To describe the cost-effectiveness analyses of medications launched in Spain for the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents.
Material and methodsSystematic review of the literature without meta-analysis. A search was made in PubMed/MEDLINE, SCOPUS, databases of the Centre for Reviews and Dissemination, and the websites of technology assessment agencies from Canada, the United Kingdom and the Spanish Platform AUnETS. Only full economic evaluations were included, considering at least methylphenidate or atomoxetine as pharmacological treatment alternatives in children and/or adolescents with ADHD.
ResultsEleven studies published in 9 articles or reports were included. The most frequent characteristics were: cost-utility analysis (82%), health system perspective (82%), short-term horizon (91%), and private funding (50%). Methylphenidate was included in all studies, and atomoxetine in 4 studies. Methylphenidate and atomoxetine are cost-effective alternatives compared to placebo or no treatment, although incremental cost-effectiveness ratios are variable. The few direct treatment-comparisons between methylphenidate and atomoxetine provided contradictory and potentially biased results.
ConclusionsThe pharmacological treatment of ADHD in children and adolescents, with the reservations arising from the generalisation of results to different settings, is probably cost-effective in the short term. The existing studies do not allow the relative efficiency of different treatments to be established, either in the long-term treatment or in patient subgroups with specific characteristics or comorbidities.
Describir los estudios de coste-efectividad sobre las alternativas farmacológicas comercializadas en España para el tratamiento del trastorno por déficit de atención e hiperactividad (TDAH) en niños y adolescentes.
Material y métodosRevisión sistemática de la literatura sin utilizar técnicas de metaanálisis. Se realizó una búsqueda en PubMed/MEDLINE, SCOPUS, bases de datos del Centre for Reviews and Dissemination y las web de agencias de evaluación de Canadá, Reino Unido y la Plataforma AUnETS. Se incluyeron las evaluaciones económicas completas que consideraran al menos metilfenidato o atomoxetina como alternativas de tratamiento farmacológico en niños y/o adolescentes con TDAH.
ResultadosSe incluyeron 11 estudios publicados en 9 artículos o informes. Las características más frecuentes fueron: análisis coste-utilidad (82%), perspectiva del sistema sanitario (82%), horizonte temporal de hasta un año (91%) y financiación privada (50%). Metilfenidato se incluía en todos los estudios y atomoxetina en 4 estudios. Metilfenidato (en cualquiera de sus formulaciones) y atomoxetina aparecen como alternativas coste-efectivas frente a placebo o no tratamiento, aunque con razones coste-efectividad incremental variables. Las escasas comparaciones directas entre metilfenidato y atomoxetina presentan resultados contradictorios pudiendo existir potenciales sesgos.
ConclusionesEl tratamiento farmacológico del TDAH en niños y adolescentes, con las salvedades derivadas de la generalización de resultados a diferentes entornos, es probablemente coste-efectivo en el corto plazo. Los estudios existentes no permiten establecer la eficiencia relativa de los diferentes tratamientos, del tratamiento a largo plazo o en subgrupos de pacientes con características o comorbilidades específicas.
Attention deficit hyperactivity disorder (ADHD) is a childhood-initiated health problem that includes a persistent pattern of behaviour including hyperactivity, impulsiveness and lack of attention. It presents when these behaviours are of greater frequency and intensity than expected in children of the same age. The disorder causes a significant deterioration in school or work performance and in activities of daily living. Children and adolescents with ADHD find it hard to control their behaviour and adjust to rules, presenting family, school and/or social adaptation difficulties.1 ADHD is one of the most common neuropsychiatric conditions in childhood and adolescence. Its course is chronic and requires long-term treatment, with the corresponding social cost. In Spain, the overall prevalence in children and adolescents is estimated to be 6.8%.2
Pharmacological treatment is not indicated in all children having ADHD. The decision to use a drug should be based on an in-depth assessment of the seriousness and persistence of the symptoms. The most frequently used pharmacological alternatives are methylphenidate (MPH) and atomoxetine (ATX). The first is moderate stimulant of the central nervous system authorised as part of a comprehensive treatment plan for ADHD in children older than 6 years and in adolescents when other measures are insufficient.3 The drug is available in immediate release (IR) tablets and extended release (ER) capsules and tablets. There are 2 ER formulations that vary in the duration of their clinical action (modified release, having 7–8h of effect, and extended release, with an effect of approximately 12h). The second drug, ATX, is a selective noradrenaline reuptake inhibitor authorised in ADHD treatment in children from the age of 6 years and in adolescents as part of a complete treatment programme that normally includes psychological, educational and social measures.4
Limits in the health resources available (greater and more visible in periods of economic crisis) make it necessary to prioritise between different actions and programmes to achieve the greatest levels of health in the population with the resources available. Economic evaluation (the comparative analysis of alternative actions in terms of health costs and results5,6) (Appendix A) is one of the most frequently used methods in such setting of priorities. Various countries, such as the United Kingdom, Australia, New Zealand, Canada and Holland, among others, use cost-effectiveness criteria derived from economic evaluations in the process of making decisions on which treatments should be included in universal health service coverage and on what prices the public health system should pay for them,7,8 independently of whether drug marketing is authorised and of the “official” drug price.
The different formulations of MPH and ATX differ in price, with costs per year of treatment (in 2010 and for the defined daily dose) of €98.6 for IR-MPH, €667.5 for ER-MPH and €1584.1€ for ATX.9 In relation to the effectiveness of the treatments, both MPH and ATX seem to be more effective than placebo in reducing clinical symptoms, as measured by various specific ADHD scales.10,11 However, it is not clear that they improve school performance.1,10,12–16 Clinical efficacy studies have not been able to establish the superiority of 1 drug over another in improving response rates in children and adolescents.10,17,18 Although they are drugs with frequent side effects, their safety profile is considered acceptable in the appropriate indications; at any rate, the liver toxicity of ATX stands out.10
The objective of this study was to systematically review the economic evaluations of the pharmacological alternatives sold in Spain for the treatment of ADHD in children and adolescents.
Material and methodsDesignQualitative synthesis (systematic review) of the literature without employing meta-analysis techniques.
Information sourcesTo identify the relevant publications, we searched the PubMed/MEDLINE and SCOPUS (which includes EMBASE) databases using terms or keywords distributed in 2 blocks:
- (1)
“attention deficit disorder with hyperactivity” [MeSH Terms] OR (“attention”[All Fields] AND “deficit”[All Fields] AND “disorder”[All Fields] AND “hyperactivity”[All Fields]) OR “adhd”[All Fields]) OR “hyperkinesis”[MeSH Terms] OR “hyperkinesis”[All Fields] OR “hyperactivity”[All Fields].
- (2)
“Economics, Pharmaceutical”[MeSH Terms] OR “Cost-Benefit Analysis”[MeSH Terms] OR “Drug Costs”[MeSH Terms] OR “Costs and Cost Analysis”[MeSH Terms] OR “Cost Savings”[MeSH Terms] OR “health resources”[MeSH Terms] OR “Quality-Adjusted Life Years”[MeSH Terms] OR “cost effectiveness”[All Fields] OR “pharmacoeconomics”[All Fields] OR “economics, medical”[MeSH Terms] OR “health economics”[All Fields].
In addition, we manually searched the databases of the University of York's Centre for Reviews and Dissemination; the databases of the health technology evaluation agencies (Canadian Agency for Drugs and Technologies in Health of Canada, National Institute for Health and Clinical Excellence of the United Kingdom, HTA program of the United Kingdom and AUnETS Platform of the Spanish National Health System); and the references of the works selected, review articles on the subject, editorials and consensus documents. A further source was the references provided by the various pharmaceutical companies holding the sales authorisations for MPH and ATX in Spain. We limited all searches to articles published through September 2011.
Criteria of inclusion and exclusionWe only included complete economic evaluations (evaluating least 2 alternatives, even though 1 of those was an alternative lacking active pharmacological treatment, and covering both costs and effectiveness) that considered MPH and/or ATX as pharmacological treatment alternatives. The studies had to include children and/or adolescents with ADHD as the target study population. Studies on adults only were excluded. We also excluded review articles, methodological article, editorials and communications at congresses, as well as studies focused on intervention programmes (e.g., psychological–social support programmes) in which the pharmacological treatment was not an essential aspect, studies that included the evaluation of pharmacological treatment but that did not consider MPH and/or ATX explicitly, and any studies that did not present cost-effectiveness ratios.
Variables studiedThe works were review by 2 investigators (FCL and MR), who extracted the information of interest independently. If there were any discrepancies, the works were reviewed by a third investigator (GSG). The information extracted included data on the methodological characteristics of the study (year published, population studied, type of analysis, perspective, alternatives compared, effectiveness and cost measures, and source used), the results of each study (incremental cost-effectiveness analysis) and conclusions.
AnalysisUsing evidence tables, a descriptive analysis was performed of the characteristics of the economic evaluations selected.
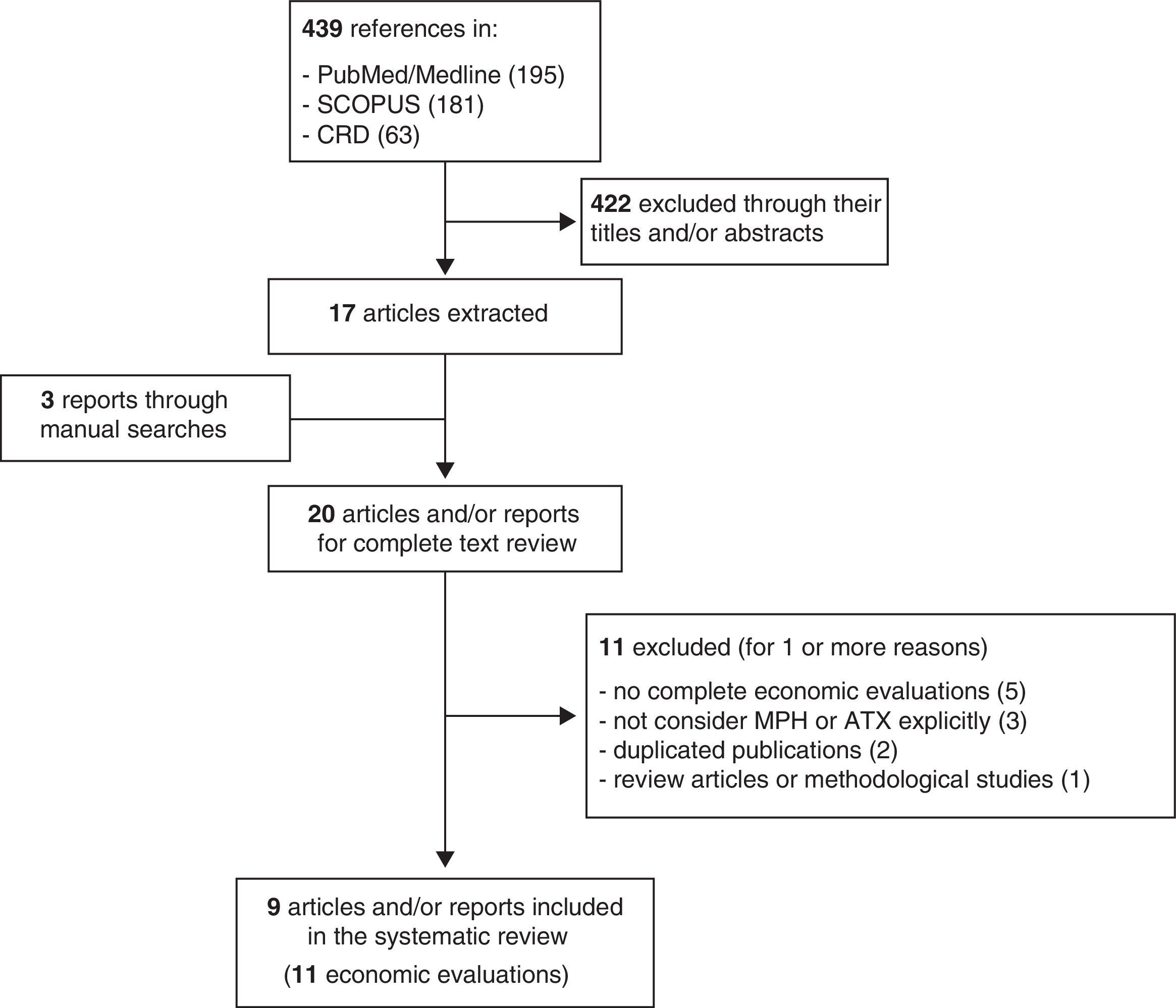
ResultsThe literature search identified a total of 439 references for preliminary review. After looking at the title, type of work and abstract, we selected 17 articles of potential interest (Fig. 1). The complementary manual search and the review of the works provided by the pharmaceutical companies that sell 1 or more of the treatments analysed made it possible to identify 3 more articles. Consequently, there were 20 works for full-text reading. From these, we excluded 9 for 1 or more of the following reasons: not being complete economic evaluations (n=5), being focused on intervention programmes or not considering MPH or ATX explicitly (n=3), being a duplicated publication of another already selected one (n=2) and corresponding to review articles or methodological articles (n=1). In the end, we included in the review 11 economic evaluations that had been published in 9 articles or reports of evaluations of health technologies.19–27
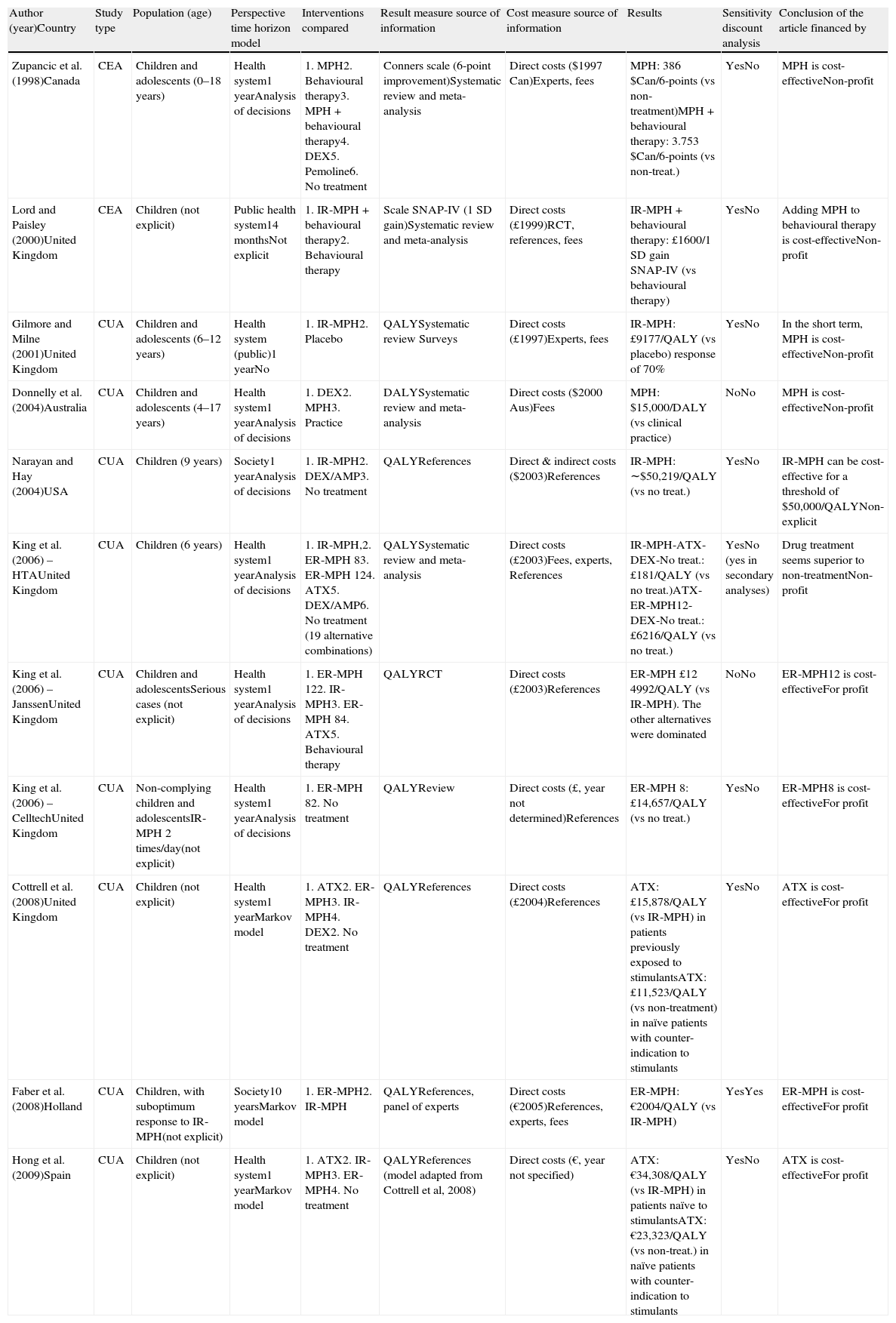
Table 1 shows the characteristics of these economic evaluations of pharmacological treatment of ADHD. The studies were published in journals specific to the areas of mental health and psychiatry (n=3), economy of health and health management (n=2) and pharmaco-epidemiology (n=1); there were also reports from agencies of technology evaluation (n=3). With respect to when the works were published, all of them except for 1 were published in the decade from 2000 on. By country, 6 studies were performed in the United Kingdom and the rest in Canada, the USA, Australia, Holland and Spain. The population studied included “children and adolescents” in 5 of the works, only “children” in 6 articles (6 evaluations did not specify the age limits included) and 8 did not mention the sex of the patients (of those that gave details, 2 studies included only males and 1 included both sexes). Nine works (82%) provided no information as to risk factors or comorbidities; in the others, 2 were carried out in a population with less than optimal response or that did not comply with the previous treatment, as well as 1 with serious cases.
Summary of the characteristics of the economic evaluations of drug treatment for ADHD.
| Author (year)Country | Study type | Population (age) | Perspective time horizon model | Interventions compared | Result measure source of information | Cost measure source of information | Results | Sensitivity discount analysis | Conclusion of the article financed by |
| Zupancic et al. (1998)Canada | CEA | Children and adolescents (0–18 years) | Health system1 yearAnalysis of decisions | 1. MPH2. Behavioural therapy3. MPH+behavioural therapy4. DEX5. Pemoline6. No treatment | Conners scale (6-point improvement)Systematic review and meta-analysis | Direct costs ($1997 Can)Experts, fees | MPH: 386 $Can/6-points (vs non-treatment)MPH+behavioural therapy: 3.753 $Can/6-points (vs non-treat.) | YesNo | MPH is cost-effectiveNon-profit |
| Lord and Paisley (2000)United Kingdom | CEA | Children (not explicit) | Public health system14 monthsNot explicit | 1. IR-MPH+behavioural therapy2. Behavioural therapy | Scale SNAP-IV (1 SD gain)Systematic review and meta-analysis | Direct costs (£1999)RCT, references, fees | IR-MPH+behavioural therapy: £1600/1 SD gain SNAP-IV (vs behavioural therapy) | YesNo | Adding MPH to behavioural therapy is cost-effectiveNon-profit |
| Gilmore and Milne (2001)United Kingdom | CUA | Children and adolescents (6–12 years) | Health system (public)1 yearNo | 1. IR-MPH2. Placebo | QALYSystematic review Surveys | Direct costs (£1997)Experts, fees | IR-MPH: £9177/QALY (vs placebo) response of 70% | YesNo | In the short term, MPH is cost-effectiveNon-profit |
| Donnelly et al. (2004)Australia | CUA | Children and adolescents (4–17 years) | Health system1 yearAnalysis of decisions | 1. DEX2. MPH3. Practice | DALYSystematic review and meta-analysis | Direct costs ($2000 Aus)Fees | MPH: $15,000/DALY (vs clinical practice) | NoNo | MPH is cost-effectiveNon-profit |
| Narayan and Hay (2004)USA | CUA | Children (9 years) | Society1 yearAnalysis of decisions | 1. IR-MPH2. DEX/AMP3. No treatment | QALYReferences | Direct & indirect costs ($2003)References | IR-MPH: ∼$50,219/QALY (vs no treat.) | YesNo | IR-MPH can be cost-effective for a threshold of $50,000/QALYNon-explicit |
| King et al. (2006) – HTAUnited Kingdom | CUA | Children (6 years) | Health system1 yearAnalysis of decisions | 1. IR-MPH,2. ER-MPH 83. ER-MPH 124. ATX5. DEX/AMP6. No treatment (19 alternative combinations) | QALYSystematic review and meta-analysis | Direct costs (£2003)Fees, experts, References | IR-MPH-ATX-DEX-No treat.: £181/QALY (vs no treat.)ATX-ER-MPH12-DEX-No treat.: £6216/QALY (vs no treat.) | YesNo (yes in secondary analyses) | Drug treatment seems superior to non-treatmentNon-profit |
| King et al. (2006) – JanssenUnited Kingdom | CUA | Children and adolescentsSerious cases (not explicit) | Health system1 yearAnalysis of decisions | 1. ER-MPH 122. IR-MPH3. ER-MPH 84. ATX5. Behavioural therapy | QALYRCT | Direct costs (£2003)References | ER-MPH £12 4992/QALY (vs IR-MPH). The other alternatives were dominated | NoNo | ER-MPH12 is cost-effectiveFor profit |
| King et al. (2006) – CelltechUnited Kingdom | CUA | Non-complying children and adolescentsIR-MPH 2 times/day(not explicit) | Health system1 yearAnalysis of decisions | 1. ER-MPH 82. No treatment | QALYReview | Direct costs (£, year not determined)References | ER-MPH 8: £14,657/QALY (vs no treat.) | YesNo | ER-MPH8 is cost-effectiveFor profit |
| Cottrell et al. (2008)United Kingdom | CUA | Children (not explicit) | Health system1 yearMarkov model | 1. ATX2. ER-MPH3. IR-MPH4. DEX2. No treatment | QALYReferences | Direct costs (£2004)References | ATX: £15,878/QALY (vs IR-MPH) in patients previously exposed to stimulantsATX: £11,523/QALY (vs non-treatment) in naïve patients with counter-indication to stimulants | YesNo | ATX is cost-effectiveFor profit |
| Faber et al. (2008)Holland | CUA | Children, with suboptimum response to IR-MPH(not explicit) | Society10 yearsMarkov model | 1. ER-MPH2. IR-MPH | QALYReferences, panel of experts | Direct costs (€2005)References, experts, fees | ER-MPH: €2004/QALY (vs IR-MPH) | YesYes | ER-MPH is cost-effectiveFor profit |
| Hong et al. (2009)Spain | CUA | Children (not explicit) | Health system1 yearMarkov model | 1. ATX2. IR-MPH3. ER-MPH4. No treatment | QALYReferences (model adapted from Cottrell et al, 2008) | Direct costs (€, year not specified) | ATX: €34,308/QALY (vs IR-MPH) in patients naïve to stimulantsATX: €23,323/QALY (vs non-treat.) in naïve patients with counter-indication to stimulants | YesNo | ATX is cost-effectiveFor profit |
AMP: amphetamine salts; ATX: atomoxetine; CEA: cost-effectiveness analysis; CUA: cost-utility analysis; DALY: disability-adjusted life years; DEX: dexamphetamine; ER: extended release; IR: immediate release; MPH: methylphenidate; QALY: quality-adjusted life years; RCT: randomised clinical trials; SD: standard deviation.
By type, 9 (82%) of the works were cost-utility analyses using quality- and/or disability adjusted life years as the measure of effectiveness, and 2 were cost-effectiveness analyses using ADHD symptom scales. As for the perspective of the analysis, 9 (82%) works took the health system perspective and 2 (18%) that of the perspective of society as a whole. The analytic horizon of the analysis presented short-term results (up to 1 year) in 10 (90%) studies, while a single long-term study had a 10-year follow-up.
As regards the pharmacological treatments, MPH (in any of its formulations) was included in the 11 evaluations identified and ATX in 4 studies (36%). A few studies considered other drugs that are not authorised for the treatment of ADHD in Spain (dexamphetamine and pemoline). Several studies carried out more than 1 comparison. MPH was evaluated against a placebo/no treatment (n=5), another formulation or presentation of MPH (n=2), standard clinical practice (n=1) and behavioural therapy (n=1). ATX was evaluated against a placebo/no treatment (n=3) and MPH (n=2). The most frequent sources of information used to quantify the effectiveness of the treatments were the literature reviews (with/without meta-analysis) and assumptions adopted by the authors. The sources of information used most often to quantify the costs and the utilisation of resources were the literature reviews carried out by the authors themselves and the opinions of experts. None of the studies used primary sources such as works associated with clinical trials in which the data on utilisation and cost of the services were obtained from the field study itself.
All of the studies included the direct costs of the drug. Most of the studies (91%) included out-patient (primary care) and specialist care costs. Only 2 studies included some type of indirect costs. With respect to discount rates, only the evaluation that went over 1 year of follow-up discounted costs and benefits. In 9 studies (82%), there was a sensitivity analysis (based on drug cost, effectiveness or other parameters); however, only 1 of them carried out a sensitivity analysis based on treatment duration over time. Only 2 studies included some type of assessment of result uncertainty, whether using P values or intervals of confidence. One study omitted the source of funding for the study. Of the 10 mentioned, 5 were funded by the pharmaceutical industry.
With respect to the results, both MPH and ATX were presented as cost-effective alternatives over placebo or no treatment in all the studies. However, the incremental cost-effectiveness reasons varied greatly in the various studies (Table 1). The few direct comparisons between MPH and ATX presented contradictory results according to the source of funding for the study: ATX was shown to be cost-effective over MPH in 2 evaluations associated with the manufacturer of ATX, while ER-MPH was cost-effective over ATX in the evaluation associated with the manufacturer of MPH. Likewise, ER-MPH appeared as cost-effective over IR-MPH in the 2 economic evaluations funded by manufacturers of extended release formulations.
In additional material available on the web (Appendix B), we present a summary of the quality of the 11 studies included in this review.
DiscussionThe results of this review demonstrate, in the first place, that both MPH (in any of its formulations) and ATX are cost-effective drugs in the face of the alternative of no treatment. This conclusion is directly applicable to the environments where the economic evaluations were performed, the type of patients considered (in general terms, cases clearly labelled with criteria of inclusion and exclusion derived from clinical trials), and in the conditions of application considered (which in many cases combined the pharmaceutical treatment with accompanying psychological or educational psychological interventions). However, the exact quantification of the cost-effectiveness relationship was difficult to specify, because the various studies presented extremely variable incremental cost-effectiveness ratios. A good part of this variability stemmed from the selection of the sources of information for the construction of the analysis models, from the assumptions on benefits and risks taken in these modelling structures (especially in the transformation of the ADHD scales in quality-adjusted life years) and, to a lesser extent, from the consideration of indirect costs. At any rate, it should be pointed out that there was great uncertainty as to the cost-effectiveness relationship of drug treatments for ADHD in lengthy periods. This is a relevant aspect because, while the standard treatment for ADHD lasts until the end of adolescence and, in some cases, until adulthood, all the economic evaluations except 1 modelled costs and treatment effectiveness during only a single year of follow-up. This problem has also been documented in other reviews on cost-effectiveness of child and youth psychopharmacology28 and represents a significant limitation in considering these economic evaluations in decision-making.
The heterogeneity among the studies that we found in this systematic review did not make performing a quantitative synthesis of the results using a meta-analysis advisable. In addition, this type of synthesis (based on the analysis of studies highly dependent upon the assumptions made in the models) would end up assigning a better cost-effectiveness relationship to the product that had more economic evaluations performed. It should be noted that the variations among studies do not depend so much on their technical quality (although some have significant defects) as on the choice of different sources of information and on the establishment of assumptions in the modelling that, even though they are apparently reasonable, in fact favour of one or other alternative.
Another interesting aspect is that both ER-MPH and ATX present an advantage in convenience in dosage, even through they did not show greater effectiveness than IR-MPH in the systematic reviews of effectiveness. The dose convenience of ER-MPH and ATX lies principally in the fact that the mid-day dose (which, in many cases, has to be administered in a school environment) can be eliminated. The real value of this advantage cannot be estimated based on the information provided by the economic evaluations reviewed and, in fact, we have not found any studies on willingness to pay for this specific benefit. At any rate, treatment costs up to 6 times a great cannot always be justified, nor is it obvious who (the health system? the families?) should assume the cost of a greater convenience in drug administration. This is particularly true considering that this convenience does not appear to translate into relevant clinical benefits.
Among the limitations of our review are, in the first place, those derived from the original studies that, as has been pointed out earlier, did not permit (or recommend) a quantitative synthesis using meta-analysis techniques. In second place, we should mention the scarcity of studies carried out in the Spanish National Health System and the limitations for generalising the studies from other countries to our environment, given that the cost-effectiveness ratios could vary notably depending on: (1) differences in the adaptation of the treatments and in the baseline risk of the patients treated in each environment, (2) differences in effectiveness of the social and health organisation as a whole, including a greater or lesser development of the psychological and educational psychological interventions, (3) differences in the prices of the drugs and in the costs of the services avoided by the treatment, and (4) differences in the preferences of the patients. In third place, we have to indicate the probable existence of a significant “sponsorship bias” in the evaluations funded by the pharmaceutical industry, which translates to the systematic presence of results favourable to the financial backer (which is especially visible in comparative evaluations). This bias includes the publication of cost-effectiveness ratios that are more favourable than those of studies with public funding and that are always lower than the cost-effectiveness threshold used in each environment. In fourth place, our study was limited by the fact that the economic evaluations reviewed lacked information that would allow us to address some specific questions that can arise in clinical practise (e.g., cost-effectiveness ratio in subgroups of children with tic disorder, Tourette syndrome, epilepsy, etc.). In these cases, the clinical practice guidelines with indications for specific subgroups of patients1 are probably more illustrative than the results of the cost-effectiveness analyses for the aggregate of the population. Finally, a limitation is that the economic evaluations reviewed did not make it possible to evaluate some arguments that are used in some practice guidelines to recommend various forms of treatment. Examples are the importance of the number of doses in non-compliance (and the association or not with worse results), the lack of collaboration of the schools in the administration of the mid-day dose, the possible stigmatisation of the child who takes medication at school, as well as a few other aspects.
The most important direct conclusions from this systematic review can be summarised as: (1) pharmacological treatment of ADHD in children and adolescents (with the exceptions derived from the generalisation of the data to different environments and in the indications and type of patients included in the reviews) is probably cost-effective in the short term, (2) existing economic evaluations do not allow establishing greater or lesser effectiveness for the various treatments, and (3) existing economic evaluations do not make it possible to establish treatment effectiveness in the long term or in subgroups of patients with specific characteristics or comorbidities.
In these circumstances, the implications of these results in making decisions on funding and prices, prescription policies and clinical practice should be evaluated in the context of all the information available on effectiveness, safety and costs of these treatments. It must be remembered that evidence of greater clinical effectiveness of some drugs over others or of some formulations of MPH with respect to others is nonexistent. Consequently, the similar safety profiles (except for the greater severity of some adverse side effects of ATX)10 and the lower cost of treatment with IR-MPH, both for the families and for society, make it reasonable to consider IR-MPH the drug of first choice for initial and maintenance treatment for children and adolescents with ADHD.29 This alternative implies a cost reduction in treatment of €569.4/year with respect to ER-MPH and €1485.5/year with respect to ATX. Note that with the current mechanisms of patient participation in the cost of medications, the families with “active population” prescriptions (the most common as children and adolescents are involved) and co-payment of 50% have to take on annual treatment costs of €333.7 and €992.0 in treatment with ER-MPH and ATX, against only €49.3 in treatment with IR-MPH. However, ER-MPH can be considered a cost-effective option in cases with important problems of compliance with IR-MPH derived from the difficulties involved in the administration of the mid-day dose that cannot be solved through interaction with parents or schools. In addition, for patients that cannot be treated with MPH due to clinical reasons, ATX is still a cost-effective alternative with respect to that of no treatment.
Ethical disclosuresProtection of persons and animalsThe authors declare that no experiments on humans or animals were performed for this research.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis review was funded through an agreement between the Commission for Economic Evaluation and Budgetary Impact (CAEIP is the Spanish acronym) of the Catalonian Health Service (CatSalut) and the Centre for Research in Economy and Health (CRES) at the Pompeu Fabra university (year de execution: 2011). The financial backers, other than selecting the objective and specifications of the project, have had no role in its development.
Conflict of interestsThe foundation “Institute of Research in Health Services” (“Instituto de Investigación en Servicios de Salud”) funded some of the research dissemination activities by means of agreements with pharmaceutical companies. The financial institutions and participants in this review do not necessarily share its contents, which are the responsibility of the authors.
Cost analysis or cost minimization analysis: Considers only the costs of the alternatives compared, and ignores or considers equivalent the health consequences.
Cost-effectiveness analysis: compares the additional costs that an alternative implies with the incremental results it provides, the latter being measured in non-monetary units (e.g., life years gained, cases detected, improvement in the symptoms, etc.).
Cost-utility analysis: compares the additional costs that an alternative implies with the incremental results it provides, using quality adjusted life years (QALY) or disability adjusted life years (DALY) as the unit of result.
Cost-benefit analysis: assesses the benefits of the alternatives in monetary units.
Please cite this article as: Catalá-López F, Ridao M, Sanfélix-Gimeno G, Peiró S. Coste-efectividad del tratamiento farmacológico del trastorno por déficit de atención e hiperactividad en niños y adolescentes: síntesis cualitativa de la evidencia científica. Rev Psiquiatr Salud Ment (Barc.). 2013;6:168–177.
The results of this study were presented in the XXXII Health Economy Sessions, Bilbao, 15–18 May 2012. The report presented to the CAEIP of CatSalut on which this article is based is available at: http://www10.gencat.cat/catsalut/archivos/farmacia/CAEIP/tdah_informe_es.pdf.