
| GAF | 71.04±12.59 | 69.05±14.35 | 0.992 | 0.322 |
| FAST | 19.01±13.20 | 26.05±18.30 | −2.850 | 0.002 |
| Quality of Life Scale | 12.43±3.42 | 11.71±2.86 | 1.331 | 0.185 |
| Estimation IQ | 94.46±13.03 | 98.21±18.62 | −1.308 | 0.129 |
| Verbal memory | 222.42±54.78 | 215.47±61.86 | 0.705 | 0.481 |
| Attention | 121.72±16.09 | 119.72±17.35 | 0.687 | 0.493 |
| Processing speed | 65.71±15.71 | 61.64±17.27 | 1.476 | 0.142 |
| Executive functions | 145.58±19.21 | 152.84±24.07 | −1.748 | 0.082 |
| Working memory | 78.17±13.32 | 80.91±16.53 | −1.056 | 0.249 |
| Verbal fluency | 41.56±8.60 | 39.47±9.47 | 1.368 | 0.173 |
| MSCEIT | 101.20±14.54 | 99.91±14.14 | 0.515 | 0.607 |
Abbreviations: M=mean, CGI-S=Clinical Global Impression-Severity, PANSS=Positive and Negative Symptom Scale, YMRS=Young Mania Rating Scale, MADRS=Montgomery-Asberg Depression Rating Scale, PSF=Positive Symptoms Factor of the PANSS, NSF=Negative Symptoms Factor of the PANSS, GAF=Global Assessment of Functioning, FAST=Functioning Assessment Short Test, IQ=Intelligence Quotient, MSCEIT=Mayer–Salovey–Caruso Emotional Intelligence Test. Significant differences (p<0.05) marked in bold.
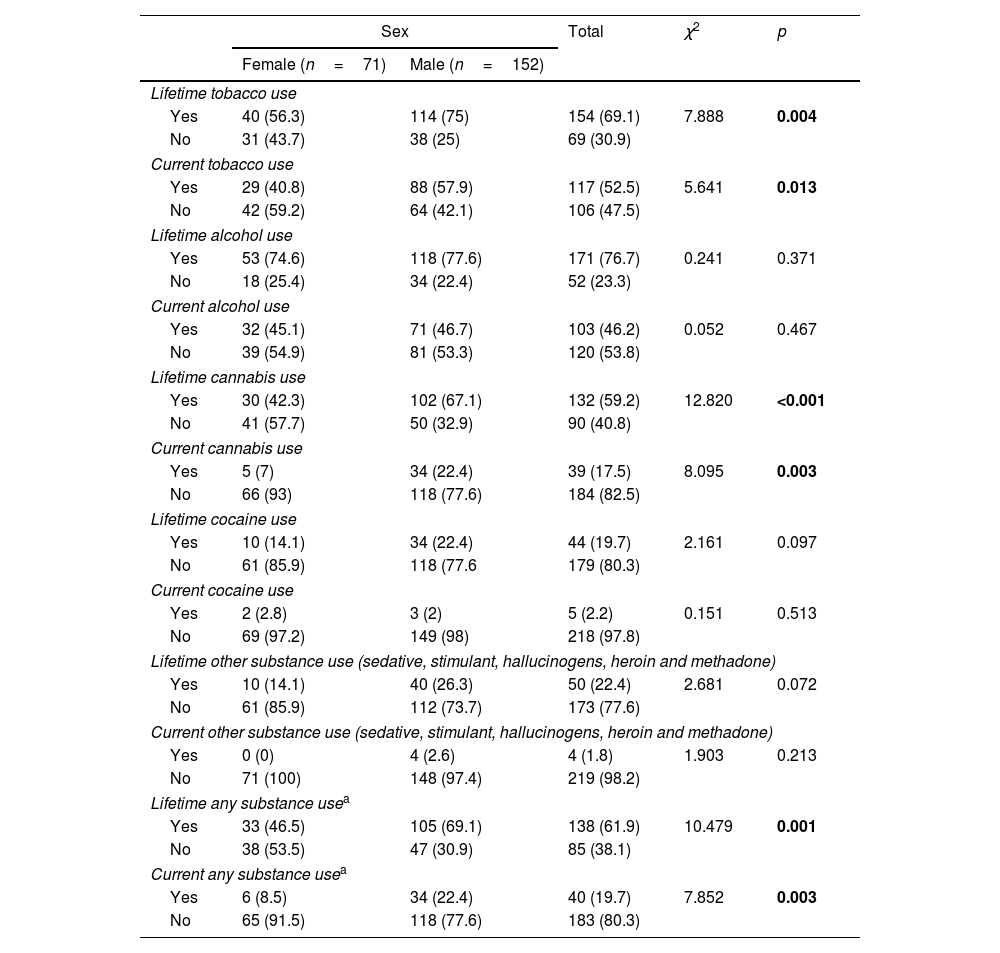
Overall lifetime and current use of any substance was less frequent in females (p=0.001 and p=0.003, respectively). Approximately 41% of female patients reported current tobacco use, 15.5% had quit smoking and 43.7% reported no lifetime tobacco use. A percentage as high as 57.9% of males smoked tobacco when they entered the study, 17.1% were lifetime (but not actual) users and 25% had never smoked tobacco. There were significant differences between female and male patients in current and lifetime tobacco use (p=0.013 and p=0.004). Females also displayed lower rates of current cannabis use (p=0.003). Only 5 females (7%) consumed cannabis at enrolment, compared to 34 men (22.7%). There were also sex differences in lifetime cannabis consumption (p<0.001), with higher rates for males than females. There were no differences in lifetime and current alcohol nor cocaine use (lifetime and current) (see Table 2). Two women (2.8%) and one man (0.7%) reported lifetime sedative use (p=0.240), four women (5.6%) and twenty men (13.2%) reported lifetime stimulant use (p=0.066) and four men current stimulant use (2.6%), whilst four women (5.6%) and sixteen men (10.6%) reported lifetime use of hallucinogens (p=0.171). Only one man reported lifetime heroin use (p=0.680), two men reported lifetime inhalant use (p=0.462) and no one reported methadone use.
Sex differences in substance use.
| Sex | Total | χ2 | p | ||
|---|---|---|---|---|---|
| Female (n=71) | Male (n=152) | ||||
| Lifetime tobacco use | |||||
| Yes | 40 (56.3) | 114 (75) | 154 (69.1) | 7.888 | 0.004 |
| No | 31 (43.7) | 38 (25) | 69 (30.9) | ||
| Current tobacco use | |||||
| Yes | 29 (40.8) | 88 (57.9) | 117 (52.5) | 5.641 | 0.013 |
| No | 42 (59.2) | 64 (42.1) | 106 (47.5) | ||
| Lifetime alcohol use | |||||
| Yes | 53 (74.6) | 118 (77.6) | 171 (76.7) | 0.241 | 0.371 |
| No | 18 (25.4) | 34 (22.4) | 52 (23.3) | ||
| Current alcohol use | |||||
| Yes | 32 (45.1) | 71 (46.7) | 103 (46.2) | 0.052 | 0.467 |
| No | 39 (54.9) | 81 (53.3) | 120 (53.8) | ||
| Lifetime cannabis use | |||||
| Yes | 30 (42.3) | 102 (67.1) | 132 (59.2) | 12.820 | <0.001 |
| No | 41 (57.7) | 50 (32.9) | 90 (40.8) | ||
| Current cannabis use | |||||
| Yes | 5 (7) | 34 (22.4) | 39 (17.5) | 8.095 | 0.003 |
| No | 66 (93) | 118 (77.6) | 184 (82.5) | ||
| Lifetime cocaine use | |||||
| Yes | 10 (14.1) | 34 (22.4) | 44 (19.7) | 2.161 | 0.097 |
| No | 61 (85.9) | 118 (77.6 | 179 (80.3) | ||
| Current cocaine use | |||||
| Yes | 2 (2.8) | 3 (2) | 5 (2.2) | 0.151 | 0.513 |
| No | 69 (97.2) | 149 (98) | 218 (97.8) | ||
| Lifetime other substance use (sedative, stimulant, hallucinogens, heroin and methadone) | |||||
| Yes | 10 (14.1) | 40 (26.3) | 50 (22.4) | 2.681 | 0.072 |
| No | 61 (85.9) | 112 (73.7) | 173 (77.6) | ||
| Current other substance use (sedative, stimulant, hallucinogens, heroin and methadone) | |||||
| Yes | 0 (0) | 4 (2.6) | 4 (1.8) | 1.903 | 0.213 |
| No | 71 (100) | 148 (97.4) | 219 (98.2) | ||
| Lifetime any substance usea | |||||
| Yes | 33 (46.5) | 105 (69.1) | 138 (61.9) | 10.479 | 0.001 |
| No | 38 (53.5) | 47 (30.9) | 85 (38.1) | ||
| Current any substance usea | |||||
| Yes | 6 (8.5) | 34 (22.4) | 40 (19.7) | 7.852 | 0.003 |
| No | 65 (91.5) | 118 (77.6) | 183 (80.3) | ||
Significant differences (p<0.05) marked in bold.
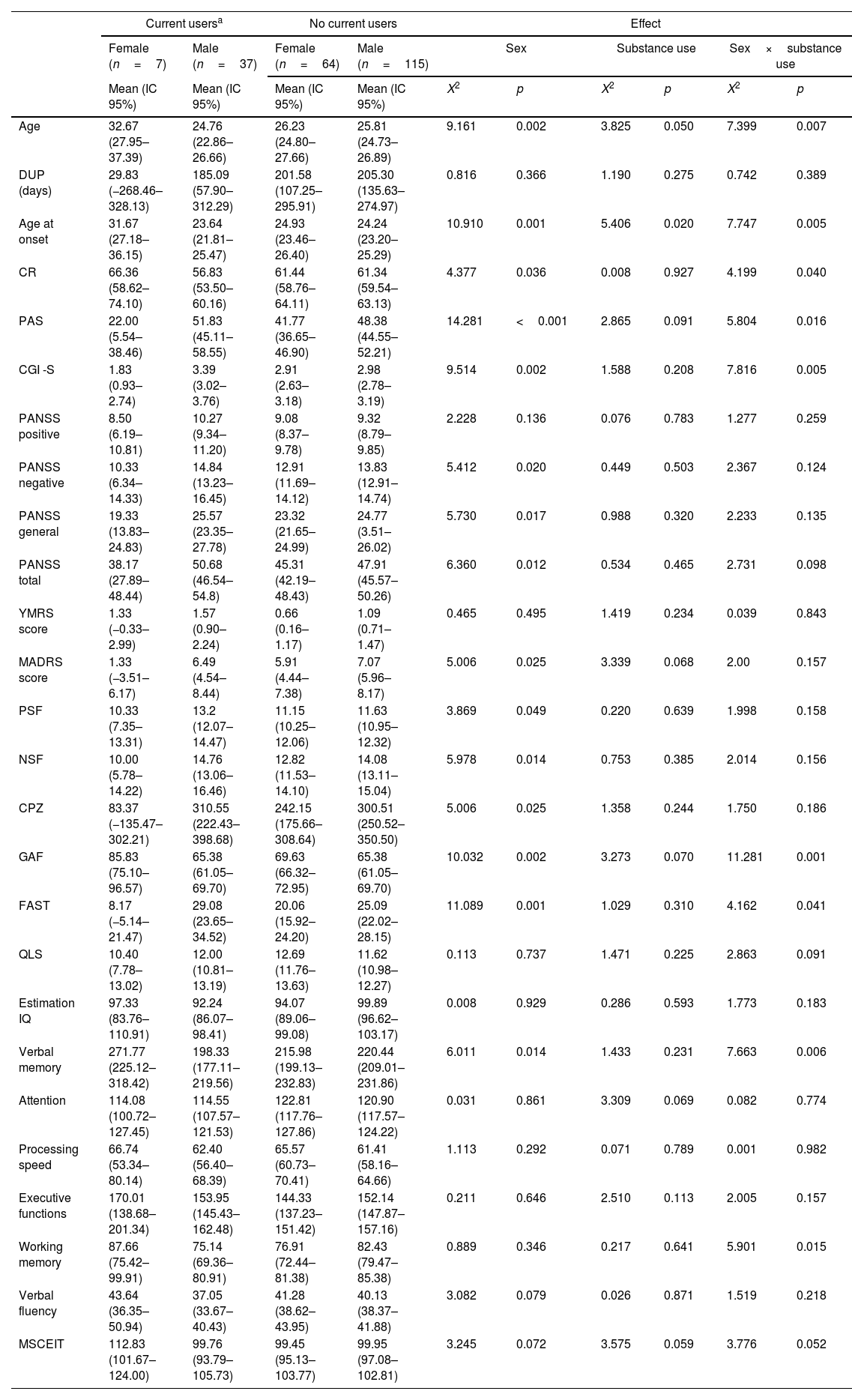
When considering the current users and non-users during the baseline assessment (see Table 3), main effects of sex were found for age (p=0.002), age at onset (p=0.001), cognitive reserve (p=0.036), premorbid adjustment (p<0.001), CGI (p=0.002), negative, general and total PANSS symptoms (p=0.020, p=0.017 and p=0.012), positive and negative symptoms factor of the PANSS (p=0.049 and p=0.014), depressive symptoms (p=0.025), CPZ (p=0.025), GAF (p=0.002), FAST (p=0.001) and verbal memory (p=0.014). Females were older, with an older age of onset, better premorbid adjustment and higher CR. Clinically, females also presented less clinical severity, lower CPZ, better psychosocial functioning and better performance on verbal memory. Regarding the main effects of the substance use group, there were effects for age (p=0.050) and age at onset (p=0.020). Finally, there was a significant substance use group by sex interaction for age (p=0.007), age at onset (p=0.005), cognitive reserve (p=0.040), premorbid adjustment (p=0.016), CGI (p=0.005), GAF (p=0.001), FAST (p=0.041), verbal memory (p=0.006) and working memory (p=0.015). These results remain significant after post-hoc analyses.
Main effects and interactions for the sociodemographic, clinical, functional and neurocognitive variables of current and non-current substance users.
| Current usersa | No current users | Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female (n=7) | Male (n=37) | Female (n=64) | Male (n=115) | Sex | Substance use | Sex×substance use | ||||
| Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | X2 | p | X2 | p | X2 | p | |
| Age | 32.67 (27.95–37.39) | 24.76 (22.86–26.66) | 26.23 (24.80–27.66) | 25.81 (24.73–26.89) | 9.161 | 0.002 | 3.825 | 0.050 | 7.399 | 0.007 |
| DUP (days) | 29.83 (−268.46–328.13) | 185.09 (57.90–312.29) | 201.58 (107.25–295.91) | 205.30 (135.63–274.97) | 0.816 | 0.366 | 1.190 | 0.275 | 0.742 | 0.389 |
| Age at onset | 31.67 (27.18–36.15) | 23.64 (21.81–25.47) | 24.93 (23.46–26.40) | 24.24 (23.20–25.29) | 10.910 | 0.001 | 5.406 | 0.020 | 7.747 | 0.005 |
| CR | 66.36 (58.62–74.10) | 56.83 (53.50–60.16) | 61.44 (58.76–64.11) | 61.34 (59.54–63.13) | 4.377 | 0.036 | 0.008 | 0.927 | 4.199 | 0.040 |
| PAS | 22.00 (5.54–38.46) | 51.83 (45.11–58.55) | 41.77 (36.65–46.90) | 48.38 (44.55–52.21) | 14.281 | <0.001 | 2.865 | 0.091 | 5.804 | 0.016 |
| CGI -S | 1.83 (0.93–2.74) | 3.39 (3.02–3.76) | 2.91 (2.63–3.18) | 2.98 (2.78–3.19) | 9.514 | 0.002 | 1.588 | 0.208 | 7.816 | 0.005 |
| PANSS positive | 8.50 (6.19–10.81) | 10.27 (9.34–11.20) | 9.08 (8.37–9.78) | 9.32 (8.79–9.85) | 2.228 | 0.136 | 0.076 | 0.783 | 1.277 | 0.259 |
| PANSS negative | 10.33 (6.34–14.33) | 14.84 (13.23–16.45) | 12.91 (11.69–14.12) | 13.83 (12.91–14.74) | 5.412 | 0.020 | 0.449 | 0.503 | 2.367 | 0.124 |
| PANSS general | 19.33 (13.83–24.83) | 25.57 (23.35–27.78) | 23.32 (21.65–24.99) | 24.77 (3.51–26.02) | 5.730 | 0.017 | 0.988 | 0.320 | 2.233 | 0.135 |
| PANSS total | 38.17 (27.89–48.44) | 50.68 (46.54–54.8) | 45.31 (42.19–48.43) | 47.91 (45.57–50.26) | 6.360 | 0.012 | 0.534 | 0.465 | 2.731 | 0.098 |
| YMRS score | 1.33 (−0.33–2.99) | 1.57 (0.90–2.24) | 0.66 (0.16–1.17) | 1.09 (0.71–1.47) | 0.465 | 0.495 | 1.419 | 0.234 | 0.039 | 0.843 |
| MADRS score | 1.33 (−3.51–6.17) | 6.49 (4.54–8.44) | 5.91 (4.44–7.38) | 7.07 (5.96–8.17) | 5.006 | 0.025 | 3.339 | 0.068 | 2.00 | 0.157 |
| PSF | 10.33 (7.35–13.31) | 13.2 (12.07–14.47) | 11.15 (10.25–12.06) | 11.63 (10.95–12.32) | 3.869 | 0.049 | 0.220 | 0.639 | 1.998 | 0.158 |
| NSF | 10.00 (5.78–14.22) | 14.76 (13.06–16.46) | 12.82 (11.53–14.10) | 14.08 (13.11–15.04) | 5.978 | 0.014 | 0.753 | 0.385 | 2.014 | 0.156 |
| CPZ | 83.37 (−135.47–302.21) | 310.55 (222.43–398.68) | 242.15 (175.66–308.64) | 300.51 (250.52–350.50) | 5.006 | 0.025 | 1.358 | 0.244 | 1.750 | 0.186 |
| GAF | 85.83 (75.10–96.57) | 65.38 (61.05–69.70) | 69.63 (66.32–72.95) | 65.38 (61.05–69.70) | 10.032 | 0.002 | 3.273 | 0.070 | 11.281 | 0.001 |
| FAST | 8.17 (−5.14–21.47) | 29.08 (23.65–34.52) | 20.06 (15.92–24.20) | 25.09 (22.02–28.15) | 11.089 | 0.001 | 1.029 | 0.310 | 4.162 | 0.041 |
| QLS | 10.40 (7.78–13.02) | 12.00 (10.81–13.19) | 12.69 (11.76–13.63) | 11.62 (10.98–12.27) | 0.113 | 0.737 | 1.471 | 0.225 | 2.863 | 0.091 |
| Estimation IQ | 97.33 (83.76–110.91) | 92.24 (86.07–98.41) | 94.07 (89.06–99.08) | 99.89 (96.62–103.17) | 0.008 | 0.929 | 0.286 | 0.593 | 1.773 | 0.183 |
| Verbal memory | 271.77 (225.12–318.42) | 198.33 (177.11–219.56) | 215.98 (199.13–232.83) | 220.44 (209.01–231.86) | 6.011 | 0.014 | 1.433 | 0.231 | 7.663 | 0.006 |
| Attention | 114.08 (100.72–127.45) | 114.55 (107.57–121.53) | 122.81 (117.76–127.86) | 120.90 (117.57–124.22) | 0.031 | 0.861 | 3.309 | 0.069 | 0.082 | 0.774 |
| Processing speed | 66.74 (53.34–80.14) | 62.40 (56.40–68.39) | 65.57 (60.73–70.41) | 61.41 (58.16–64.66) | 1.113 | 0.292 | 0.071 | 0.789 | 0.001 | 0.982 |
| Executive functions | 170.01 (138.68–201.34) | 153.95 (145.43–162.48) | 144.33 (137.23–151.42) | 152.14 (147.87–157.16) | 0.211 | 0.646 | 2.510 | 0.113 | 2.005 | 0.157 |
| Working memory | 87.66 (75.42–99.91) | 75.14 (69.36–80.91) | 76.91 (72.44–81.38) | 82.43 (79.47–85.38) | 0.889 | 0.346 | 0.217 | 0.641 | 5.901 | 0.015 |
| Verbal fluency | 43.64 (36.35–50.94) | 37.05 (33.67–40.43) | 41.28 (38.62–43.95) | 40.13 (38.37–41.88) | 3.082 | 0.079 | 0.026 | 0.871 | 1.519 | 0.218 |
| MSCEIT | 112.83 (101.67–124.00) | 99.76 (93.79–105.73) | 99.45 (95.13–103.77) | 99.95 (97.08–102.81) | 3.245 | 0.072 | 3.575 | 0.059 | 3.776 | 0.052 |
IC=Lower–Upper values within Wald Confidence Interval of 95%, DUP=Duration of Untreated Psychosis, CR=Cognitive Reserve, PAS=Premorbid Adjustment Scale, CGI-S=Clinical Global Impression-Severity, PANSS=Positive and Negative Symptom Scale, YMRS=Young Mania Rating Scale, MADRS=Montgomery–Asberg Depression Rating Scale, PSF=Positive Symptoms Factor of the PANSS, NSF=Negative Symptoms Factor of the PANSS, CPZ=Chlorpromazine equivalents, GAF=Global Assessment of Functioning, FAST=Functioning Assessment Short Test, QLS=Quality of Life Scale, IQ=Intelligence Quotient, MSCEIT=Mayer–Salovey–Caruso Emotional Intelligence Test. Significant differences (p<0.05) marked in bold.
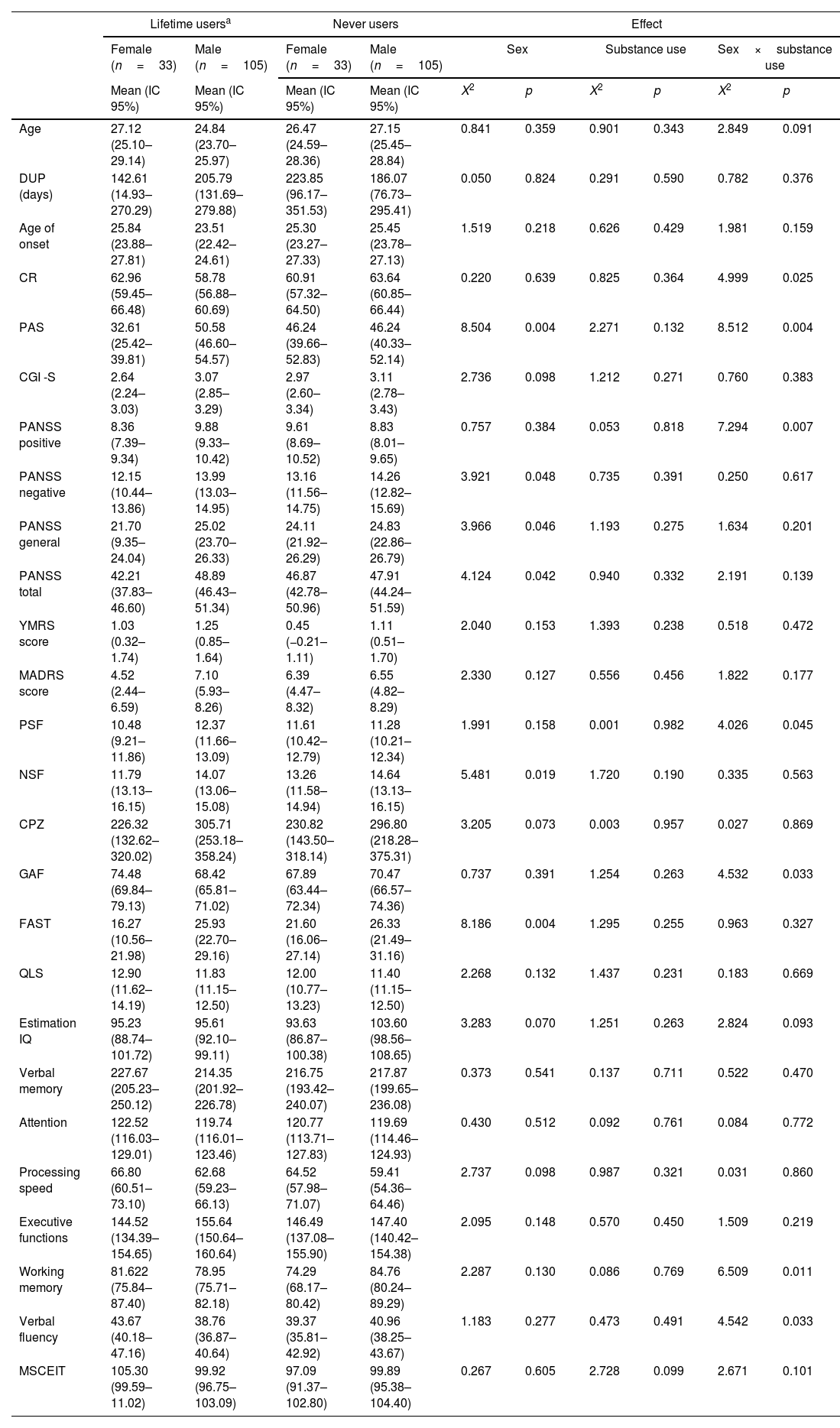
As for the interactions of substance use (those FEP who reported at least one substance other than alcohol and tobacco during lifetime and those never users), there were main effects of sex for premorbid adjustment (p=0.004), negative, general and total PANSS symptoms (p=0.048, p=0.046 and p=0.042), negative symptoms factor of the PANSS (p=0.019) and FAST (p=0.004). There were no significant main effects of substance use group for any variable. Finally, there was a significant substance use group by sex interaction for CR (p=0.025), PAS (p=0.004), positive symptoms (p=0.007 and p=0.045), GAF (p=0.033), working memory (p=0.011) and verbal fluency (p=0.033) (see details in Table 4). These results remain significant after post-hoc analyses.
Main effects and interactions for the sociodemographic, clinical, functional and neurocognitive variables on lifetime and never substance users.
| Lifetime usersa | Never users | Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female (n=33) | Male (n=105) | Female (n=33) | Male (n=105) | Sex | Substance use | Sex×substance use | ||||
| Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | X2 | p | X2 | p | X2 | p | |
| Age | 27.12 (25.10–29.14) | 24.84 (23.70–25.97) | 26.47 (24.59–28.36) | 27.15 (25.45–28.84) | 0.841 | 0.359 | 0.901 | 0.343 | 2.849 | 0.091 |
| DUP (days) | 142.61 (14.93–270.29) | 205.79 (131.69–279.88) | 223.85 (96.17–351.53) | 186.07 (76.73–295.41) | 0.050 | 0.824 | 0.291 | 0.590 | 0.782 | 0.376 |
| Age of onset | 25.84 (23.88–27.81) | 23.51 (22.42–24.61) | 25.30 (23.27–27.33) | 25.45 (23.78–27.13) | 1.519 | 0.218 | 0.626 | 0.429 | 1.981 | 0.159 |
| CR | 62.96 (59.45–66.48) | 58.78 (56.88–60.69) | 60.91 (57.32–64.50) | 63.64 (60.85–66.44) | 0.220 | 0.639 | 0.825 | 0.364 | 4.999 | 0.025 |
| PAS | 32.61 (25.42–39.81) | 50.58 (46.60–54.57) | 46.24 (39.66–52.83) | 46.24 (40.33–52.14) | 8.504 | 0.004 | 2.271 | 0.132 | 8.512 | 0.004 |
| CGI -S | 2.64 (2.24–3.03) | 3.07 (2.85–3.29) | 2.97 (2.60–3.34) | 3.11 (2.78–3.43) | 2.736 | 0.098 | 1.212 | 0.271 | 0.760 | 0.383 |
| PANSS positive | 8.36 (7.39–9.34) | 9.88 (9.33–10.42) | 9.61 (8.69–10.52) | 8.83 (8.01–9.65) | 0.757 | 0.384 | 0.053 | 0.818 | 7.294 | 0.007 |
| PANSS negative | 12.15 (10.44–13.86) | 13.99 (13.03–14.95) | 13.16 (11.56–14.75) | 14.26 (12.82–15.69) | 3.921 | 0.048 | 0.735 | 0.391 | 0.250 | 0.617 |
| PANSS general | 21.70 (9.35–24.04) | 25.02 (23.70–26.33) | 24.11 (21.92–26.29) | 24.83 (22.86–26.79) | 3.966 | 0.046 | 1.193 | 0.275 | 1.634 | 0.201 |
| PANSS total | 42.21 (37.83–46.60) | 48.89 (46.43–51.34) | 46.87 (42.78–50.96) | 47.91 (44.24–51.59) | 4.124 | 0.042 | 0.940 | 0.332 | 2.191 | 0.139 |
| YMRS score | 1.03 (0.32–1.74) | 1.25 (0.85–1.64) | 0.45 (−0.21–1.11) | 1.11 (0.51–1.70) | 2.040 | 0.153 | 1.393 | 0.238 | 0.518 | 0.472 |
| MADRS score | 4.52 (2.44–6.59) | 7.10 (5.93–8.26) | 6.39 (4.47–8.32) | 6.55 (4.82–8.29) | 2.330 | 0.127 | 0.556 | 0.456 | 1.822 | 0.177 |
| PSF | 10.48 (9.21–11.86) | 12.37 (11.66–13.09) | 11.61 (10.42–12.79) | 11.28 (10.21–12.34) | 1.991 | 0.158 | 0.001 | 0.982 | 4.026 | 0.045 |
| NSF | 11.79 (13.13–16.15) | 14.07 (13.06–15.08) | 13.26 (11.58–14.94) | 14.64 (13.13–16.15) | 5.481 | 0.019 | 1.720 | 0.190 | 0.335 | 0.563 |
| CPZ | 226.32 (132.62–320.02) | 305.71 (253.18–358.24) | 230.82 (143.50–318.14) | 296.80 (218.28–375.31) | 3.205 | 0.073 | 0.003 | 0.957 | 0.027 | 0.869 |
| GAF | 74.48 (69.84–79.13) | 68.42 (65.81–71.02) | 67.89 (63.44–72.34) | 70.47 (66.57–74.36) | 0.737 | 0.391 | 1.254 | 0.263 | 4.532 | 0.033 |
| FAST | 16.27 (10.56–21.98) | 25.93 (22.70–29.16) | 21.60 (16.06–27.14) | 26.33 (21.49–31.16) | 8.186 | 0.004 | 1.295 | 0.255 | 0.963 | 0.327 |
| QLS | 12.90 (11.62–14.19) | 11.83 (11.15–12.50) | 12.00 (10.77–13.23) | 11.40 (11.15–12.50) | 2.268 | 0.132 | 1.437 | 0.231 | 0.183 | 0.669 |
| Estimation IQ | 95.23 (88.74–101.72) | 95.61 (92.10–99.11) | 93.63 (86.87–100.38) | 103.60 (98.56–108.65) | 3.283 | 0.070 | 1.251 | 0.263 | 2.824 | 0.093 |
| Verbal memory | 227.67 (205.23–250.12) | 214.35 (201.92–226.78) | 216.75 (193.42–240.07) | 217.87 (199.65–236.08) | 0.373 | 0.541 | 0.137 | 0.711 | 0.522 | 0.470 |
| Attention | 122.52 (116.03–129.01) | 119.74 (116.01–123.46) | 120.77 (113.71–127.83) | 119.69 (114.46–124.93) | 0.430 | 0.512 | 0.092 | 0.761 | 0.084 | 0.772 |
| Processing speed | 66.80 (60.51–73.10) | 62.68 (59.23–66.13) | 64.52 (57.98–71.07) | 59.41 (54.36–64.46) | 2.737 | 0.098 | 0.987 | 0.321 | 0.031 | 0.860 |
| Executive functions | 144.52 (134.39–154.65) | 155.64 (150.64–160.64) | 146.49 (137.08–155.90) | 147.40 (140.42–154.38) | 2.095 | 0.148 | 0.570 | 0.450 | 1.509 | 0.219 |
| Working memory | 81.622 (75.84–87.40) | 78.95 (75.71–82.18) | 74.29 (68.17–80.42) | 84.76 (80.24–89.29) | 2.287 | 0.130 | 0.086 | 0.769 | 6.509 | 0.011 |
| Verbal fluency | 43.67 (40.18–47.16) | 38.76 (36.87–40.64) | 39.37 (35.81–42.92) | 40.96 (38.25–43.67) | 1.183 | 0.277 | 0.473 | 0.491 | 4.542 | 0.033 |
| MSCEIT | 105.30 (99.59–11.02) | 99.92 (96.75–103.09) | 97.09 (91.37–102.80) | 99.89 (95.38–104.40) | 0.267 | 0.605 | 2.728 | 0.099 | 2.671 | 0.101 |
IC=Lower–Upper values within Wald Confidence Interval of 95%, DUP=Duration of Untreated Psychosis, CR=Cognitive Reserve, PAS=Premorbid Adjustment Scale, CGI-S=Clinical Global Impression-Severity, PANSS=Positive and Negative Symptom Scale, YMRS=Young Mania Rating Scale, MADRS=Montgomery–Asberg Depression Rating Scale, PSF=Positive Symptoms Factor of the PANSS, NSF=Negative Symptoms Factor of the PANSS, CPZ=Chlorpromazine equivalents, GAF=Global Assessment of Functioning, FAST=Functioning Assessment Short Test, QLS=Quality of Life Scale, IQ=Intelligence Quotient, MSCEIT=Mayer–Salovey–Caruso Emotional Intelligence Test. Significant differences (p<0.05) marked in bold.
Regarding the number of different substances used, 138 of the patients (61.9%) had used at least one substance other than alcohol and tobacco during their lifetime. Of those 138 individuals, 88 (39.5%) had used only one substance during their lifetime, while 24 people (10.8%) had used two substances and 26 (11.7%) had used more than two substances. Regarding sex differences, 53.5% of females and 30.9% of males reported that they had never consumed any substance and 33.8% of females and 42.4% of males had used only one substance during their lifetime (χ2=15.61, p=0.008).
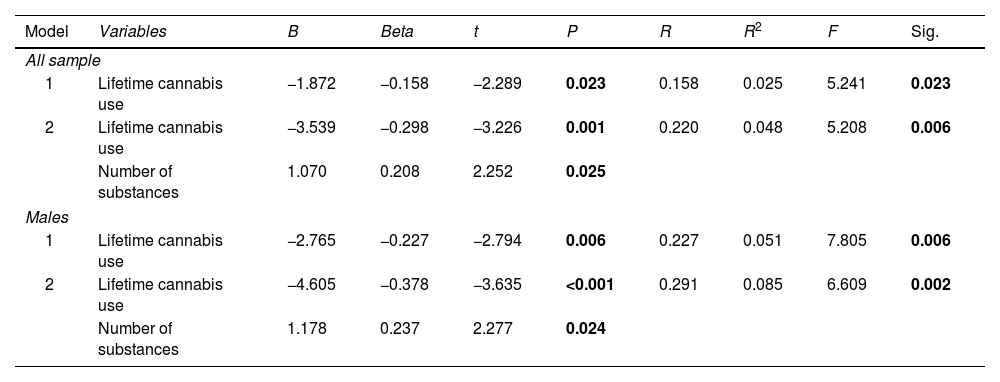
Sex, lifetime use of each substance, and number of substances used were entered into a stepwise regression model to test their ability to predict age at onset of psychosis. Two models were significant: model 1 included only lifetime cannabis use (p=0.023), while model 2 included lifetime cannabis use and the number of substances used (p=0.006) (see Table 5).
Prediction of age at onset of psychosis.
| Model | Variables | B | Beta | t | P | R | R2 | F | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| All sample | |||||||||
| 1 | Lifetime cannabis use | −1.872 | −0.158 | −2.289 | 0.023 | 0.158 | 0.025 | 5.241 | 0.023 |
| 2 | Lifetime cannabis use | −3.539 | −0.298 | −3.226 | 0.001 | 0.220 | 0.048 | 5.208 | 0.006 |
| Number of substances | 1.070 | 0.208 | 2.252 | 0.025 | |||||
| Males | |||||||||
| 1 | Lifetime cannabis use | −2.765 | −0.227 | −2.794 | 0.006 | 0.227 | 0.051 | 7.805 | 0.006 |
| 2 | Lifetime cannabis use | −4.605 | −0.378 | −3.635 | <0.001 | 0.291 | 0.085 | 6.609 | 0.002 |
| Number of substances | 1.178 | 0.237 | 2.277 | 0.024 | |||||
Significant differences (p<0.05) marked in bold.
Later, separated sex models were run with the same covariates. On them, only models applied to males were significant (p=0.006 for model 1 and p=0.002 for model 2). In this case, we observed similar results to those found when considering the overall sample.
DiscussionThree findings emerged from the present study. Firstly, female FEPs showed fewer negative symptoms, a better premorbid adjustment, better psychosocial functioning and verbal fluency performance than male FEPs. Secondly, overall lifetime and current substance use was less frequent for females. When analyzing the potential interaction between group (current vs non-current users) and sex (male and female), we found an interaction in age, age at onset, CR, premorbid adjustment, CGI-S, functioning, verbal memory and working memory. And thirdly, using several substances, but especially cannabis, were the variables that most significantly contributed to an earlier age at onset of psychosis, only for males.
As expected, females showed a lower severity of negative, general and total symptoms, better premorbid adjustment and greater functionality.1–3 No significant differences were found regarding neuropsychological performance.4 Differences in functioning were only observed when it was measured by FAST scale but not by GAF. This is probably due to the fact that GAF scores primarily reflect symptom severity whereas the FAST scale is designed to assess the main functioning problems experienced by psychiatric patients.43 A similar explanation would be given in the case of negative symptoms, males showed more negative symptoms than women measured by NSF (p=0.031) but there was only a tendency towards significance when measured by PANSS scale (p=0.058). Although PANSS is a widely-used instrument for measuring symptomatology in patients with schizophrenia, it seems that NSF has several aspects of improved content validity in comparison to the original negative PANSS subscale.37 Factor analytic studies in PANSS found that two items (Difficulty in abstract thinking (N5) and stereotyped thinking (N7)) should no longer be considered part of the negative symptom domain.57–60
Overall, not unexpectedly, lifetime and current substance use was less frequent for females.9,12,13 When analyzing those patients who reported being current users and never users, in line with previous literature we found that females were older,61 with a delayed age of onset,62,63 better premorbid adjustment2 and higher CR, lower clinical symptoms and better functional outcomes3 and verbal memory performance than males.64 Whereas in the lifetime and never users groups there were fewer sex differences, females showed better premorbid adjustment, lower negative, general and total symptoms and better psychosocial functioning than males, without differences in age, CR or neurocognitive performance. A possible explanation of these gender differences is the “estrogen hypothesis” which postulates that estrogen raise the vulnerability threshold for the outbreak of the illness and plays a protective role against schizophrenia and severity of the illness.65–67 This could explain the fact that women often have a later age of onset68–69 and lower negative symptoms.
There were significant substance use group by sex interaction for CR, premorbid adjustment, positive symptoms, functioning, working memory and verbal fluency. Thus, it seems that sex is more relevant than substance use in explaining the clinical heterogeneity of non-affective FEP patients. However, the interaction is an important element, especially for CR, premorbid adjustment, functioning and neurocognitive performance. The small sample size of the FEP who have never consumed any substance hampers the generalizability of the findings, thus further research should be conducted to validate them.
Regarding the prediction of age at onset of psychosis, our results suggest that using several substances but especially cannabis, were the variables that most significantly contributed to an earlier age at onset of psychosis, but only for males. This result is in accordance with a previous study of 114 FEPs which found that being male and using several substances were the variables that contributed to an earlier age at onset.11,70 The literature suggests that substance use is associated with an earlier onset of psychosis,71 especially in those male cannabis users.11,62 It is well-known that cannabis is a risk factor for psychosis.41 Since it has also been related to an earlier age of onset, which in turn is associated with a worse prognosis, reducing substance use should be prioritized as the target of preventive strategies, especially for the male population. The implementation of early intervention programmes on substance use could be beneficial in preventing or reducing the impact of illness. The implementation of preventive drug education programmes in schools would be recommended as a primary preventive strategy.
This study has certain limitations, which must be taken into account. Firstly, substance use was self-reported by participants, without using an objective measure such as a urine drug screen. Secondly, no specific scale was used to assess negative symptomatology, due to constraints associated with the PANSS scale. Future studies making use of improved negative symptom scales—such as the Brief Negative Symptom Scale (BNSS)72 or the Clinical Assessment Interview for Negative Symptoms (CAINS)73—might differentiate between primary and secondary negative symptoms,74 which is an unmet need of current research on FEP. However, it is a naturalistic and multicentric study with a representative sample of non-affective FEP in a stable clinical phase in Spain. Furthermore, there is a considerable number of females, representing more than one quarter of the sample.
In conclusion, clinical presentation of FEP varies by sex and especially when considering substance use. Our results suggest that early interventions need to be tailored to the different clinical needs of males and females and according to substance consumption in first episode psychosis, or even earlier on a population level by improving preventive educational interventions during youth and adolescence.
Data availability statementThe data that support the findings of this study are available on request from the corresponding authors.
Conflict of interestM. Garriga has received research support from or served as consultant, adviser or speaker for Ferrer, Janssen, Lundbeck, and the Spanish Ministry of Science and Innovation (CIBERSAM).
E. Vieta has received research support from or served as consultant, adviser or speaker for AB-Biotics, Actavis, Allergan, Angelini, AstraZeneca, Bristol-Myers Suibb, Dainippon Sumitomo Pharma, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Telefónica, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute.
M. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of AB-Biotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Roviand Takeda.
R. Rodriguez-Jimenez has been a consultant for, spoken in activities of, or received grants from: Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid Regional Government (S2010/BMD-2422 AGES, S2017/BMD-3740), JanssenCilag, Lundbeck, Otsuka, Pfizer, Ferrer, Juste, Takeda, Exeltis, Casen-Recordati, Angelini.
D. Berge has received financial support to attend meetings, travel support and serve as a speaker for Otsuka and Janssen Cilag.
A. Ibáñez has received research support from or served as speaker or advisor for Janssen-Cilag, Lundbeck and Otsuka.
C. De-la-Camara received financial support to attend scientific meetings from Janssen, Almirall, Lilly, Lundbeck, Rovi, Esteve, Novartis, Astrazeneca and Pfizer.
J. Saiz-Ruiz has been as speaker for and on the advisory boards of Adamed, Lundbeck, Servier, Medtronic, Casen Recordati, Neurofarmagen, Otsuka, Indivior, Lilly, Schwabe, Janssen and Pfizer, outside the submitted work.
The rest of authors report no biomedical financial interests or potential conflicts of interest.
FundingThis study is part of a coordinated-multicenter Project, funded by the Ministerio de Economía y Competitividad (PI08/0208, PI11/00325, PI14/00612), Instituto de Salud Carlos III – Fondo Europeo de Desarrollo Regional, Unión Europea. Una manera de hacer Europa, Centro de Investigación Biomédica en Red de salud Mental, CIBERSAM, by the CERCA Programme/Generalitat de Catalunya AND Secretaria d’Universitats i Recerca del Departament d’Economia I Coneixement (2017SGR1355). Departament de Salut de la Generalitat de Catalunya, en la convocatòria corresponent a l’any 2017 de concessió de subvencions del Pla Estratègic de Recerca i Innovació en Salut (PERIS) 2016-2020, modalitat Projectes de recerca orientats a l’atenció primària, amb el codi d’expedient SLT006/17/00345. M. Bernardo is also grateful for the support of the Institut de Neurociències, Universitat de Barcelona.
S. Amoretti has been supported by a Sara Borrell contract (CD20/00177), funded by Instituto de Salud Carlos III (ISCIII) and co-funded by European Social Fund “Investing in your future”.
The study has been supported by a BITRECS project conceded to N. Verdolini. BITRECS project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754550 and from “La Caixa” Foundation.
E. Vieta thanks the support of the Spanish Ministry of Science, Innovation and Universities (PI15/00283) integrated into the Plan Nacional de I+D+I y cofinanciado por el ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER), CIBERSAM, and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365) and the project SLT006/17/00357, from PERIS 2016-2020 (Departament de Salut). CERCA Programme/Generalitat de Catalunya.
A. Ibánez thanks the support by the Madrid Regional Government (R&D Activities in Biomedicine S2017/BMD3740: AGES-CM 2-CM) and European Union Structural Funds, the support by CIBERSAM and by the Spanish Ministry of Science, Innovation and Universities (PI11/02252, PI16/00834 and PI19/01295), co-financed by ERDF Funds from the European Commission, “A way of making Europe”.
We also would like to thank the authors of the PEPs group who participated in the development of this manuscript, namely, G. Mezquida3, M. Serra2, L. Pina-Camacho7, J. Merchán-Naranjo7, I. Corripio11, A. Alonso-Solís11, I. González-Ortega8,18, I. Zorrilla8, L. Martínez-Saduriní9, A. Trabsa9, L. Sanchez-Pastor10, O. Jiménez-Rodríguez10, E. Pomarol-Clotet12, M.A. García-León12, A. Butjosa14, M. Pardo14, A. Sánchez-Torres5,6, L. Moreno-Izco5,6, J. Saiz-Ruiz13, L. León-Quismondo13, M.J. Escartí Fabra19, F. Contreras20, C. De-la-Cámara21, A. Zabala15, P. Portilla22.
PEPs Group additional affiliations:
18 The National Distance Education University (UNED), Vitoria, Spain.
19 Department of Psychiatry, Hospital Clínico Universitario de Valencia, CIBERSAM, Biomedical Research Institute INCLIVA, Valencia, Spain.
20 Bellvitge Biomedical Research Institute IDIBELL, Department of Psychiatry, Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain.
21 Department of Medicine and Psychiatry. Universidad de Zaragoza, Hospital Clinico Universitario and Instituto de Investigación Sanitaria (IIS) Aragón, CIBERSAM, Zaragoza, Spain.
22 Psychiatry Department, Medical Center of Asturias, Oviedo, Spain.
We are extremely grateful to all participants.