The PEPs study is multicenter, naturalistic, prospective, longitudinal study designed to evaluate clinical, neuropsychological, neuroimaging, biochemical, environmental and pharmacogenetic variables in a sample of nearly 350 first episode of psychosis patients and 250 healthy controls. The PEPs project was conducted in Spain from January 2009 to December 2011.
This article describes the rationale for the measurement approach adopted, providing an overview of the selected clinical and functional measures. The main objectives are: (a) the thorough clinical and neurocognitive characterization of a sample of first episodes of psychosis and (b) the study of the interactions between the genetic and environmental variables selected to predict clinical and brain structural outcomes, and to determine the relationship of genetic polymorphisms involved in the pharmacokinetics and pharmacodynamics, and the responses and adverse effects of treatment.
PEPs es un estudio multicéntrico, naturalístico, prospectivo y longitudinal diseñado para evaluar las variables clínicas, neuropsicológicas, de neuroimagen, bioquímicas, ambientales y genéticas en una muestra de casi 350 pacientes con un primer episodio psicótico y 250 controles sanos. El proyecto PEPs ha sido realizado en España desde Enero de 2009 hasta Diciembre de 2011.
En este artículo se describe la justificación de los métodos de evaluación adoptados, proporcionando una breve descripción de las medidas clínicas y funcionales seleccionadas. Los objetivos principales son: a) el examen clínico y la caracterización neuropsicológica de una muestra de primeros episodios de psicosis y b) el estudio de las interacciones entre las variables genéticas y ambientales seleccionadas para predecir los resultados clínicos y de estructura cerebral y determinar la relación de polimorfismos genéticos implicados en la farmacocinética y la farmacodinámica, y la respuesta en los efectos adversos del tratamiento.
A psychotic episode is characterized by the presence of positive (delusions, hallucinations and strange conduct) and frequently the presence of negative (e.g. apathy and alogy), cognitive and affective symptoms.
Around 3% of the general population suffers a psychotic episode along its life.1 The first episode of psychosis (FEP) is usually somewhere between 15 and 30 years. In these ages academic, professional and social skills are in their major expansion. Males show an increased earlier emergence age. The early onset is associated with a higher genetic load, severe cognitive deterioration and worse evolution and prognosis.2–5
The clinical evolution after a FEP tends to be chronic and variable, causing a huge loss in quality of life of patients and their families, in their physical health, and a high cost to society, representing 11.2% of the global burden of brain disorders in Europe.6 Complete remission only occurs in one-third of the patients.7 Up to 80% of the patients relapse during the next five years after a FEP, with a major risk to become resistant to treatment.8
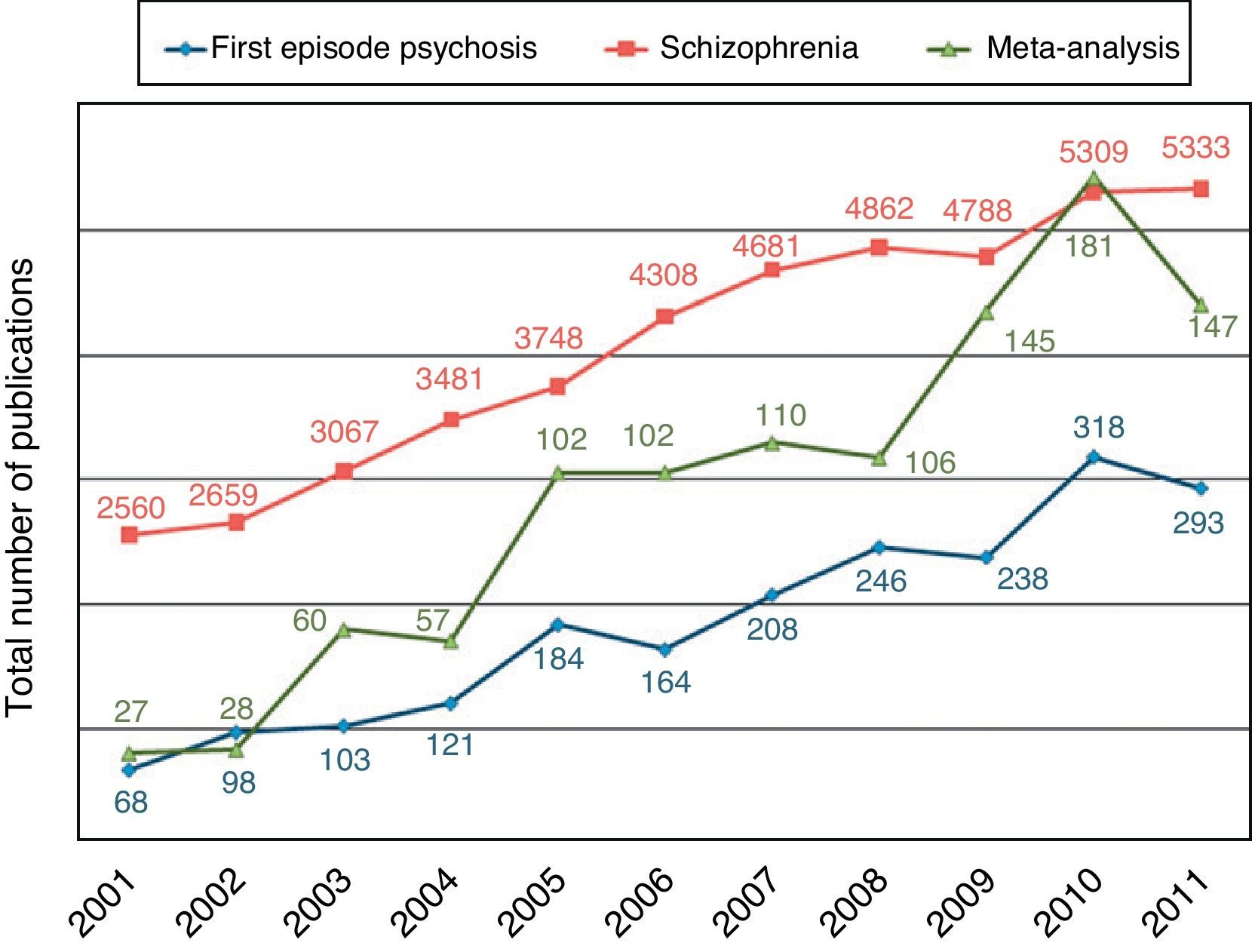
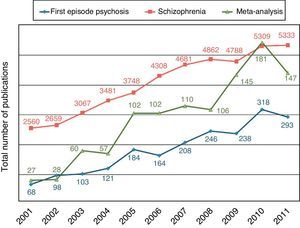
Although the population with chronic schizophrenia has been studied in large naturalistic studies with real-life patients,9 the FEP population represents a unique opportunity to evaluate in a sharper way the clinical variables and the functional outcomes of psychotic disorders. The characterization of the population of FEP has become a priority area for recent research of growing interest, having conducted large studies in the United States,10 Europe11–13 and also in Spain14–16 with this subpopulation. In fact, the number of publications about FEP, schizophrenia and meta-analyzes on schizophrenia has maintained an ascending line in the last decade (Fig. 1).
Moreover, some monographic journals about this subject have appeared recently,17 there is a nosological debate about their representation in the next edition of the DSM-V,18,19 and have greatly increased the economic investment of health systems to create specific assistance programs for this population.20
The conduct of longitudinal research in the onset of illness is especially significant because it avoids the effect of confounding variables such as the influence of antipsychotic treatment or chronicity.11 Such variables cause long-term structural changes and may be one reason for the inconsistency of the findings so far.15 Patients with a first psychotic episode (FEP) are therefore an excellent group to study the risk factors linked to the development of schizophrenia and other psychotic disorders related to neural stress processes.
General design of the studies with FEP presents certain challenges that must be taken into account when designing new studies. The samples are usually heterogeneous, they often do not include children and adolescents, the assessment instruments are different among various studies and usually exclude cases with comorbid substance use or risk of suicide, both common conditions in FEP.11,15 That is why realistic studies are needed to represent the entire population of FEP.
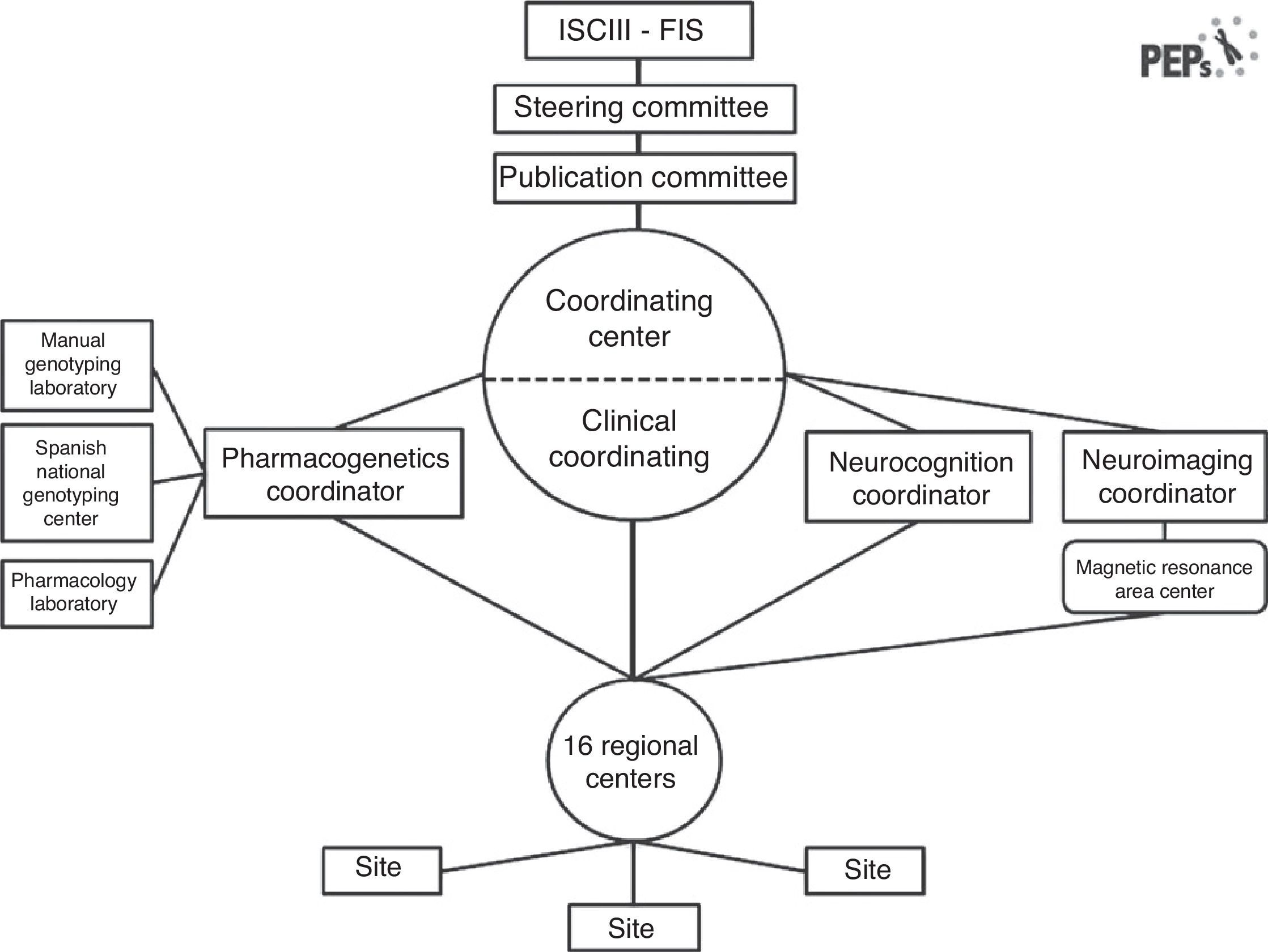
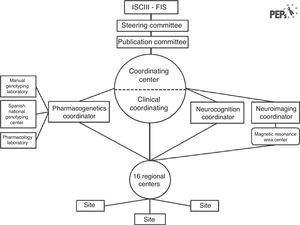
The “Phenotype–genotype and environmental interaction. Application of a predictive model in first psychotic episodes.” (PEPs study, from the Spanish abbreviator) is a multicenter, prospective, longitudinal, naturalistic, follow-up study, designed to evaluate clinical, neuropsychological, neuroimaging, biochemical and genetic variables in a sample of 350 first psychotic episode patients in Spain, matched with healthy controls by age, sex and socio-economic status. This project is funded by the Spanish public health system by means of the Public National Agency (FIS). Patients have been recruited from sixteen centers located throughout the Spanish territory with experience in performing and assessing diagnoses, and in evaluation and treatment with semi-structured interviews and clinical scales. This study is a joint effort in the emerging collaborative research in Spain,21 and a suitable example of the synergy from cross-discipline approaches in mental health.22 Fourteen of these teams are members of the Centro de Investigaciones Biomédicas en Red en Salud Mental (CIBERSAM), a Spanish network of translational research in neuroscientific aspects related to health and mental illness (www.cibersam.es). The other two hospitals are collaborator centers. Its aim is to assess clinical characteristics, functional prognostic factors, diagnostic specificities of findings, and pathophysiological changes in the brain during the first 2 years after the psychotic episode through an integrative and translational approach. The study organization is illustrated in Fig. 2.
The research of recent decades demonstrates the heterogeneity of the etiology of psychotic disorders, where both genetic and environmental factors play a key role. Genetics play a big role in psychotic disorders, as evidenced by the existence of families with multiple affected individuals or monozygotic twin studies.23 The heritability of the disorder is estimated around 80%,24 with higher prevalence of pathology at a higher genetic load shared with the affected relative.25,26 However, these studies do not evaluate the contribution of the interaction between genes and environment.27–30
As in other complex disorders, common genetic variants tend to have low penetrance and a moderate effect on risk, while rare genetic variants have a major effect to explain a lower proportion of cases.31,32 Moreover, the associations obtained in these studies are more related to disease mechanisms, evolution and classification than to the risk to develop the disorder.33
In recent years the classical concept of the psychotic illness has been reformulated,34,35 being seen as an heterogeneous disorder with a multisystemic impact from the beginning, in addition to its psychiatric expression.36–42 In newly diagnosed patients with non-affective psychosis have been described a considerable number of cardiometabolic abnormalities before the start of the antipsychotic medication, comprising a shortened life expectancy. Between these findings there are abnormal glucose tolerance and diabetes,36–42 telomere shortening and increased pulse pressure,43 metabolic syndrome,44–47 increased visceral fat,43,48 some angiotensin converting enzyme gene polymorphisms,49 increased rate of sudden death with a cardiac origin50,51 as well accelerated aging processes.52
The literature has detected as the main environmental risk conditions for psychosis prenatal stress, high paternal age, malnutrition, infections during pregnancy, perinatal hypoxia, presence of traumatic events, urbanicity, poverty, being membership of a minority ethnic group and cannabis use.53,54 In the case of cannabis, increased risk for psychosis is associated with the environmental exposure before adulthood, suggesting an interaction with development.55 Following the concept of sensibilization,56 there is evidence that the exposure to certain environmental factors interacting with genetic factors can alter dopaminergic transmission, neuroendocrine and cognitive functioning, patterns of interpersonal interaction and processing affective, and may therefore lead to an increased worsening of psychopathology.56–59
Unlike other previous studies with a more trimmed approach, and given the diversity of symptom presentations and global functioning associated with psychotic disorders, one of the key challenges facing the PEPs study was the selection of efficient assessment measures appropriate to this longitudinal, naturalistic, follow-up study.
A study like this, performed during real conditions of treatment, should employ measures of high relevance and fast to be administered in clinical practice. Moreover, the PEPs study also offers an opportunity to test the efficiency of this assessment measures and potentially introduce new assessments that might represent novel assessment domains and methods. The main objective of this assessment is the accurate characterization of this sample of first episode psychosis, in order to optimize the evolution monitoring, the therapeutic tools and the functional prognostic.
The breadth and level of detail of the study assessments were reconsidered according to the time required to collect the information from each subject, as well as the importance of domains to the general and ambient interaction hypotheses, the reliability of the instrument, and the frequency with which the instrument has been previously used in clinical and community studies of psychotic disorders.
Obviously, the costs of conducting a study are in large part directly related to the number and frequency of study assessments. Being a public funding project, this aspect was specially taken into account.
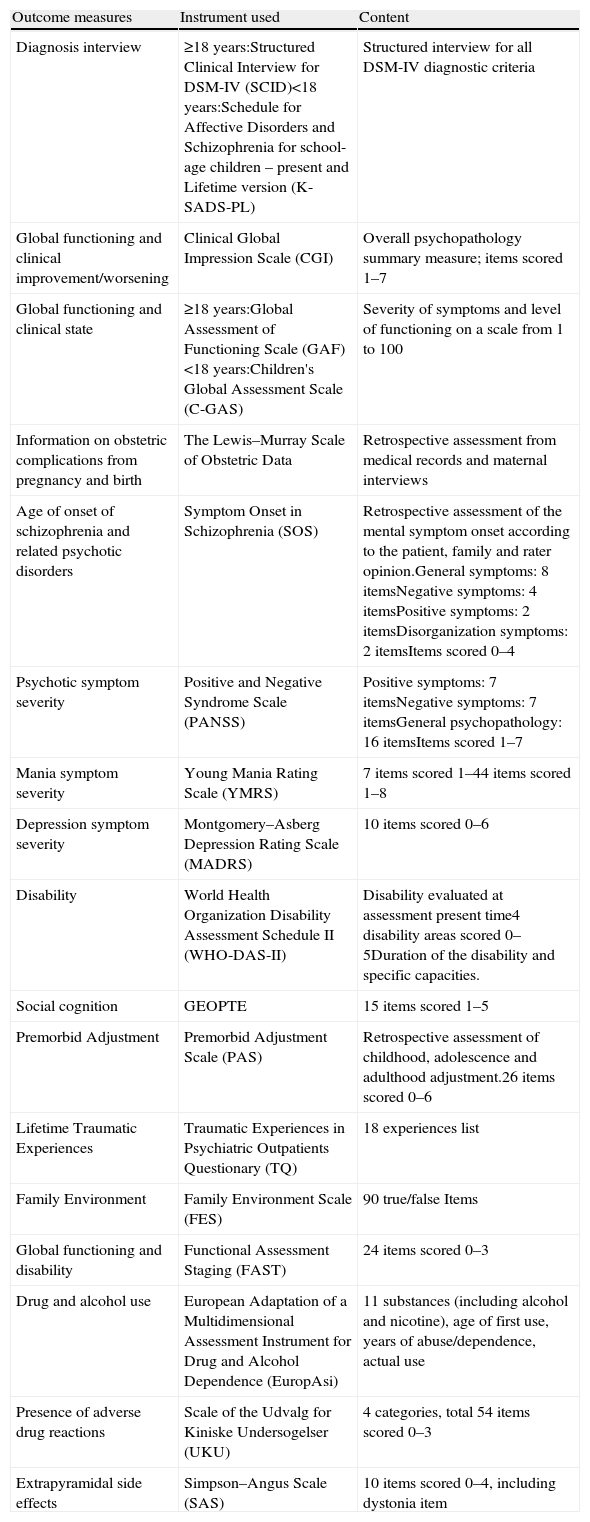
This article will describe the PEPs study's measures of assessment, which are listed in Table 1 and discussed in detail below.
PEPs study clinical and functional outcome measures.
| Outcome measures | Instrument used | Content |
| Diagnosis interview | ≥18 years:Structured Clinical Interview for DSM-IV (SCID)<18 years:Schedule for Affective Disorders and Schizophrenia for school-age children – present and Lifetime version (K-SADS-PL) | Structured interview for all DSM-IV diagnostic criteria |
| Global functioning and clinical improvement/worsening | Clinical Global Impression Scale (CGI) | Overall psychopathology summary measure; items scored 1–7 |
| Global functioning and clinical state | ≥18 years:Global Assessment of Functioning Scale (GAF)<18 years:Children's Global Assessment Scale (C-GAS) | Severity of symptoms and level of functioning on a scale from 1 to 100 |
| Information on obstetric complications from pregnancy and birth | The Lewis–Murray Scale of Obstetric Data | Retrospective assessment from medical records and maternal interviews |
| Age of onset of schizophrenia and related psychotic disorders | Symptom Onset in Schizophrenia (SOS) | Retrospective assessment of the mental symptom onset according to the patient, family and rater opinion.General symptoms: 8 itemsNegative symptoms: 4 itemsPositive symptoms: 2 itemsDisorganization symptoms: 2 itemsItems scored 0–4 |
| Psychotic symptom severity | Positive and Negative Syndrome Scale (PANSS) | Positive symptoms: 7 itemsNegative symptoms: 7 itemsGeneral psychopathology: 16 itemsItems scored 1–7 |
| Mania symptom severity | Young Mania Rating Scale (YMRS) | 7 items scored 1–44 items scored 1–8 |
| Depression symptom severity | Montgomery–Asberg Depression Rating Scale (MADRS) | 10 items scored 0–6 |
| Disability | World Health Organization Disability Assessment Schedule II (WHO-DAS-II) | Disability evaluated at assessment present time4 disability areas scored 0–5Duration of the disability and specific capacities. |
| Social cognition | GEOPTE | 15 items scored 1–5 |
| Premorbid Adjustment | Premorbid Adjustment Scale (PAS) | Retrospective assessment of childhood, adolescence and adulthood adjustment.26 items scored 0–6 |
| Lifetime Traumatic Experiences | Traumatic Experiences in Psychiatric Outpatients Questionary (TQ) | 18 experiences list |
| Family Environment | Family Environment Scale (FES) | 90 true/false Items |
| Global functioning and disability | Functional Assessment Staging (FAST) | 24 items scored 0–3 |
| Drug and alcohol use | European Adaptation of a Multidimensional Assessment Instrument for Drug and Alcohol Dependence (EuropAsi) | 11 substances (including alcohol and nicotine), age of first use, years of abuse/dependence, actual use |
| Presence of adverse drug reactions | Scale of the Udvalg for Kiniske Undersogelser (UKU) | 4 categories, total 54 items scored 0–3 |
| Extrapyramidal side effects | Simpson–Angus Scale (SAS) | 10 items scored 0–4, including dystonia item |
The sixteen centers participating in the PEPs project prospectively recruited patients from each center and included those with a diagnosis of a first psychotic episode and matched healthy control subjects from April 2009 to April 2011.
Patients who met the inclusion criteria that were attended at these facilities during the recruitment period were invited to participate in the study.
The inclusion criteria for patients were: age between 7 and 35 years at the time of first evaluation, presence of psychotic symptoms of less than 12 months’ duration and speak Spanish correctly and sign the informed consent.
The exclusion criteria for patients were: mental retardation according to DSM-IV60 criteria (including not only an IQ below 70 but also impaired functioning), history of head trauma with loss of consciousness and organic disease with mental repercussions.
The patients matched with healthy controls by age (±10%), sex and parental socio-economic status (SES), measured by the Hollingshead–Redlich scale (±1 level). Controls had to speak Spanish correctly and sign the informed consent.
The exclusion criteria for controls were: presence of past or present psychotic disorder, major depression disorder or mental retardation (including not only an IQ below 70 but also impaired functioning) according to DSM-IV criteria,60 head trauma with loss of consciousness, organic disease with mental repercussions and having a first degree relative with psychotic disorder history.
The study was approved by the investigation ethics committees of all participating clinical centers. Informed consent was obtained from all participants. In case of children under 16 years of age, parents or legal guardians gave written informed consent before the beginning of their participation in the study, and patients assented to participate. The genetic part had a specific informed consent form. When requested, participants in the study were given a report on the results of the tests.
Since this was a naturalistic study, there were no guidelines for the treatment administered (drugs and psychotherapy).
Clinical outcome measuresMeasure selection began by identifying the measurement domains with the most relevance to a general and ambient interaction study. The main investigators, reducing areas of overlap and duplication, proposed available measures for the selected domains. After deciding the main evaluation instruments, the protocol assessment measures were then broadly discussed with external consultants and with the center investigators. These discussions led to recommendations for refined measurement and further reduction of unnecessary and redundant measures.
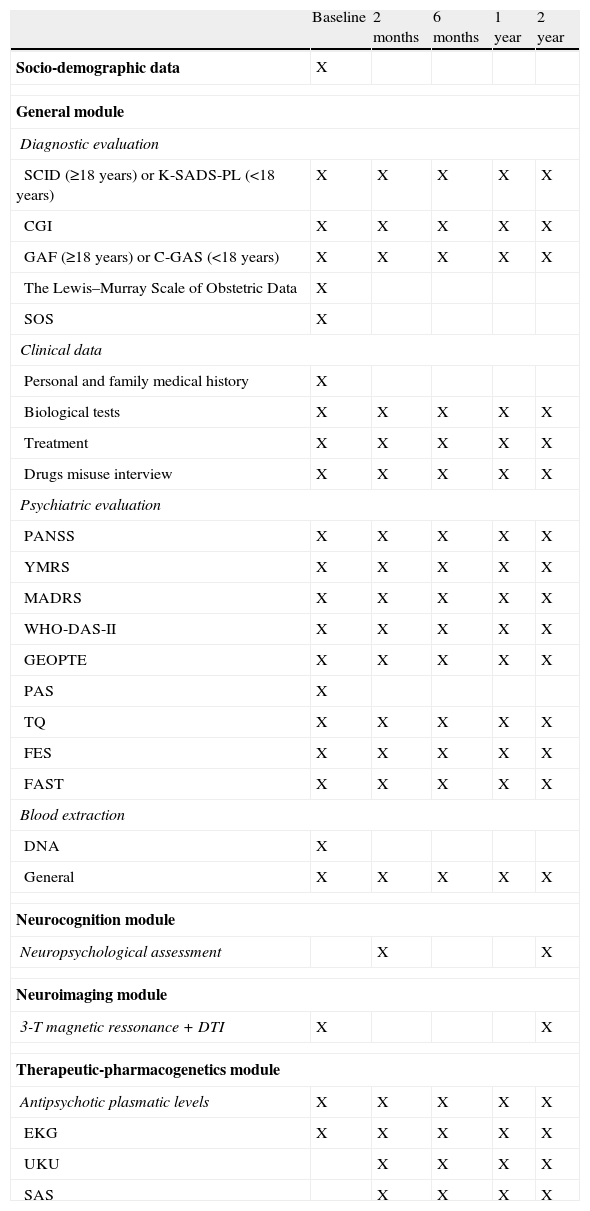
General moduleAll groups participated in this module. At baseline, a complete evaluation (structured interview, clinical scales, family environment, prognostic and premorbid adjustment scales, genetic and analytic) was performed (Table 1). All scales (except those self-administered) were administered by expert clinicians. The pharmacological treatment and Adverse Drug Reactions (ADRs) were also recorded as it will be described later. Clinical, functional and disability scales were administered again at 2, 6, 12 and 24 months (Table 2).
Assessment frequency and timing.
| Baseline | 2 months | 6 months | 1 year | 2 year | |
| Socio-demographic data | X | ||||
| General module | |||||
| Diagnostic evaluation | |||||
| SCID (≥18 years) or K-SADS-PL (<18 years) | X | X | X | X | X |
| CGI | X | X | X | X | X |
| GAF (≥18 years) or C-GAS (<18 years) | X | X | X | X | X |
| The Lewis–Murray Scale of Obstetric Data | X | ||||
| SOS | X | ||||
| Clinical data | |||||
| Personal and family medical history | X | ||||
| Biological tests | X | X | X | X | X |
| Treatment | X | X | X | X | X |
| Drugs misuse interview | X | X | X | X | X |
| Psychiatric evaluation | |||||
| PANSS | X | X | X | X | X |
| YMRS | X | X | X | X | X |
| MADRS | X | X | X | X | X |
| WHO-DAS-II | X | X | X | X | X |
| GEOPTE | X | X | X | X | X |
| PAS | X | ||||
| TQ | X | X | X | X | X |
| FES | X | X | X | X | X |
| FAST | X | X | X | X | X |
| Blood extraction | |||||
| DNA | X | ||||
| General | X | X | X | X | X |
| Neurocognition module | |||||
| Neuropsychological assessment | X | X | |||
| Neuroimaging module | |||||
| 3-T magnetic ressonance + DTI | X | X | |||
| Therapeutic-pharmacogenetics module | |||||
| Antipsychotic plasmatic levels | X | X | X | X | X |
| EKG | X | X | X | X | X |
| UKU | X | X | X | X | |
| SAS | X | X | X | X | |
SCID: Structured Clinical Interview for DSM-IV; K-SADS-PL: Kids Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime version; CGI: Clinical Global Impression Scale; GAF: Global Assessment of Functioning Scale; C-GAS: Children's Global Assessment Scale (C-GAS); SOS: Symptom Onset in Schizophrenia; PANSS: Positive and Negative Syndrome Scale; YMRS: Young Mania Rating Scale; MADRS: Montgomery–Asberg Depression Rating Scale; WHO-DAS-II: World Health Organization Disability Assessment Schedule version II; GEOPTE: Social cognition scale from the Grupo Español para la Optimización y Tratamiento de la Esquizofrenia; PAS: Premorbid Adjustment Scale; TQ: Traumatic Experiences in Psychiatric Outpatients Questionary; FES: Family Environment Scale; FAST: Functional Assessment Staging; DNA: Genetic sample extraction; 3-T: 3 Teslas; DTI: Diffusion Tensor Imaging; EKG: Electrocardiogram; UKU: Scale of the Udvalg for Kiniske Undersogelser; SAS: Simpson–Angus Scale.
One of the key points of the PEPs study was to confirm the diagnosis of psychotic disorder. For this, semi-structured interviews appropriate to the patient's age were used.
The K-SADS-PL is a semi-structured diagnostic interview designed to assess current and past psychopathology in children and adolescents according to DSM-IV criteria.60 We used the Spanish translation of the K-SADS-PL.61
The SCID-I and II, with Spanish translation available, are semi-structured diagnostic interview designed to assess current and past psychopathology and personality disorders in adults, according to DSM-IV criteria.60,62–65 Use of a semistructured interview such as the SCID has been shown to improve the reliability of diagnostic assessments and thus helps ensure that all patients included in the study do, in fact, meet DSM-IV criteria for a psychotic disorder.66
Despite the research efforts and clinical need, little is known about the initial stage of psychotic disorders. Prodromal symptoms, including disturbances of perceptions, beliefs, cognition, affect, and behavior, are often the first symptoms of a psychotic disorder. In order to retrospectively characterize and date the initial symptoms of a psychotic illnesses the Symptom Onset in Schizophrenia (SOS) inventory was used.67
Global clinical statusThe course and outcome of psychotic disorders is more characterized by an unexplained heterogeneity rather than uniform poor outcome,68 indicating the importance of assessing the global functioning outcomes. For this purpose four different scales were used, in order to get the highest quality of global functioning information.
The Clinical Global Impression Scale (CGI)69 assesses severity and improvement of global symptomatology. It is particularly helpful for repeated evaluations of global psychopathology.
The Global Assessment of Functioning Scale (GAF) and the Children's Global Assessment Scale (C-GAS), which measure the severity of symptoms and the level of functioning.70,71
The Spanish version of the World Health Organization Disability Assessment Schedule II (WHO-DAS-II)72 is an instrument that assesses difficulties in maintaining personal care, performing occupational tasks, and functioning in family and social settings.
The Functional Assessment Staging (FAST),73 evaluates the patient's degree of difficulty in autonomy, work functioning, cognitive functioning, finance, interpersonal relationships and free time functioning.
General demographic, clinical and environmental factorsAt baseline, a complete personal and family history was performed, including drug history.
In every evaluation, information of which drugs the subject was taking, dosage and presence of severe ADRs (known as those that made the clinician change the usual practice by, for example, requesting an analytical or sending the patient to emergencies room) was collected.
Antipsychotic plasmatic levels were determined at each visit, being an indirect measure of the level of pharmacological treatment adherence of the patients.
In every evaluation the weight, height, body mass index, blood pressure and abdominal perimeter were also obtained, with the aim of monitoring physical health indicators. To study the possible appearance of cardiac abnormalities secondary to pharmacologic treatment (e.g. QT segment elongation), patients underwent an electrocardiogram during each visit.
In women, the age at menarche and the date of last period were registered. Women were asked whether they were pregnant or had used a pregnancy test in the previous month.
Since the inclusion of children and adolescents was allowed in the study, in subjects younger than eighteen the sexual maturity was established by the Tanner scale.74
Environmental factorsDifferent environmental variables have been linked to the risk of developing psychosis.53 Being a study of gene x environment interaction, in addition to recording the urbanicity of the subjects, assessments on family background, traumatic experiences and obstetric complications were included.
The family background was assessed by the Family Environment Scale (FES),75 a self-report scale which includes 10 subscales reflecting socio-environmental characteristics of the families.
The number of traumatic experiences was collected from the list of events that appear in the Traumatic Experiences in Psychiatric Outpatients Questionary (TQ).76 It is a self-report questionary.
Obstetric complications were recorded using the Lewis–Murray Scale of Obstetric Data scale.77 The evaluator retrospectively rates information on obstetric complications. For the majority of pre and peri-natal events, it has been studied that mothers provide accurate reports in comparison to information from medical records.78
Alcohol and drug usePeople experiencing a first episode of psychosis frequently have comorbid substance use disorders, usually involving alcohol and cannabis, which put them at risk for prolonged psychosis, psychotic relapse, and other adverse outcomes.79 Alcohol and drug use are key risk factors for violence, noncompliance, relapse, and other poor outcomes in schizophrenia.80
Drug abuse was evaluated in every visit by a fragment of the European Adaptation of a Multidimensional Assessment Instrument for Drug and Alcohol Dependence (EuropAsi).81 In the inclusion visit a systematic register of the drug misuse habits was performed.
Clinical assessment scalesWe used scales originally designed for use in adult samples in order to study adolescents longitudinally, with these assessments as baseline comparisons.
The scales used were the following:
Psychotic symptomsSymptom severity and functional status were assessed using different assessments. The Positive and Negative Symptom Scale (PANSS)82 comprises 3 subscales. The PANSS was chosen because of its widespread use in clinical studies of psychosis and its demonstrated reliability in assessing psychopathology across a range of patient populations. To enhance reliability, the PANSS includes a well-developed anchor system. In addition, validated criteria of schizophrenia remission are based in some of their items.83,84 We used the validated Spanish version.85
Affective symptomsInclusion criteria did not exclude patients with affective symptoms added to the psychotic symptoms. For this reason, two instruments were selected to evaluate both the manic and depressive symptoms.
The Young Mania Rating Scale (YMRS), designed to assess the maniac symptoms severity, was used in its Spanish validated version.86,87
For depression severity evaluation, the Spanish validated version of the Montgomery–Asberg Depression Rating Scale (MADRS) was chosen.88,89 Co-occurring depression can demonstrably affect outcomes in patients with psychotic disorders.
Social cognitionSocial cognition seems to mediate a significant indirect relationship between neurocognition and functional outcome.90 For measuring social cognition the GEOPTE scale characterized by its simplicity of use and design was used.91
Premorbid adjustmentPremorbid adjustment is one of the most studied factors in relationship with the prognosis of psychotic disorders.92 The Premorbid Adjustment Scale (PAS93) explores sociability and withdrawal, peer relationships, school achievement, adaptation to school, and ability to establish socio-affective and sexual relationships. The scale considers different age ranges: childhood (up to 11 years), early adolescence (12–15 years), late adolescence (16–18 years), and adulthood (19 years and older). This scale was completed based on information obtained from the patient and parents.
Adverse drug reactions (ADRs) evaluationAll treatments, both pharmacological and non pharmacological, can cause ADRs. Some antipsychotic drugs often have side effects that may in themselves cause symptoms similar to those of schizophrenia. For example, cognitive slowing, decreased motivation and emotional dulling, may occur as part of an antipsychotic-induced parkinsonian syndrome. Some subjects may develop side effects that are new symptoms, such as galactorrhea, disrupted sexual function, or sedation. In some instances, the subjective distress and functional impairment that result from the drugs side effect determinate the poor adherence to these treatments.94
There were considered as a ADRs those that made the clinician change their usual conduct (e.g. admitting the patient to the hospital, requesting an unexpected blood test, etc.), were also recorded.
In order to assess in detail the adverse drug reactions, two procedures were followed: (a) Spontaneous reports of ADRs; (b) systematic assessment of the effects targeted (like metabolic syndrome, cardiotoxicity or extrapyramidal symptoms) from physical examination (electrocardiogram, antipsychotic plasmatic levels and general blood tests) and two scales were administrated in every visit, except in baseline.
General ADRs of psychotropic drugsThe Scale of the Udvalg for Kiniske Undersogelser (UKU),95 a comprehensive rating scale designed to assess general side effects of psychotropic drugs.
Extrapyramidal ADRsThe second scale used to assess side effects was the Simpson–Angus Scale (SAS),96 more oriented to evaluate the extrapyramidal side effects. Drug-induced parkinsonism is a common and poorly tolerated adverse effect of typical antipsychotics and occurs with atypical antipsychotics, especially at higher doses. There are few comparisons of drug-induced parkinsonism among atypical antipsychotics.
NeuroimagingMagnetic resonance imaging (MRI) scans were acquired at baseline and 2 years in 6 different scanners: 1 Siemens Symphony 1.5T, 2 General Electric Signa, and 1 Philips Achieva 3T, 1 Philips intera 1.5T and 1 Siemens Magneton Trio 3T. Data were collected from each center and processed at one site. Sequences were acquired in axial orientation for each subject, a T1-weighted 3D gradient echo (voxel size 1mm×1mm×1.5mm) and a T2-weighted Turbo-Spin-Echo (voxel size 1mm×1mm×3.5mm).
In multicenter MRI studies, pooling of volumetric data requires a prior evaluation of compatibility between the different machines used. In the first phase of the study we tested the compatibility of the six different scanners by repeating the scans of six volunteers at each of the sites. Using a semiautomatic method based on the Talairach atlas, and SPM algorithms for tissue segmentation (multimodal T1 and T2, or T1-only), we obtained volume measurements of the main brain lobes (frontal, parietal, occipital, temporal) and for each tissue type and subject. MRI images were processed using locally developed software incorporating a variety of image processing and quantification tools.97,98 Our preliminary results show that the variability derived from including 6 different machines was 20%, versus the 80% of variability derived from the participants themselves.
Neuropsychological assessmentThe neuropsychological battery employed in this study was designed to address different cognitive domains by means of standardized neuropsychological tests that have proven sensibility and specificity. The battery is extensive and covers the areas proposed by the NIMH MATRICS consensus, except verbal memory.99,100 These tests have been previously used in the cognitive assessment of this type of patient and were administrated by specialized neuropsychologists.
Neuropsychological assessment in children and adolescent must take into account their different developmental levels. In order to estimate global functioning in the form of IQ, vocabulary subtest of the WISC-IV101 or WAIS-III102 was used for patients and controls under and over 16 years of age, respectively.
The potentially impaired cognitive domains were assessed with the following instruments:
- •
Attention, by means of the Conners’ Continuous Performance Test-II,103 direct order of the Digit Span Subtest of the WAIS-III102 in adults or WISC-IV101 in children, Stroop test104 and Trail Making Test–part A.105
- •
Working memory, by means of the reverse order of the Digit Span Subtest, the Letters and Numbers Subtest of the WAIS-III in adults or WISC-IV101 in children.
- •
Executive functions, by means of the Wisconsin Card Sorting Test,106,107 the Trail Making Test–part B.105
- •
Verbal fluency,108 with phonetic and categorical mark, memory and verbal learning, by means of the España-Complutense Verbal Learning Test-TAVEC109 for adults and children-TAVECi.110
- •
Social cognition, with the Mayer-Salovey-Caruso Emotional Intelligence Test–MSCEIT111 included in the MATRICS99,100,112 battery.
The assessment and analysis of handedness was made by The Edinburgh Inventory.113
The neuropsychological assessment was made in the second month evaluation in order to ensure that the patient was psychopathologically stable. The neuropsychological battery was repeated in the two years visit.
To evaluate the differences between raters, an interrater reliability study was also conducted among different neuropsychologists at each center. Those who failed the first evaluation were reassessed. The complete method and the detailed results of the interrater reliability study will be described in a specific work.
Data processingThe tool Gridsam was used for data entry. Its conception follow the PsyGrid philosophy,114 defining a Service-Oriented Architecture (SOA) on which are built several web applications that interact with a central database. The Gridsam allows for the capture of data by means of a multi-grid computerized system, which not only integrates all the available information but also facilitates a more efficient data exploitation and management.115
DiscussionIn this article we report the assessment adopted to evaluate the clinical and functional outcomes in the PEPs study. An objective of the present study was to try to minimize the clinical heterogeneity of other studies, looking for an efficient, valid and relevant design. Only longitudinal studies in early phases like the PEPs study are vital to solve these problems. In this study, the selection of the sample tried to be as close as possible to the “real-world” patients with a FEP. In our opinion, this design will provide this study of a unique validity compared to other previous trials which have used larger selected samples. This is a key factor to assess the applicability of the results to daily clinical practice. Moreover, the size of the sample, with nearly 350 FEP and 250 controls, is crucial to assess the applicability of the results to daily clinical practice.
We therefore designed a study trying to balance an efficient and clinically relevant protocol administered in a structured manner, with the necessity of capturing the complexity of the heterogeneous clinical picture of FEP and their environmental influences.
Another key feature of this study is that the age of inclusion is wider than in other previous works, including child and adolescent patients as well as adults. This fact made an increased number of patients in the group of <20 years. This wide window of age pointed to the fact that the average age of this sample (23.63±5.9) tends to be inferior to other studies with large FEP cohorts (OPUS trial: 26.6±6.4; EUFEST trial: 26±5.6).11,12
One strong point of the PEPs study is the broad neuropsychological battery used. Compared to previous first-episode of psychosis projects as EUFEST, that used brief assessment battery (including processing speed, motor skills, verbal memory and cognitive flexibility),116,117 the neuropsychological battery employed in this study is extensive and covers the areas proposed by the NIMH MATRICS consensus (except verbal memory).99,100
One benefit for patients who agreed to enter into this study is that they are being completely followed-up for two years, with analytical tests, electrocardiograms, two MRIs and two cognitive assessments. This non-invasive monitoring provided patients with a clinic better characterization and care of each patient comparing to the usual attention, regardless of the results obtained after the conclusion of the project. In our opinion, this fact explains why most patients who were offered participation in this protocol finally accepted.
Due to naturalistic design, drug treatment was not controlled. The study participants maintained their usual treatment. Although this may limit the evaluation of some variables, this method eases the recruitment and also gives a global picture of the usual treatment and outcome in these patients.
An additional problem is the use of certain adult scales whose language is not adapted to children, although a big effort has been made in order to use mainly adapted scales, both clinical and cognitive assessment. This problem will require further consideration and, for some analyses, the younger children may need to be excluded.
Another limitation of the study is that there was no specific assessment of insight, apart from certain items in some included scales (e.g. G12 item of the PANSS scale “lack of judgment and insight” or item 11 of the YOUNG scale “Insight and self-awareness”). Besides, the antipsychotic plasmatic levels determinations at each visit constituted an indirect approximation to medication adherence (and therefore insight), noting that it only measures a partial and indirect aspect of the awareness of having a mental illness (particularly in minors). A deep evaluation of insight and adherence should be been included in further studies, given its relationship with the risk of suffering a relapse in patients with a first psychotic episode.8
Since CIBERSAM, the Spanish network of mental health, was created in 2007, this is the largest collaborative project ever undertaken in our country.21 The magnitude of a trial like this should increase the chances of finding relevant and applicable results for the daily management of psychotic patients, while also elevating the overall complexity of the project.
Characterization of a Spanish sample of patients who have had a FEP is of great interest, knowing the high risk of presenting a second psychotic episode.8
The approach to clinical and functional outcomes assessment adopted for this gene–environment interaction study in first episode of psychosis is designed to meet the needs of a geographically dispersed case–control follow up naturalistic study.22 The measurement approach attempts to give equal weight to clinical and functional outcomes as efficiently as possible. The rich data collected should prove valuable to a broad range of investigators interested in treatment outcomes in this population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
FundingThis study was partly supported by the Spanish Ministry of Economy and Competiveness, Instituto de Salud Carlos III, European Union FEDER, Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2009SGR1295), Comissionat per Universitats i Recerca del Department d’Innovació, Universitats i Empresa (DIUE) (2009SGR1295), Esther Koplowitz Center and CIBERSAM.
Conflict of interestThe authors have no conflict of interest to declare.
Principal Investigators of FIS project “Phenotype–genotype and environmental interaction. Application of a predictive model in first psychotic episodes” formed the PEPs GROUP: Miguel Gutiérrez, Vicent Balanzá, Salvador Sarró, Ana Gonzalez-Pinto A, Gabriel Rubio, Judith Usall, Iluminada Corripio, Manuel Bousoño, Carmen Leal, Fernando Contreras, Antonio Bulbena, Antonio Lobo.
Information about PEPS Group is available in Appendix 1.
Please cite this article as: Bernardo M, et al. Criterios de valoración clínicos y de funcionamiento en un estudio de interacción gen-ambiente en primeros episodios psicóticos (PEPs). Rev Psiquiatr Salud Ment (Barc.). 2013;6:4–16.