In the era of new antipsychotic drugs the severe symptomatology known by the name of neuroleptic malignant syndrome (NMS) continues to have a high incidence and mortality. We review its origin, pathophysiology, diagnostic criteria and staging, particularly with electroconvulsive therapy (ECT), and proposing a less restrictive use and more adjusted to the updated knowledge of this technique.
In particular, we consider the justified use of bilateral lead placement, a frequency of three sessions per week, and loads calculated for age, which would ensure effective seizures with an early response, thus avoiding the use of repeated sub-seizure stimuli to calculate the threshold by titration. We believe that there is little evidence on the fear of the risk of increasing malignant hyperthermia in NMS due to the substances used in anaesthesia, but is justified to use non-depolarising relaxants due to the risk of hyperkalaemia on being exposed to succinylcholine.
Finally we believe that it is essential to familiarise the other specialists involved in the treatment with ECT, to increase the availability of the technique and our training in this to the currently available complexity.
En la era de los nuevos antipsicóticos, el grave cuadro conocido con el nombre de síndrome neuroléptico maligno (SNM) sigue presentando elevada incidencia y mortalidad. Revisamos su origen, fisiopatología, criterios diagnósticos y de estadificación, tratamiento general y especialmente con terapia electroconvulsiva (TEC), proponiendo un uso menos restrictivo y más ajustado al conocimiento actualizado de esta técnica.
En concreto, consideramos justificado el uso de localizaciones bilaterales de entrada, frecuencia de tres sesiones semanales y cargas calculadas por edad, que aseguren crisis eficaces con respuesta temprana, evitando el uso de estímulos subconvulsivos repetidos al calcular el umbral por titulación. Creemos poco fundamentado el temor al riesgo incrementado de hipertermia maligna en SNM por sustancias empleadas en la anestesia, pero sí justificado emplear relajantes no despolarizantes por el riesgo de hiperpotasemia al exponerse a succinilcolina.
Consideramos finalmente imprescindible familiarizar con la TEC a los otros especialistas implicados en el tratamiento, e incrementar la disponibilidad de la técnica y nuestra formación en ella en el nivel de complejidad actualmente disponible.
The existing literature on neuroleptic malignant syndrome (NMS) was reviewed through a PubMed bibliographic search, including articles published between 1965 and 2010 in English, French, and Spanish, from published cases to narrative reviews. General information was obtained from all the articles found. The search was then narrowed and focused on those articles relating to the treatment of NMS, specifically the use of electroconvulsive therapy (ECT) in treating NMS (). All articles on the treatment of NMS were thoroughly reviewed, as well as the cross references, and thereby the search was expanded to include articles in which the use of different anaesthetic agents in ECT was reviewed.
Neuroleptic malignant syndromeWhat is known today as neuroleptic malignant syndrome (NMS) was first described by Delay in 1960 and, at that time, referred to as hypertonic akinetic syndrome.1 It is defined as an uncommon but potentially lethal idiosyncratic reaction to neuroleptic therapy. Although both the pathophysiology and symptoms of this syndrome are related to reactions triggered by other drugs and toxic substances, and even though it is possible to conceptualise it within the larger framework of malignant catatonia2–4 (the APA currently admits that NMS is a form of malignant catatonia that is clearly precipitated by a toxic substance), the DSM-IV-TR definition is the one used in practice, which limits it to an adverse reaction that occurs following the use of antipsychotics.5
There is considerable variation in the data on incidence from different sources—from 2.2% to 0.07% of patients treated with antipsychotics,6,7 the acceptable mean being about 1%.8 Although the trend would seem to suggest that the number and severity of cases is declining5 due to the widespread use of atypical antipsychotics, the prescribing of lower doses, and perhaps early detection of this syndrome,7 it remains a serious condition, with a mortality of about 10%,2,7,9 and is most likely underdiagnosed here in our country.
The initial optimism based on the side effects of the new antipsychotics, known as the “atypicals,” has steadily subsided on that score: although it appears that there is still a lower risk than with conventional antipsychotics, cases abound in which the atypicals play a leading role in NMS.10–13 Among the cases of atypical antipsychotic-induced NMS, clozapine may be the antipsychotic with less risk of developing NMS because its pharmacodynamic profile is different (low affinity for D2 receptors and high affinity for D4; 5-HT 2A, 2C, 6, and 7; H1; and muscarinic receptors), but no comparative studies have been done on the incidence of NMS with atypical antipsychotics that would support this hypothesis. However, there is indeed a certain difference in how clozapine-induced NMS manifests compared to the NMS induced by other antipsychotics—this difference being less rigidity and tremor and greater changes in blood pressure and diaphoresis, with less probability of extrapyramidal symptoms appearing prior to the onset of fever.14Other risk factors besides the use of antipsychotics have been described, none of which have predictive capacity in clinical practice. Agitation, dehydration, iron deficiency, the use of physical restraints, and high environmental temperatures are associated with increased risk.15–17
In 15–20% of cases, the patient has a history of NMS.18 There are little data on a possible genetic susceptibility to NMS. Findings have been published suggesting that DRD2 Taq1A polymorphism is associated with a predisposition to NMS, perhaps related to increased DRD2 blocking. The only documentable finding is the increased A1 allele frequency in patients with NMS.18,19
Regarding the pathophysiology of NMS, 20 years ago, Henderson and Wooten proposed hyperthermia-producing, hypothalamic dopaminergic blockade as the central cause. There is concrete evidence to support this mechanism: (1) related drugs cause dopaminergic blocking, (2) the drugs used in treating NMS are pro-dopaminergic, and (3) similar symptoms are seen in patients with lesions of the dopaminergic pathways and also following abrupt suspension of l-dopa therapy.9
Consistent with the foregoing mechanism would be the extrapyramidalism-provoking nigrostriatal blockade and the frontal/mesocortical—a possible factor in the cognitive changes.20 The elevated catecholamines could be related to a direct action upon the adrenergic axis.21 In short, there are multiple neurochemical and neuroendocrine changes that would contribute to a hypermetabolic syndrome.3
Some hypotheses mention that, in addition to changes at the central level, there may be direct damage to muscle such that there is impaired calcium transport through the sarcoplasmic reticulum in the myocytes; perhaps, under conditions of dehydration, agitation, or exhaustion, the muscle becomes sensitive to this possible harmful effect of the neuroleptics.7,9
Rigidity and hyperthermia are 2 symptoms given in the DSM-IV-TR as requisites for the diagnosis of this syndrome.22 To these should be added at least 2 symptoms from the following list: diaphoresis, dysphagia, tremor, incontinence, changes in level of consciousness, mutism, tachycardia, elevated or labile blood pressure, leukocytosis, and laboratory evidence of muscle damage (CPK, among others). It is also necessary to rule out a mental disorder, medical or neurological changes, and other types of drugs or substances (phencyclidine, among others) as the cause of the symptoms.
With regard to laboratory tests, changes are non-specific, and values are normal in 90% of cases. There are no pathological findings in the CSF, neuroimaging studies are normal, and the only finding on EEG is a generalized slowing.
Symptoms are usually triggered within the first 30 days following administration of the antipsychotic—16% within the first 24h and 66% within the first week23—and, in the majority of cases, the change in mental status and the neurological signs precede the other changes.18 It is important to remember that, if it is a depot antipsychotic, this period of time could be prolonged. If the syndrome spontaneously resolves when the antipsychotic is discontinued, typically it does so within the first 30 days, with 63% recovering within the first week. In some patients, the catatonia and Parkinsonism may persist for weeks after the metabolic resolution.
NMS is a diagnosis of exclusion. Among the various conditions that may have a similar presentation, we will highlight viral encephalitis, which differs in that it presents with severe headache, meningeal signs, and laboratory (CSF) and neuroimaging changes.6 Extreme psychotic symptoms, such as stuporous mania or malignant catatonia, are indistinguishable in one-fourth of cases—NMS being conceptualised as a drug-induced form of malignant catatonia.2,5,8,24–26 Treatment measures will be similar: discontinuing the antipsychotics because they are ineffective and using ECT, this being a proper indication because ECT with benzodiazepines (BDZ) is the treatment of choice in malignant catatonia as well as for severe or prolonged NMS.
Strokes can present with hyperthermia, confusion, tachycardia, and tachypnea, and it may be difficult to distinguish a stroke in psychiatric patients who are taking antipsychotics. Some cases of non-convulsive status epilepticus also comes to resemble NMS.
Dopaminergic blockers that are not used as antipsychotics (anti-emetics) and the withdrawal of dopaminergic drugs (amantadine, l-dopa) can provoke a genuine NMS without antipsychotics. Serotonergic drugs, such as the SSRI, ADT, MAOI (including linezolid), and triptans can cause a serotonergic syndrome, which usually presents as symptoms of agitated delirium but, in many cases, resembles an NMS.
Some substances, such as cocaine and amphetamines (MDMA), can cause an NMS-like picture. Hallucinogen intoxication and withdrawal from alcohol or sedatives can give rise to symptoms similar to those of NMS, and there is an increased risk in alcohol-dependent patients under treatment with tiapride or droperidol.9 NMS must also be differentiated from intraoperative, drug-induced (succinylcholine, inhalation anaesthetic agents) malignant hyperthermia (MH).7,26
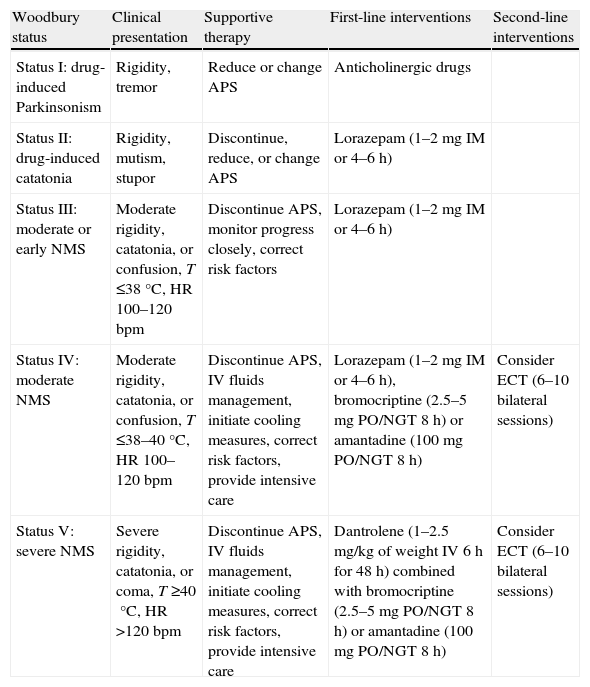
Treatment of neuroleptic malignant syndromeAs an initial measure, the drug that has triggered it must be discontinued, and individualized supportive therapy must be started. The algorithm proposed by Woodbury27 in 1992 may be useful (Table 1). Rehydration and regulation of electrolyte changes should be basic treatment objectives, and measures may be taken to reduce the degree and duration of the hyperthermia. Various causes of death have been described: cardiac arrhythmias, acute MI, aspiration pneumonia, PTE, nosocomial pneumonia, and myoglobinuric renal failure among the most common. Therefore, cardiac, respiratory, and renal functions should be monitored, with the necessary supportive care given, and coagulopathies should be prevented.
Algorithm proposed for individualized support therapy.
| Woodbury status | Clinical presentation | Supportive therapy | First-line interventions | Second-line interventions |
| Status I: drug-induced Parkinsonism | Rigidity, tremor | Reduce or change APS | Anticholinergic drugs | |
| Status II: drug-induced catatonia | Rigidity, mutism, stupor | Discontinue, reduce, or change APS | Lorazepam (1–2mg IM or 4–6h) | |
| Status III: moderate or early NMS | Moderate rigidity, catatonia, or confusion, T ≤38°C, HR 100–120bpm | Discontinue APS, monitor progress closely, correct risk factors | Lorazepam (1–2mg IM or 4–6h) | |
| Status IV: moderate NMS | Moderate rigidity, catatonia, or confusion, T ≤38–40°C, HR 100–120bpm | Discontinue APS, IV fluids management, initiate cooling measures, correct risk factors, provide intensive care | Lorazepam (1–2mg IM or 4–6h), bromocriptine (2.5–5mg PO/NGT 8h) or amantadine (100mg PO/NGT 8h) | Consider ECT (6–10 bilateral sessions) |
| Status V: severe NMS | Severe rigidity, catatonia, or coma, T ≥40°C, HR >120bpm | Discontinue APS, IV fluids management, initiate cooling measures, correct risk factors, provide intensive care | Dantrolene (1–2.5mg/kg of weight IV 6h for 48h) combined with bromocriptine (2.5–5mg PO/NGT 8h) or amantadine (100mg PO/NGT 8h) | Consider ECT (6–10 bilateral sessions) |
APS: antipsychotics; ECT: electroconvulsive therapy; HR: heart rate; IV: intravenous; NGT: nasogastric tube; NMS: Neuroleptic Malignant Syndrome. PO: by mouth; T: temperature.
In terms of pharmacotherapy, in some cases, it may be enough just to discontinue the triggering drug; however, complementary interventions will often be required, to a degree proportional to the severity.27,28
The first step routinely suggested is the BDZ. Results have been inconsistent, but in mild to moderate cases, by the oral as well as the parenteral route (lorazepam 1–2mg), they may alleviate symptoms and hasten recovery.29
Second-line pharmacotherapy includes the dopaminergic agonists. Amantadine and bromocriptine, alone or in combination, emerge in case series and meta-analyses as drugs that hasten recovery and reduce mortality. The usual dose of amantadine varies from 200 to 400mg/day in divided doses given orally or via nasogastric tube. Bromocriptine (2.5mg orally, 2–3 times per day, up to a maximum of 45mg) may exacerbate the underlying psychosis and cause hypotension; it may also cause vomiting, which means increased risk of aspiration in patients with reduced level of consciousness.30,31
In cases of NMS presenting with vomiting and achalasia, which complicates the oral administration of bromocriptine and amantadine, possible treatment with apomorphine has been described. A D2 agonist was used in Parkinson's disease and in sexual dysfunction, apomorphine was administered subcutaneously in a dose of 2mg every 3h for 3 days, and 2mg every 6h for 2 days,32 in the cases reviewed.
Dantrolene, which acts as a muscle relaxant by inhibiting the release of calcium from the sarcoplasmic reticulum, is also used in severe cases. It is used successfully in cases of MH secondary to anaesthetic agents. It should be used only in cases of severe rigidity, hyperthermia, and hypermetabolism. Although the hyperthermia and rigidity usually disappear quickly, discontinuing the drug too soon may cause them to reappear.
It is administered intravenously at a dose of 1–2.5mg/kg, initially, followed by 1mg/kg every 6h and switching to the oral route in a few days, if patient responds. Another option is a continuous intravenous infusion of dantrolene, which appears to be effective in patients with severe NMS with acute renal failure. This presents the risk of hepatotoxicity beyond 10mg/kg/day.
In comparison with supportive treatment alone, the use of dantrolene shows faster recovery and lower mortality,25,26 although some studies contradict this.33
There are cases published of good response to intravenous valproate (250mg the first day and 250mg every 12h starting on the second day); blood levels after 2 weeks were 87μg/mL.34
Electroconvulsive therapy in neuroleptic malignant syndromeThe effects of pharmacotherapy are thought to appear within the first few days, usually, and if they do not, then the drug is not likely to be effective.7,9,26,35 It is at this juncture where ECT gains popularity, for it appears to be effective in not only severe but also long-standing cases, with a marked reduction in mortality.36 Despite the traditional resistance to the use of ECT, there is enough data nowadays to have made it second-line treatment in NMS.14Hermesh et al.,37 in 1987, were the first to describe a case of NMS that responded to ECT. Subsequently, in their case series, Scheftner and Shulman38 and Trollor et al.39 proposed it as an effective treatment modality when drugs are not effective. Scheftner published responses with a latency of less than 72h following the first session, while Trollor found that there was usually an improvement starting with the sixth ECT session.39–41
Situations are described where it is recommended that ECT be used as first-line treatment, without waiting to see whether pharmacotherapy is effective. These would include not only severe cases with a high risk of complications but also cases where a diagnosis of lethal catatonia cannot be ruled out, or where the acute, NMS-related metabolic symptoms have been resolved but Parkinsonian or catatonia-like symptoms persist.7 One final indication proposed for the early use of ECT is when psychotic depression is the underlying disease.39,41
ECT reduces mortality in these situations. In severe cases, there is little chance of success with pharmacotherapy. When motor/catatonic symptoms predominate or persist, remember that NMS is linked with the concept of lethal catatonia, as we have mentioned. In view of conservative ECT consensus guidelines, such as those of the Royal College for 2005,42 that endorse ECT as the treatment of choice for malignant catatonia, it seems reasonable to begin treatment with ECT when the predominance of symptoms suggests the differential diagnosis or places them within the context of this global framework.
Data on efficacy are limited, inconsistent, and difficult to interpret because of the conceptual heterogeneity and variability of treatments.
Addonizio et al.43 obtained positive data on ECT with 43 of their patients. Trollor et al.39 reviewed 46 cases in the literature and 9 patients of their own. In 31 cases (56%), ECT was used after pharmacotherapy had failed, while in 40 cases (73%), it was the first-line treatment. Full recovery was seen in 25 cases (63%) and partial recovery in 11 cases (26%), with a total of 36 patients (90%) benefiting from the treatment.43,44
There is no obvious answer to the question of ECT's mechanism of action in NMS—a particularly persistent question that, as Reid45 points out, comes up whenever ECT is discussed. Data collected to date, however, seems to point in the direction of a mechanism based on an increased sensitivity to the neurotransmitter dopamine—and perhaps increased dopamine release, as well—which would link it to its long-established use in fully evolved Parkinsonism.14 There appears to be a boosting of serotonergic and noradrenergic transmission, as well.44
The use of ECT in NMS is not without risk, however. Because of both their severity and their frequency, the primary risks are cardiovascular complications due to ECT, MH due to substances used in anaesthesia, and hyperkalemia.32
Remember that, after the electrical stimulation, both blood pressure and heart rate drop, initially, and then come back up. The vagal stimulation that is triggered causes bradycardia and, on occasion, periods of asystole or even electrical silence43; this is quickly replaced by a tachycardia secondary to stimulation of the hypothalamus that, because of the increased cardiac work and the reduction in oxygen supply due to a shorter diastole, may result in an ischaemic accident and various types of arrhythmia.46,47
If, in addition, sub-convulsive stimuli are used, lengthy spells of bradycardia may occur—an argument often raised by critics of the titration method for individualized calculation of the initial stimulus dose.47–50 The risk of asystole increases with the use of beta blockers, and atropine is used—far too routinely, perhaps—to attenuate the bradycardia.
Thus, patients with a history of heart disease would be at greater risk for cardiovascular complications. In 2000, Brodaty et al.51 did not correlate the number and severity of adverse cardiovascular effects with the patient's age. Most of the cardiovascular changes are transitory. In 1993, Zielinski et al.52 published that the use of ECT in patients with severe heart disease is quite safe. In 2001, the APA21 upheld the safety of ECT use in the majority of cardiovascular patients. We have found no concrete data on increased cardiovascular risk with ECT in NMS.
There is disagreement as to the risk of MH from substances used in the anaesthesia for ECT, stemming from the resemblance between the two clinical Pictures53; their pathogeneses are distinct, however, and this risk has not been confirmed with either clinical or experimental data.50 Thus, as we have shown, while the changes in NMS stem from an essentially central effect due to dopaminergic hypoactivity, MH is believed to be a familial disorder due to an inositol 1,4,5-trisphosphate phosphatase deficiency, which would mean that there is a risk of hypercontractility of the muscle fibre with exposure to substances such as halothane, caffeine, or the depolarising muscle relaxants such as succinylcholine—but not with the antipsychotics.
The contradictory data from in vitro studies54–57 on muscle fibres of patients with MH and NMS—which, in both conditions and in comparison with healthy controls, would show hypercontractility upon administration of halothane—may be interpreted as a risk that, in patients with NMS, would justify excluding certain anaesthetic agents that may trigger MH. However, other authors58 propose that these findings could be read differently: in patients who have suffered both conditions, there would be an anomalous response of the sensitised muscle tissue upon exposure (in vitro) to substances such as halothane—without there being any connection between NMS and MH.
When that shared hypothetical risk is considered59,60—and when, because of the known risks of unmodified ECT, foregoing the relaxants is not an option9,37—the recommended alternative to the customary succinylcholine is the non-depolarising relaxants. Vecuronium, atracurium, and mivacurium have been used,8,61,62 but these options are not risk-free, either: the use of this type of relaxant significantly prolongs the anaesthesia time required and, consequently, increases the dose of anaesthetic agent, with its proportional increase in risks.
The use of succinylcholine has also been associated with the risk of hyperkalemia in various neuromuscular conditions, such as myopathies, traumatic muscle injuries, and toxic muscle damage.57,59,62–64 If we assume that rigidity is present in NMS and that this means muscle fibre damage, possibly to the point of myonecrosis, and if we also take into account the hypothetical direct damage to susceptible muscle fibres caused by the neuroleptics, with the ensuing release of potassium to the extracellular fluid due to membrane alterations, then consideration must be given to the possible risk in using depolarising muscle relaxants such as succinylcholine, for its mechanism involves a massive depolarisation of muscle fibres, with the requisite outflow of potassium and inflow of sodium from the external medium for achieving the exhaustion and ultimate relaxation of the myocyte following an exaggerated stimulus. These 2 factors in combination may elevate blood potassium to a level that could be arrhythmogenic. The alternative would be the non-depolarising muscle relaxants (atracurium, vecuronium, mivacurium, etc.), which would not provoke that massive outflow of potassium.8
The concomitant use of sugammadex, a selective reversal agent for rocuronium, is an alternative that would solve the problem of long-lasting effects from the non-depolarising relaxants that are substituted for succinylcholine in ECT for NMS. Recently introduced on the market, it enables muscle relaxation to be achieved quickly and without the adverse effects of the acetylcholinesterase inhibitors. The only drawbacks would be its high cost and the fact that it is contraindicated in severe renal insufficiency.65
The specific technical aspects of how ECT is applied in NMS—the same as in other circumstances except, perhaps, the depressive disorders—are based on intuitive consensus rather than on tests. Generally speaking, high-dose ECT is recommended, with bilateral placement, clearly suprathreshold stimuli, and lengthy series, which are justified in that the severity of the symptoms treated outweighs the risks taken.66
There is currently ongoing discussion about electrode placement67; while at the research level, it does make sense, at the clinical level, there is a clear consensus for ignoring the possible greater risk of cognitive impairment with bitemporal placement and taking advantage of its faster and safer response when it is a life-threatening situation.42,67
In the same regard, at a frequency of not 2 but 3 applications weekly—standard practice in most countries—an early response is ensured with an acceptable level of risk, given the severity of the situation.42
Although, as a general rule, we support calculating the threshold by titration, in situations like this where there is response urgency and increased medical risk, we believe that the exception listed—even in criteria like those of Britain's Royal College for 200442—is justified. We will calculate the stimulus dose on the basis of age (Petrides and Fink's “half of the age” method68) with other possible clinical or pharmacological variables taken into account, thereby avoiding the repeated sub-convulsive stimuli and lengthy sessions that are inevitable when the threshold is calculated by titration.
The use of “multiple ECT”—repeating 2–3 effective stimulations in a single anaesthesia session—has been proposed, with a certain amount of controversy. McKinney and Kellner69 present a heterogeneous case series suggesting that it is efficacious to repeat the stimulation in the same anaesthesia session. As Ray points out,66 this method is different from the one called MMECT, described by Maletzky70 in 1981, in which 4–6 convulsions are routinely produced per session. The evidence supporting 2–3 repetitions of the stimulus in the same anaesthesia session currently exists only in published case series, so it is not normally used in routine practice.
The response to ECT is typically seen early—generally after 6 sessions. The patient's sex, age, psychiatric diagnosis, and other diagnoses do not predict the response to ECT.37 In general, the recommendations are for 6–10 sessions—daily, if necessary—maintaining the minimum of 6 sessions, even if there is a response beforehand, to minimize the risk of relapse. The frequent use of BDZ in NMS may mean an elevated threshold, which will require adjustment of the stimulus dose, anaesthetics such as etomidate, or reversal with flumazenil.8 It is generally recommended that the BDZ could be continued during the application of ECT in NMS because, even though they raise the convulsive threshold, they appear to be synergistic with the ECT, and there is a beneficial effect that speeds recovery from NMS.71
Finally, once the NMS is resolved, there is the matter of re-establishing antipsychotic therapy, for which a preliminary 2-week waiting period is suggested.7 It is recommended that low-potency or atypical antipsychotics be given, starting with low doses and gradually titrating; clozapine is the agent of choice because it has less D2 affinity.34 Despite the precautions, resumption of antipsychotic drugs following NMS is associated with a 30% incidence of recurrence.
One alternative to resuming antipsychotics in patients who are at high risk for recurrence of NMS41,43 would be to use maintenance ECT in patients whose case was resolved this way—or even after resolution with pharmacotherapy.
ConclusionsNMS is a condition that is probably underdiagnosed in mild cases and at risk for misinterpretation in severe cases; still exists in this era of the new antipsychotics; and still carries a mortality rate of 10%. In addition to early diagnosis and smooth management of the routine drugs, we stress that proper thought must be given to ECT, in terms of both timing and technique.
The seriousness of these situations calls for ordering it early and using a technique that ensures its efficacy from the very first moment. We recommend bitemporal placement from the outset; a frequency of 3 times weekly; and clearly suprathreshold stimuli to ensure effective seizures with early response for at least 6 sessions.
It is also necessary to work closely with the anaesthesia team, carefully evaluating the increased cardiovascular risk and individualizing the use of atropine and beta blockers. While we do not think there is much reason to fear the increased risk of MH in NMS because of substances used in anaesthesia, we believe that there is good reason to use non-depolarising relaxants because of the risk of hyperkalemia upon exposure to succinylcholine.8
Finally, we believe that it is absolutely essential so that other specialists involved in the treatment become familiar with ECT; that the technique be more widely available; and that our training in it would be consistent with the level of complexity currently available.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Verdura Vizcaíno EJ, et al. Terapia electroconvulsiva como tratamiento del síndrome neuroléptico maligno. Rev Psiquiatr Salud Ment (Barc.). 2011;4:169–76.