Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosWe analyzed the impact of age, sex, vaccination against COVID-19, immunosuppressive treatment, and comorbidities on patients' risk of requiring hospital admission or of death.
MethodsPopulation-based observational retrospective study conducted on a cohort of 19,850 patients aged 12 years or more, who were diagnosed with COVID-19 between June 1st and December 31st, 2021, in the island of Gran Canaria.
ResultsHypertension (18.5%), asthma (12.8%) and diabetes (7.2%) were the most frequent comorbidities; 147 patients died (0.7%). The combination of advanced age, male sex, cancer, coronary heart disease, immunosuppressive treatment, hospital admission, admission to the intensive care unit, mechanical ventilation and lack of complete COVID-19 vaccination or booster dose was strongly predictive of mortality (p < 0.05); 831 patients required hospital admission and it was more frequent in men, older age groups, and patients with cancer, diabetes, arterial hypertension, chronic obstructive pulmonary disease, congestive heart failure or immunosuppressive treatment. The COVID-19 vaccine booster dose was associated with a lower risk of death ([OR] 0.11, 95% CI 0.06–0.21, p < 0.05) or hospital admission ([OR] 0.36, 95% CI 0.29–0.46, p < 0.05).
ConclusionsCancer, coronary heart disease, and immunosuppressive treatment were associated with increased COVID-19 mortality. More complete vaccination was associated with lower risk of hospital admission or death. Three doses of the SARS-CoV-2 vaccine were highly associated with the prevention of death and hospital admission in all age groups. These findings suggest that COVID-19 vaccination can help bring the pandemic under control.
Analizamos el impacto de la edad, el sexo, la vacunación frente a la COVID-19, el tratamiento inmunosupresor y las comorbilidades en el riesgo de los pacientes de precisar ingreso hospitalario o de fallecer.
MétodosEstudio retrospectivo observacional de base poblacional realizado sobre una cohorte de 19.850 pacientes de 12 años o más, que fueron diagnosticados de COVID-19 entre el 1 de junio y el 31 de diciembre de 2021, en la isla de Gran Canaria.
ResultadosLa hipertensión arterial (18,5%), el asma (12,8%) y la diabetes (7,2%) fueron las comorbilidades más frecuentes; Fallecieron 147 pacientes (0,7%). La combinación de edad avanzada, sexo masculino, cáncer, cardiopatía coronaria, tratamiento inmunosupresor, ingreso hospitalario, ingreso en unidad de cuidados intensivos, ventilación mecánica y la falta de vacunación completa contra el COVID-19 o dosis de refuerzo fue fuertemente predictiva de mortalidad (p < 0,05); 831 pacientes requirieron ingreso hospitalario y fue más frecuente en hombres, grupos de mayor edad y pacientes con cáncer, diabetes, hipertensión arterial, enfermedad pulmonar obstructiva crónica, insuficiencia cardiaca congestiva o tratamiento inmunosupresor. La dosis de refuerzo contra la vacuna del COVID-19 se asoció con un menor riesgo de muerte ([OR] 0.11, IC 95% 0.06–0.21, p < 0,05) o ingreso hospitalario ([OR] 0.36, IC 95% 0.29–0.46; p < 0,05).
ConclusionesEl cáncer, la enfermedad coronaria y el tratamiento inmunosupresor se asociaron con una mayor mortalidad por COVID-19. Una vacunación más completa se asoció con un menor riesgo de hospitalización o muerte. Tres dosis de la vacuna contra el SARS-CoV-2 se asociaron a una mayor prevención de la muerte y el ingreso hospitalario relacionados con la COVID-19 en todos los grupos de edad. Estos hallazgos sugieren que la vacunación contra el COVID-19 puede ayudar a controlar la pandemia.
The respiratory infection caused by SARS-CoV-2 was first documented by the end of December 2019 in Wuhan,1 from where it spread globally, and caused a pandemic with unprecedented consequences.2 As of June 22nd, 2022, there have been more than 545 million cases and more than 6,3 million deaths worldwide.3
Although most patients with SARS-CoV-2 infections develop mild to moderate symptoms, those with severe respiratory failure requiring admission to the intensive care unit (ICU) are at a higher risk of morbidity and show higher mortality rates.4–6 Several cohort studies have been published on the characteristics and outcomes of the COVID-19 pneumonia in such critical patients.4 However, most of the studies published up to date have been conducted with patient cohorts from North America6,7 and China,8 which may not represent the overall picture. In addition, many studies include a relatively small number of patients, which may hinder estimations of the outcome and burden of disease in these patients.9
The characteristics of COVID-19 patients that require hospitalization in Gran Canaria are not well known. This study analyzed the impact of COVID-19 patients' age, sex, COVID-19 vaccination, immunosuppressive therapy, and comorbidities on the risk of requiring hospital or ICU admission and on the risk of death. Our hypothesis was that patients admitted for COVID-19 would show high morbidity and mortality rates and that pre-existing comorbid conditions would be associated with a high risk of death.
MethodsDesign. Population-based, observational retrospective cohort study.
Study area. A cohort of 19,850 patients who lived in the island of Gran Canaria, Spain. 876.200 people resided in this island at the time of the study.
Eligibility criteria. The inclusion criteria were patients aged 12 years or more, who were diagnosed with COVID-19 between June 1st and December 31st, 2021, in the island of Gran Canaria. The exclusion criteria was as follows: age < 12 years.
Definitions. Patients were classified as suffering from: diabetes, if they had basal glycemia ≥126 mg/dl or were on anti-diabetes treatment; obesity, if they had BMI ≥ 30 kg/m2; hypertension, if they had diastolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or were on anti-hypertension treatment. The participants were defined as known OSAS when there was a previous sleep study and/or the initiation of treatment documented by a physician.
Confirmed COVID-19 cases. Patients who met the clinical criteria for suspected COVID-19 and showed positive results in AIDT (active infection diagnostic test); or asymptomatic patients with positive AIDT plus negative or not undertaken IgG-test. Suspected COVID-19 cases: Patients with acute respiratory infection of sudden onset of any degree of severity, who presented with fever, cough or shortness of breath, among other signs. Further signs or symptoms like odynophagia, anosmia, ageusia, muscle pain, diarrhea, chest pain, headache and others were also considered as symptoms of suspected SARS-CoV-2 infection, depending on the doctor's criterion.
Severe COVID-19 progression was defined as the need for hospital admission, ICU admission or mechanical ventilation.
Complete vaccination schedule. Patients were considered to be fully vaccinated if 1) they had received 2 doses of the vaccine separated by a minimum of: 19 days if the first dose was BNT162b2 mRNA (Pfizer-BioNTech), 21 days if it was ChAdOx1 nCoV-19 (AstraZeneca-University of Oxford), or 25 days if it was mRNA-1273 (Moderna); and 2) if the minimum time elapsed since the last dose was: 7 days if the last dose was Pfizer, or 14 days if it was AstraZeneca or Moderna. Patients were also considered to be fully vaccinated if they had received one dose of Ad26.COV2.S (Janssen) more than 14 days before. Patients up to 65 years old, were also considered as fully vaccinated if they had passed the disease and subsequently received a dose of any of the vaccines, after the corresponding mentioned period for the second dose. Subjects vaccinated with a heterologous schedule consisting of a first dose of AstraZeneca and a second dose of an mRNA vaccine were considered as fully vaccinated after 7 days, if the second dose was Pfizer or 14 days if it was Moderna.10
Variables. Personal history (asthma, cancer, dementia, diabetes, coronary heart disease, chronic obstructive pulmonary disease (COPD), auricular fibrillation (AF), hypertension, congestive heart failure (CHF), obesity), date of first, second and third doses of COVID-19 vaccine, type of COVID-19 vaccine (Pfizer, Moderna, AstraZeneca, Janssen), mechanical ventilation, hospital admission, ICU admission, immunosuppressive therapy, death.
Data source and collection. The identification data of all patients who were vaccinated against COVID-19 in Gran Canaria (from December 28th, 2020, to December 31st, 2021) were obtained from REGVACU (the registry of vaccination against COVID-19 in Spain). The identification data of all COVID-19 cases in Gran Canaria that were notified to the Epidemiological Surveillance Network of the Canary Islands (REVECA), were obtained from the General Directorate of Public Health (DGSP) (period: June 1st, 2021, to December 31st, 2021). Post-vaccination COVID-19 cases reported to the DGSP were identified by combining both databases. The clinical information of patients diagnosed with COVID-19 was obtained from their Primary Care electronic medical records (DRAGO AP). DRAGO is the healthcare management system of the Canary Islands.
Statistical analysis. A descriptive analysis of the results was carried out using frequency and percentages for categorical variables; and mean and standard deviation (SD) for analytical determinations or quantitative variables. Bivariate analysis of qualitative variables was carried out with the χ2 test, using the Likelihood Ratio when necessary. In addition to the bivariate analysis, a multivariable logistic regression model adjusting for predefined covariates was used to estimate the propensity scores for cohort participants. The models were used to determine the predictive values of death and hospitalization, which were defined as the dependent categorical variables in the analysis, adjusted by age, sex, immunosuppressive treatment, type of COVID-19 vaccine, complete vaccination schedule, booster dose (3rd dose) by age and comorbidities, including diabetes, coronary heart disease, auricular fibrillation, hypertension, COPD, asthma, CHF, cancer, obesity, OSAS and dementia. Statistical significance was established at 5% (p < 0.05), and the level of confidence was set at 95%. Data were analyzed with the Statistical Package for the Social Sciences (SPSS) v20 and Microsoft® Excel (2010).
Informed Consent Statement. This study was approved by the Ethics Committee for Research of the University Hospital of Gran Canaria Dr. Negrín (registration number 2021–355-1 COVID19) and it was compliant with the local laws and regulations, the Declaration of Helsinki, and the Good Clinical Practices. Patient consent was waived due to anonymization/dissociation of patient data and the results did not affect the clinical management of patients.
ResultsThe study included 19,850 patients diagnosed with COVID-19 between June and December, 2021, of whom 10,505 (52.9%) were women. The mean age was 40.7 years (SD 17.7), women being older than men on average (41.1 years vs. 40.2 years). The predominant age group in the sample was 18 to 49 years; 35% of patients presented some risk factor; the most frequent one was hypertension (18.5%), followed by asthma (12.8%) and diabetes (7.2%); 4.2% of patients were admitted to hospital; 1.1% required admission to the ICU, 0.3% required mechanical ventilation and 0.7% died.
Between June 1st and December 31st, 2021, a total of 513,295 subjects were vaccinated in Gran Canaria. The mean time elapsing between the completion of the vaccination schedule and the diagnóstico of COVID-19 was 135.7 days (SD 64.7).
The mean age of hospitalized COVID-19 patients was 60.5 years (interquartile range 44–67 years). More than half of them were men (54.8%, 455/831); 85.7% (712/831) were discharged to home; and 14.3% (119/831) died. The mean age of COVID-19 patients admitted to the ICU was 55.3 years (interquartile range 46–76 years). More than half of them were men (67.3%, 140/208); 85.6% (178/208) were discharged to home; and 14.4% (30/208) died. In survivors discharged to home and deceased patients, the proportion of subjects that needed ICU care or invasive mechanical ventilation was higher among 50-or-older ones than among 12–17 or 18–49 year-old ones; 0.46% (14/3051) of patients who had received a booster dose died, as compared to 0.80% (133/16,652) of those who had not (<0.05).
The mean hospital stay was 12.5 days (median 8 days) with a maximum of 130 days. The mean ICU stay was 13.5 days (median 9 days) with a maximum of 83 days.
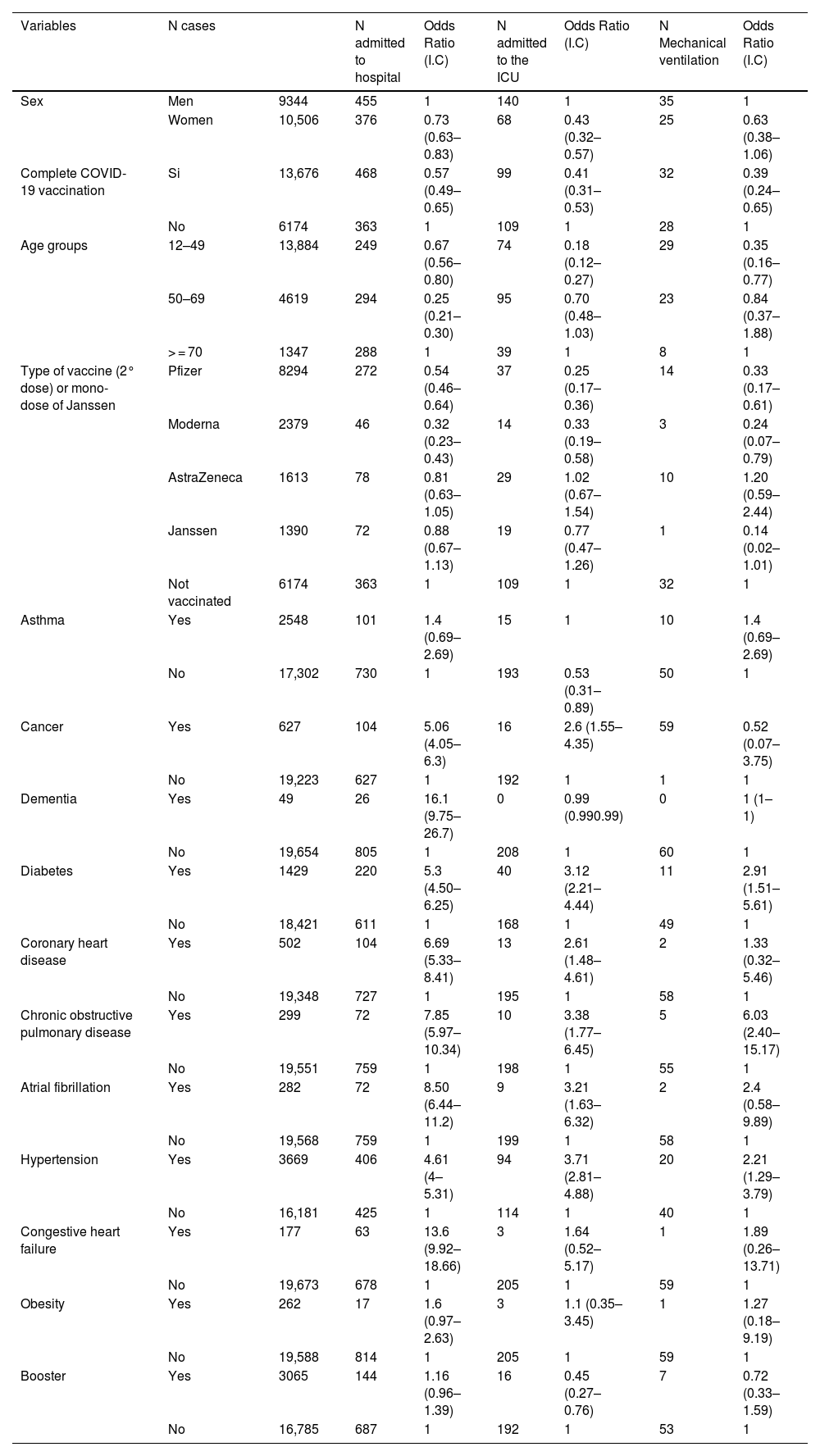
Subjects who had received mRNA vaccines (Pfizer/ BioNTech or Moderna) were at lower risk of needing hospital admission, ICU care or mechanical ventilation (p < 0.05) than those who were not vaccinated. No differences were found between the Janssen and Astrazeneca vaccines (p > 0.05). Table 1 illustrates the impact of patient's age, sex, COVID-19 vaccination, immunosuppressive treatment or comorbidities on the risk of requiring hospital admission, ICU care or mechanical ventilation. The risk of death was lower in patients vaccinated with Moderna or Janssen (p < 0.05) than in those who were not vaccinated (Table 2).
Bivariate analysis. Associations with hospital admission, admission to the ICU and mechanical ventilation.
| Variables | N cases | N admitted to hospital | Odds Ratio (I.C) | N admitted to the ICU | Odds Ratio (I.C) | N Mechanical ventilation | Odds Ratio (I.C) | |
|---|---|---|---|---|---|---|---|---|
| Sex | Men | 9344 | 455 | 1 | 140 | 1 | 35 | 1 |
| Women | 10,506 | 376 | 0.73 (0.63–0.83) | 68 | 0.43 (0.32–0.57) | 25 | 0.63 (0.38–1.06) | |
| Complete COVID-19 vaccination | Si | 13,676 | 468 | 0.57 (0.49–0.65) | 99 | 0.41 (0.31–0.53) | 32 | 0.39 (0.24–0.65) |
| No | 6174 | 363 | 1 | 109 | 1 | 28 | 1 | |
| Age groups | 12–49 | 13,884 | 249 | 0.67 (0.56–0.80) | 74 | 0.18 (0.12–0.27) | 29 | 0.35 (0.16–0.77) |
| 50–69 | 4619 | 294 | 0.25 (0.21–0.30) | 95 | 0.70 (0.48–1.03) | 23 | 0.84 (0.37–1.88) | |
| > = 70 | 1347 | 288 | 1 | 39 | 1 | 8 | 1 | |
| Type of vaccine (2° dose) or mono-dose of Janssen | Pfizer | 8294 | 272 | 0.54 (0.46–0.64) | 37 | 0.25 (0.17–0.36) | 14 | 0.33 (0.17–0.61) |
| Moderna | 2379 | 46 | 0.32 (0.23–0.43) | 14 | 0.33 (0.19–0.58) | 3 | 0.24 (0.07–0.79) | |
| AstraZeneca | 1613 | 78 | 0.81 (0.63–1.05) | 29 | 1.02 (0.67–1.54) | 10 | 1.20 (0.59–2.44) | |
| Janssen | 1390 | 72 | 0.88 (0.67–1.13) | 19 | 0.77 (0.47–1.26) | 1 | 0.14 (0.02–1.01) | |
| Not vaccinated | 6174 | 363 | 1 | 109 | 1 | 32 | 1 | |
| Asthma | Yes | 2548 | 101 | 1.4 (0.69–2.69) | 15 | 1 | 10 | 1.4 (0.69–2.69) |
| No | 17,302 | 730 | 1 | 193 | 0.53 (0.31–0.89) | 50 | 1 | |
| Cancer | Yes | 627 | 104 | 5.06 (4.05–6.3) | 16 | 2.6 (1.55–4.35) | 59 | 0.52 (0.07–3.75) |
| No | 19,223 | 627 | 1 | 192 | 1 | 1 | 1 | |
| Dementia | Yes | 49 | 26 | 16.1 (9.75–26.7) | 0 | 0.99 (0.990.99) | 0 | 1 (1–1) |
| No | 19,654 | 805 | 1 | 208 | 1 | 60 | 1 | |
| Diabetes | Yes | 1429 | 220 | 5.3 (4.50–6.25) | 40 | 3.12 (2.21–4.44) | 11 | 2.91 (1.51–5.61) |
| No | 18,421 | 611 | 1 | 168 | 1 | 49 | 1 | |
| Coronary heart disease | Yes | 502 | 104 | 6.69 (5.33–8.41) | 13 | 2.61 (1.48–4.61) | 2 | 1.33 (0.32–5.46) |
| No | 19,348 | 727 | 1 | 195 | 1 | 58 | 1 | |
| Chronic obstructive pulmonary disease | Yes | 299 | 72 | 7.85 (5.97–10.34) | 10 | 3.38 (1.77–6.45) | 5 | 6.03 (2.40–15.17) |
| No | 19,551 | 759 | 1 | 198 | 1 | 55 | 1 | |
| Atrial fibrillation | Yes | 282 | 72 | 8.50 (6.44–11.2) | 9 | 3.21 (1.63–6.32) | 2 | 2.4 (0.58–9.89) |
| No | 19,568 | 759 | 1 | 199 | 1 | 58 | 1 | |
| Hypertension | Yes | 3669 | 406 | 4.61 (4–5.31) | 94 | 3.71 (2.81–4.88) | 20 | 2.21 (1.29–3.79) |
| No | 16,181 | 425 | 1 | 114 | 1 | 40 | 1 | |
| Congestive heart failure | Yes | 177 | 63 | 13.6 (9.92–18.66) | 3 | 1.64 (0.52–5.17) | 1 | 1.89 (0.26–13.71) |
| No | 19,673 | 678 | 1 | 205 | 1 | 59 | 1 | |
| Obesity | Yes | 262 | 17 | 1.6 (0.97–2.63) | 3 | 1.1 (0.35–3.45) | 1 | 1.27 (0.18–9.19) |
| No | 19,588 | 814 | 1 | 205 | 1 | 59 | 1 | |
| Booster | Yes | 3065 | 144 | 1.16 (0.96–1.39) | 16 | 0.45 (0.27–0.76) | 7 | 0.72 (0.33–1.59) |
| No | 16,785 | 687 | 1 | 192 | 1 | 53 | 1 | |
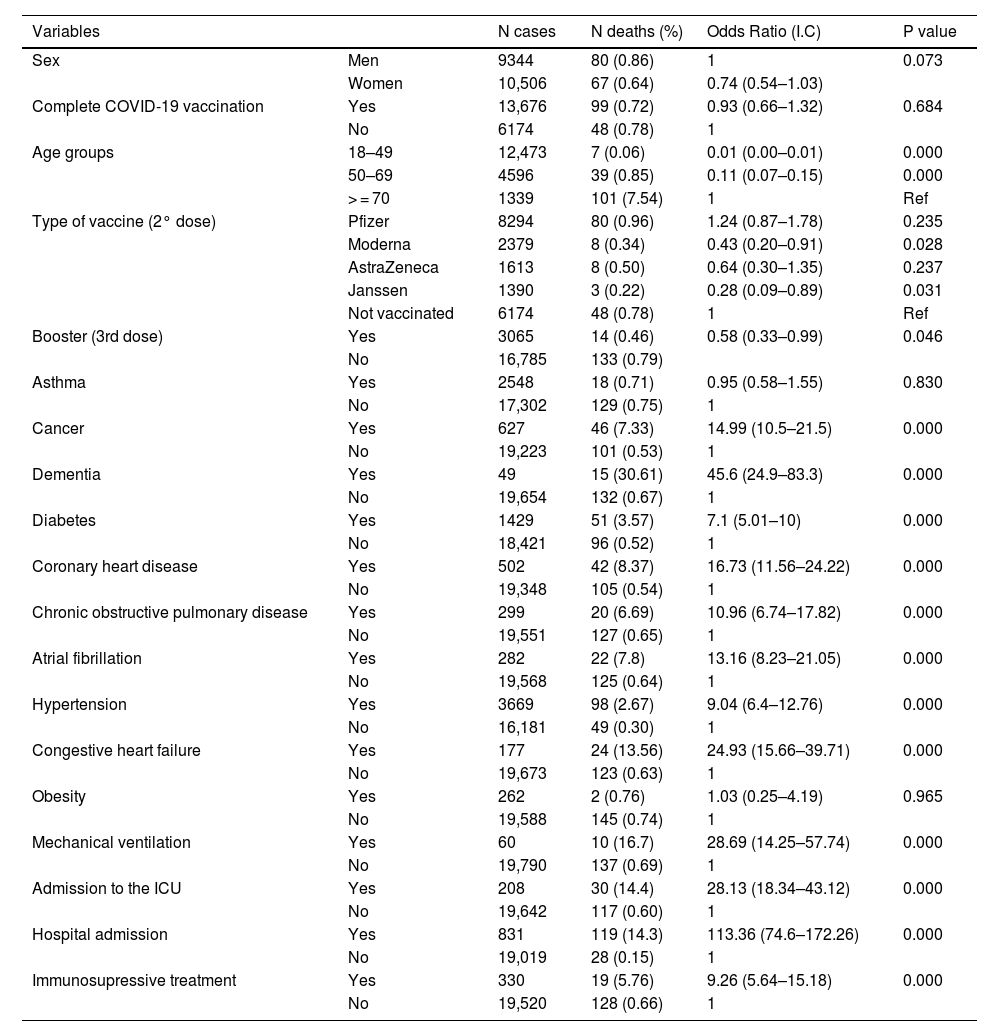
Bivariate analysis. Associations with mortality in the 147 deaths in a 19.850 population.
| Variables | N cases | N deaths (%) | Odds Ratio (I.C) | P value | |
|---|---|---|---|---|---|
| Sex | Men | 9344 | 80 (0.86) | 1 | 0.073 |
| Women | 10,506 | 67 (0.64) | 0.74 (0.54–1.03) | ||
| Complete COVID-19 vaccination | Yes | 13,676 | 99 (0.72) | 0.93 (0.66–1.32) | 0.684 |
| No | 6174 | 48 (0.78) | 1 | ||
| Age groups | 18–49 | 12,473 | 7 (0.06) | 0.01 (0.00–0.01) | 0.000 |
| 50–69 | 4596 | 39 (0.85) | 0.11 (0.07–0.15) | 0.000 | |
| > = 70 | 1339 | 101 (7.54) | 1 | Ref | |
| Type of vaccine (2° dose) | Pfizer | 8294 | 80 (0.96) | 1.24 (0.87–1.78) | 0.235 |
| Moderna | 2379 | 8 (0.34) | 0.43 (0.20–0.91) | 0.028 | |
| AstraZeneca | 1613 | 8 (0.50) | 0.64 (0.30–1.35) | 0.237 | |
| Janssen | 1390 | 3 (0.22) | 0.28 (0.09–0.89) | 0.031 | |
| Not vaccinated | 6174 | 48 (0.78) | 1 | Ref | |
| Booster (3rd dose) | Yes | 3065 | 14 (0.46) | 0.58 (0.33–0.99) | 0.046 |
| No | 16,785 | 133 (0.79) | |||
| Asthma | Yes | 2548 | 18 (0.71) | 0.95 (0.58–1.55) | 0.830 |
| No | 17,302 | 129 (0.75) | 1 | ||
| Cancer | Yes | 627 | 46 (7.33) | 14.99 (10.5–21.5) | 0.000 |
| No | 19,223 | 101 (0.53) | 1 | ||
| Dementia | Yes | 49 | 15 (30.61) | 45.6 (24.9–83.3) | 0.000 |
| No | 19,654 | 132 (0.67) | 1 | ||
| Diabetes | Yes | 1429 | 51 (3.57) | 7.1 (5.01–10) | 0.000 |
| No | 18,421 | 96 (0.52) | 1 | ||
| Coronary heart disease | Yes | 502 | 42 (8.37) | 16.73 (11.56–24.22) | 0.000 |
| No | 19,348 | 105 (0.54) | 1 | ||
| Chronic obstructive pulmonary disease | Yes | 299 | 20 (6.69) | 10.96 (6.74–17.82) | 0.000 |
| No | 19,551 | 127 (0.65) | 1 | ||
| Atrial fibrillation | Yes | 282 | 22 (7.8) | 13.16 (8.23–21.05) | 0.000 |
| No | 19,568 | 125 (0.64) | 1 | ||
| Hypertension | Yes | 3669 | 98 (2.67) | 9.04 (6.4–12.76) | 0.000 |
| No | 16,181 | 49 (0.30) | 1 | ||
| Congestive heart failure | Yes | 177 | 24 (13.56) | 24.93 (15.66–39.71) | 0.000 |
| No | 19,673 | 123 (0.63) | 1 | ||
| Obesity | Yes | 262 | 2 (0.76) | 1.03 (0.25–4.19) | 0.965 |
| No | 19,588 | 145 (0.74) | 1 | ||
| Mechanical ventilation | Yes | 60 | 10 (16.7) | 28.69 (14.25–57.74) | 0.000 |
| No | 19,790 | 137 (0.69) | 1 | ||
| Admission to the ICU | Yes | 208 | 30 (14.4) | 28.13 (18.34–43.12) | 0.000 |
| No | 19,642 | 117 (0.60) | 1 | ||
| Hospital admission | Yes | 831 | 119 (14.3) | 113.36 (74.6–172.26) | 0.000 |
| No | 19,019 | 28 (0.15) | 1 | ||
| Immunosupressive treatment | Yes | 330 | 19 (5.76) | 9.26 (5.64–15.18) | 0.000 |
| No | 19,520 | 128 (0.66) | 1 | ||
A multivariate logistic regression analysis (Tables 3 and 4) revealed that older patients, men, subjects with personal history issues (cancer or coronary heart disease), and those under immunosuppressive treatment were more likely to develop severe post-vaccine COVID-19 (often requiring hospital admission, ICU care or mechanical ventilation) or to die (p < 0.05). The COVID-19 vaccine booster dose was associated with a lower risk of death ([OR] 0.11, 95% CI 0.06–0.21, p < 0.05) or hospital admission ([OR] 0.36, 95% CI 0.29–0.46, p < 0.05). No association was found with asthma, obesity, hypertension or diabetes (p > 0.05).
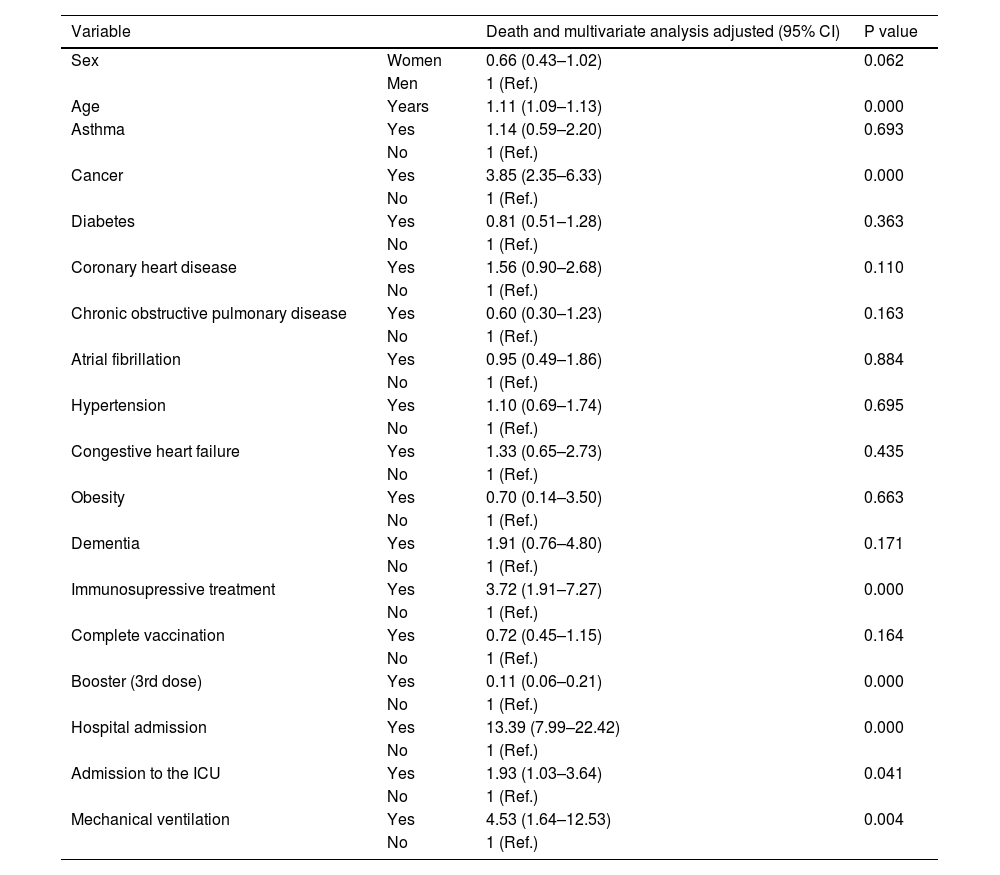
Death according to gender and age, and association with obesity, diabetes, hypertension, cancer, coronary heart disease, COPD, CHD and dementia in 110,726 in-patients positive for SARS-CoV-2.
| Variable | Death and multivariate analysis adjusted (95% CI) | P value | |
|---|---|---|---|
| Sex | Women | 0.66 (0.43–1.02) | 0.062 |
| Men | 1 (Ref.) | ||
| Age | Years | 1.11 (1.09–1.13) | 0.000 |
| Asthma | Yes | 1.14 (0.59–2.20) | 0.693 |
| No | 1 (Ref.) | ||
| Cancer | Yes | 3.85 (2.35–6.33) | 0.000 |
| No | 1 (Ref.) | ||
| Diabetes | Yes | 0.81 (0.51–1.28) | 0.363 |
| No | 1 (Ref.) | ||
| Coronary heart disease | Yes | 1.56 (0.90–2.68) | 0.110 |
| No | 1 (Ref.) | ||
| Chronic obstructive pulmonary disease | Yes | 0.60 (0.30–1.23) | 0.163 |
| No | 1 (Ref.) | ||
| Atrial fibrillation | Yes | 0.95 (0.49–1.86) | 0.884 |
| No | 1 (Ref.) | ||
| Hypertension | Yes | 1.10 (0.69–1.74) | 0.695 |
| No | 1 (Ref.) | ||
| Congestive heart failure | Yes | 1.33 (0.65–2.73) | 0.435 |
| No | 1 (Ref.) | ||
| Obesity | Yes | 0.70 (0.14–3.50) | 0.663 |
| No | 1 (Ref.) | ||
| Dementia | Yes | 1.91 (0.76–4.80) | 0.171 |
| No | 1 (Ref.) | ||
| Immunosupressive treatment | Yes | 3.72 (1.91–7.27) | 0.000 |
| No | 1 (Ref.) | ||
| Complete vaccination | Yes | 0.72 (0.45–1.15) | 0.164 |
| No | 1 (Ref.) | ||
| Booster (3rd dose) | Yes | 0.11 (0.06–0.21) | 0.000 |
| No | 1 (Ref.) | ||
| Hospital admission | Yes | 13.39 (7.99–22.42) | 0.000 |
| No | 1 (Ref.) | ||
| Admission to the ICU | Yes | 1.93 (1.03–3.64) | 0.041 |
| No | 1 (Ref.) | ||
| Mechanical ventilation | Yes | 4.53 (1.64–12.53) | 0.004 |
| No | 1 (Ref.) | ||
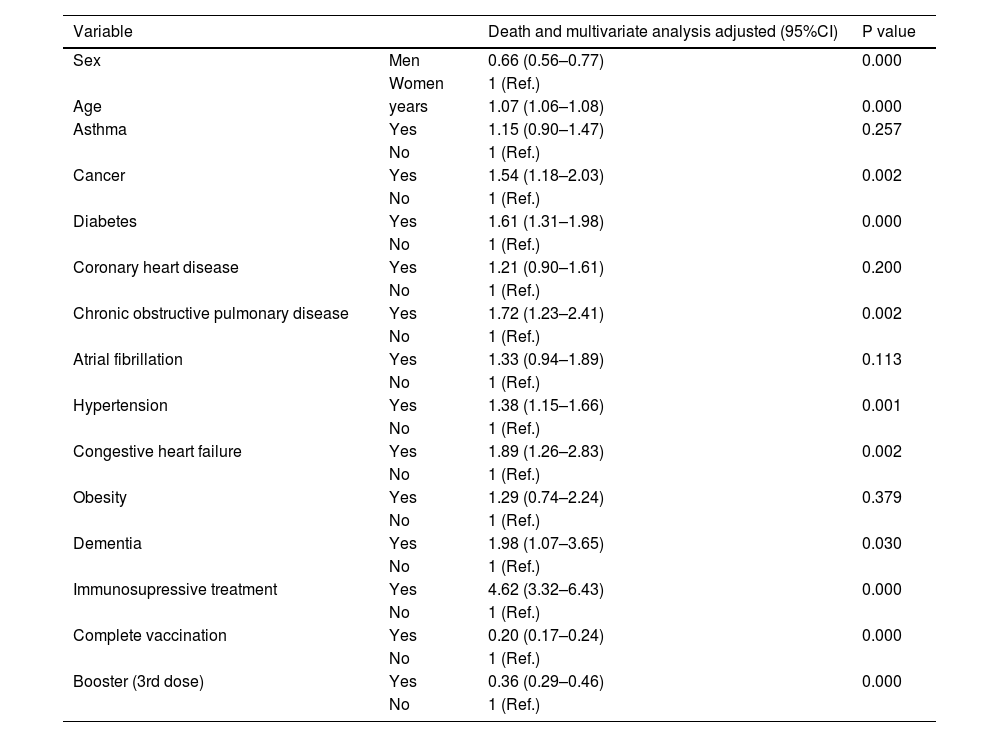
Hospitalization according to gender and age, and association with obesity, diabetes, hypertension, cancer, coronary heart disease, COPD, CHD and dementia in 110,726 in-patients positive for SARS-CoV-2.
| Variable | Death and multivariate analysis adjusted (95%CI) | P value | |
|---|---|---|---|
| Sex | Men | 0.66 (0.56–0.77) | 0.000 |
| Women | 1 (Ref.) | ||
| Age | years | 1.07 (1.06–1.08) | 0.000 |
| Asthma | Yes | 1.15 (0.90–1.47) | 0.257 |
| No | 1 (Ref.) | ||
| Cancer | Yes | 1.54 (1.18–2.03) | 0.002 |
| No | 1 (Ref.) | ||
| Diabetes | Yes | 1.61 (1.31–1.98) | 0.000 |
| No | 1 (Ref.) | ||
| Coronary heart disease | Yes | 1.21 (0.90–1.61) | 0.200 |
| No | 1 (Ref.) | ||
| Chronic obstructive pulmonary disease | Yes | 1.72 (1.23–2.41) | 0.002 |
| No | 1 (Ref.) | ||
| Atrial fibrillation | Yes | 1.33 (0.94–1.89) | 0.113 |
| No | 1 (Ref.) | ||
| Hypertension | Yes | 1.38 (1.15–1.66) | 0.001 |
| No | 1 (Ref.) | ||
| Congestive heart failure | Yes | 1.89 (1.26–2.83) | 0.002 |
| No | 1 (Ref.) | ||
| Obesity | Yes | 1.29 (0.74–2.24) | 0.379 |
| No | 1 (Ref.) | ||
| Dementia | Yes | 1.98 (1.07–3.65) | 0.030 |
| No | 1 (Ref.) | ||
| Immunosupressive treatment | Yes | 4.62 (3.32–6.43) | 0.000 |
| No | 1 (Ref.) | ||
| Complete vaccination | Yes | 0.20 (0.17–0.24) | 0.000 |
| No | 1 (Ref.) | ||
| Booster (3rd dose) | Yes | 0.36 (0.29–0.46) | 0.000 |
| No | 1 (Ref.) | ||
This study is the study with the largest number of subjects up to date, to describe the clinical and epidemiological characteristics of hospitalized COVID-19 patients in Gran Canaria. The data, corresponding to the last 7 months (June to December 2021) illustrate the new reality of the disease in a population with high vaccination rates.
The main findings of our study showed that more complete vaccination was associated with less frequent risk of death or hospital admission. These findings suggest that COVID-19 vaccination can help in bringing the pandemic under control.
Factors associated with greater probability of requiring hospital admission included: older age, male sex, diabetes, hypertension, cancer, CHF, COPD, and immunosuppressive treatment. These findings can help healthcare professionals identify patients at higher risk of hospitalization, who may require closer monitorization and care, and those who may benefit from specific preventive or therapeutic interventions.
Among patients who required hospital admission, mortality was 14.3%. The mortality rate in this study was lower than the rates reported for other hospitalized patient cohorts, in earlier studies (approximately 15% to >20%).10–15
Our results showed that 25% (95% CI 22.2–28.1%) of hospitalized patients required ICU admission. The mortality rate for these patients was 14.5% (95% CI 10.3–19.8%). These findings are in agreement with those of a meta-analysis published by Rodríguez et al., in which 20.3% (95% CI 10.0–30.6%) required ICU admission and the mortality rate was 13.9% (95% CI 6.2–21.5%).16
In our analysis, the magnitude of the risk of hospitalization for COVID-19 was lower in patients with asthma, obesity, dementia, auricular fibrillation, and coronary heart disease than in those with other medical conditions (e.g., COPD). This finding is in line with the results of Aveyard et al., who showed that the risk of severe COVID-19 in people with asthma was relatively low. Subjects with COPD appeared to have a moderately higher risk of suffering a severe illness or requiring hospital admission, but their risk of death from COVID-19 at the height of the pandemic was generally lower than the normal risk of death from any cause.17
Although it is considered a risk factor for the acquisition of COVID-19, the role of immunosuppression after a SARS-CoV-2 infection has not been extensively studied. In this study, receiving immunosuppressive therapy before COVID-19 diagnóstico was identified as a unique risk factor for hospital admission, ICU admission, mechanical ventilation, and death from COVID-19; in agreement with Akama-Garren et al.,18 who found that the use of immunosuppressive treatment could be associated with a slightly increased risk of severe COVID-19 or death. These findings demonstrate that COVID-19 is more severe in patients who are already taking immunosuppressive medication and emphasize the need of providing aggressive monitoring and supportive care to immunocompromised patients diagnosed with COVID-19.19
Subjects with comorbidities and older subjects (who often present comorbidities) are especially vulnerable to acquire acute COVID-19 infection and to meet the criteria for severity during the acute phase, with the consequent aftereffects for survivors. Increased morbidity and mortality in older patients and in patients with comorbidities have been associated with both comorbidities and frailty, which entail poorer immune response.20
There may also be protective factors for the post-COVID-19 condition, since the results of a recent study suggested that vaccines may offer protection.21 In our study, receiving two doses of the COVID-19 vaccine was associated with a decreased risk of death. Arbel et al. demonstrated that participants who had received a booster dose at least 5 months after a second dose of Pfizer-BioNTech had 90% less short-term Covid-19 mortality than participants who did not receive a booster.22
Complete COVID-19 vaccination was significantly less frequent than no-vaccination among patients with outcomes of hospital admission, mechanical ventilation or death. These findings are consistent with the literature.23,24
A systematic review revealed that patients undergoing cancer treatment, such as chemotherapy, had a higher risk of death from COVID-19.25 In our study, the overall prevalence of active cancer as a comorbidity was 3.2%, and it was an independent factor associated with mortality in a multivariate analysis. Other authors like Xiaochen Li et al. provided substantial statistical evidence for the value of coronary heart disease as a predictor of COVID-19 like a risk factor for severe cases on admission.26
Our findings showed that diabetes and hypertension were comorbidities associated with an increased risk of hospitalization. These findings are consistent with those of Cascella et al. who concluded that 49% of the cases that required ICU admission for COVID-19 suffered from pre-existing comorbidities; and with the results of Mughal et al. who showed that comorbidities such as obesity, diabetes or hypertension increased the severity and the mortality rates (10.5% with comorbidity vs. 0.9% without comorbidity).27,28
CHF was associated with an increased risk of hospital admission in a multivariate analysis. Angeli et al. also found that this condition was an independent predictor of adverse prognosis and death in COVID-19 patients.29
The severity of COVID-19 has changed through the successive epidemic waves, likely due to increasing population immunity (caused both by vaccination and by ongoing virus circulation) and possibly to a different intrinsic virulence of SARS-CoV-2 variants. While Delta variant was generally (though inconsistently) associated to increased risk of severe disease compared to the previously dominant Alpha variant, results from different countries have pointed to a lower severity of Omicron. On the other hand, vaccine effectiveness against severe COVID-19 with Delta variant was found well preserved compared to Alpha, but evidence for severe COVID-19 with Omicron is less consistent.
There are contextual factors that may affect the estimates of variant severity as well as the variant-specific vaccine effectiveness, such as the intensity of previous circulation of other SARS-CoV-2 variants in the territory or particular characteristics of the COVID-19 vaccination rollout.30
In the period in which this study was carried out, the Alpha variant was the dominant one in Gran Canaria until week 22 of the year 2021, until later the Delta variant became dominant until week 50 of the same year. Finally, the Ómicron variant of COVID-19 was the dominant one in Gran Canaria, accounting for 54.8% of infections at the end of 2021 and coinciding with the final period of this study.31
Our study has some limitations. The description of severe cases is limited by the reduced number of patients in this category. There are also certain epidemiological limitations to be considered when interpreting the data like the heterogeneity of samples in terms of age (younger vs. older age groups), or severity of COVID-19 (patients with mild forms vs. patients admitted to hospital or to the ICU). The main strength of this study is the size of the sample, which consisted of a large number of participants, much higher than most of the Spanish studies on this subject.
Receiving a complete vaccination schedule against SARS-CoV-2 and a booster dose effectively reduced hospitalization and death from COVID-19. These findings highlight the benefits of providing SARS-CoV-2 immunization including a support dose for a complete vaccination.
In conclusion, cancer, coronary heart disease, and immunosuppressive treatment were associated with increased COVID-19 mortality. More complete vaccination was associated with lower risk of hospital admission and death. Two doses of the SARS-CoV-2 vaccine were highly effective in preventing COVID-19-related deaths and hospital admission in all age groups.
Sources of supportWe would like to thank the following entities for collaboration and funding: Fundación DISA (A38445839) and Fundación Española de Calidad Asistencial (G74295718), without whom this study would not have been carried out. We would also like to thank to all the people who voluntarily participated in the study
Authors' participationAll the authors participated in the design of the study, data collection and preparation of the manuscript, and they declare that they approve its final version and are publicly responsible for its content.