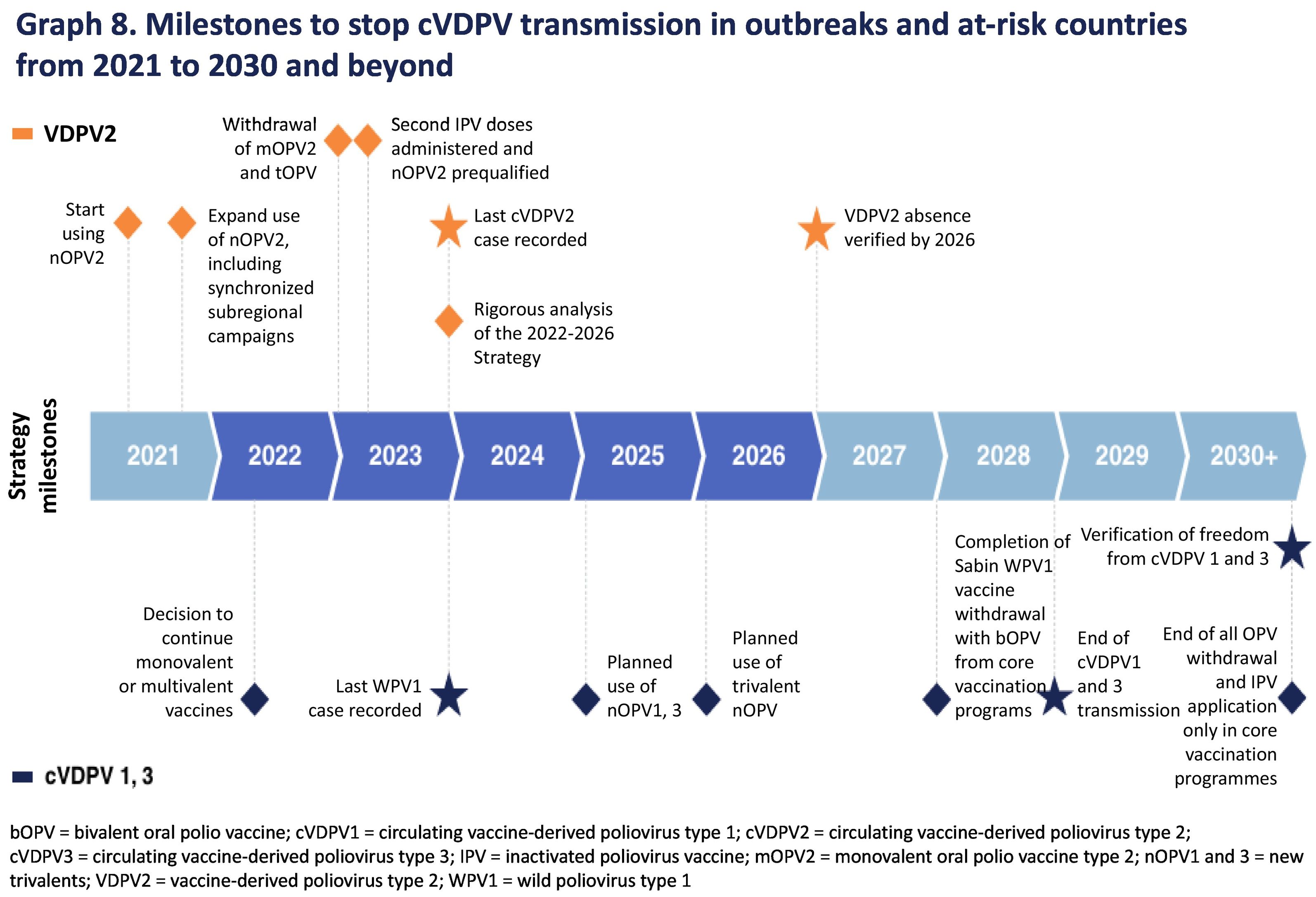
array:22 [ "pii" => "S244514602400075X" "issn" => "24451460" "doi" => "10.1016/j.vacune.2024.10.004" "estado" => "S200" "fechaPublicacion" => "2024-10-30" "aid" => "376" "copyrightAnyo" => "2024" "documento" => "article" "crossmark" => 0 "subdocumento" => "sco" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "Traduccion" => array:1 [ "es" => array:19 [ "pii" => "S157698872400089X" "issn" => "15769887" "doi" => "10.1016/j.vacun.2024.09.003" "estado" => "S300" "fechaPublicacion" => "2024-10-01" "aid" => "376" "copyright" => "Elsevier España, S.L.U." "documento" => "article" "crossmark" => 1 "subdocumento" => "sco" "cita" => "Vacunas. 2024;25:436-8" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "es" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Editorial</span>" "titulo" => "Vacuna polio oral de nueva generación ¿solución para la erradicación?" "tienePdf" => "es" "tieneTextoCompleto" => "es" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "436" "paginaFinal" => "438" ] ] "titulosAlternativos" => array:1 [ "en" => array:1 [ "titulo" => "Next-generation oral polio vaccine: The solution to reach eradication?" ] ] "contieneTextoCompleto" => array:1 [ "es" => true ] "contienePdf" => array:1 [ "es" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:8 [ "identificador" => "f0005" "etiqueta" => "Figura 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 1995 "Ancho" => 3160 "Tamanyo" => 469781 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "al0005" "detalle" => "Figura " "rol" => "short" ] ] "descripcion" => array:1 [ "es" => "<p id="sp9055" class="elsevierStyleSimplePara elsevierViewall">Hitos para detener la transmisión de cVDPV en los brotes epidémicos y los países en riesgo de 2021 a 2030 y más.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Nuria Torner" "autores" => array:1 [ 0 => array:2 [ "nombre" => "Nuria" "apellidos" => "Torner" ] ] ] ] ] "idiomaDefecto" => "es" "Traduccion" => array:1 [ "en" => array:9 [ "pii" => "S244514602400075X" "doi" => "10.1016/j.vacune.2024.10.004" "estado" => "S200" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "en" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S244514602400075X?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S157698872400089X?idApp=UINPBA00004N" "url" => "/15769887/0000002500000004/v1_202410301224/S157698872400089X/v1_202410301224/es/main.assets" ] ] "itemSiguiente" => array:17 [ "pii" => "S2445146024000748" "issn" => "24451460" "doi" => "10.1016/j.vacune.2024.10.003" "estado" => "S200" "fechaPublicacion" => "2024-10-31" "aid" => "367" "documento" => "article" "crossmark" => 0 "subdocumento" => "fla" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:12 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Original article</span>" "titulo" => "Importance of optimizing routine immunization schedules in pediatric heart transplantation" "tienePdf" => "en" "tieneTextoCompleto" => "en" "tieneResumen" => array:2 [ 0 => "en" 1 => "es" ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Importancia de la optimización de los calendarios de vacunación en el trasplante cardíaco infantil" ] ] "contieneResumen" => array:2 [ "en" => true "es" => true ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:8 [ "identificador" => "f0010" "etiqueta" => "Fig. 2" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr2.jpeg" "Alto" => 1486 "Ancho" => 2313 "Tamanyo" => 150507 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "al0010" "detalle" => "Fig. " "rol" => "short" ] ] "descripcion" => array:1 [ "en" => "<p id="sp0020" class="elsevierStyleSimplePara elsevierViewall">Vaccine coverage for each vaccine after HT.</p> <p id="sp0025" class="elsevierStyleSimplePara elsevierViewall">Individual analysis and grouped into funded and non-funded. All of them are inactivated since no attenuated vaccines are administered post-HT.</p> <p id="sp0030" class="elsevierStyleSimplePara elsevierViewall">DTP: diphtheria–tetanus–pertussis; HBV: hepatitis B; Hib: <span class="elsevierStyleItalic">Haemophilus influenzae</span> type b; MenACWY: meningococcal ACWY; MenB: meningococcal B; MenC: meningococcal C; Polio: polio; T Td: tetanus–diphtheria; TV: triple virus; VCN13: pneumococcal conjugate 13-valent; VPN23: pneumococcal polysaccharide 23; VZV: varicella zoster.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Sofía Bassy Navarro, Nuria Gil Villanueva, Manuela Camino-López, Roberto Alonso Fernández, Beatriz Lázaro-Martín, Alicia Hernanz-Lobo, Marisa Navarro Gómez" "autores" => array:7 [ 0 => array:2 [ "nombre" => "Sofía" "apellidos" => "Bassy Navarro" ] 1 => array:2 [ "nombre" => "Nuria" "apellidos" => "Gil Villanueva" ] 2 => array:2 [ "nombre" => "Manuela" "apellidos" => "Camino-López" ] 3 => array:2 [ "nombre" => "Roberto" "apellidos" => "Alonso Fernández" ] 4 => array:2 [ "nombre" => "Beatriz" "apellidos" => "Lázaro-Martín" ] 5 => array:2 [ "nombre" => "Alicia" "apellidos" => "Hernanz-Lobo" ] 6 => array:2 [ "nombre" => "Marisa" "apellidos" => "Navarro Gómez" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S1576988724000712" "doi" => "10.1016/j.vacun.2024.07.002" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S1576988724000712?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2445146024000748?idApp=UINPBA00004N" "url" => "/24451460/unassign/S2445146024000748/v1_202410310612/en/main.assets" ] "itemAnterior" => array:18 [ "pii" => "S2445146024000724" "issn" => "24451460" "doi" => "10.1016/j.vacune.2024.10.001" "estado" => "S200" "fechaPublicacion" => "2024-10-30" "aid" => "361" "copyright" => "The Authors" "documento" => "article" "crossmark" => 0 "subdocumento" => "rev" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:12 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Review article</span>" "titulo" => "Review of vaccine packaging formats and their key features" "tienePdf" => "en" "tieneTextoCompleto" => "en" "tieneResumen" => array:2 [ 0 => "en" 1 => "es" ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Revisión de los formatos de presentación de vacunas y sus principales características" ] ] "contieneResumen" => array:2 [ "en" => true "es" => true ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:8 [ "identificador" => "f0010" "etiqueta" => "Fig. 2" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr2.jpeg" "Alto" => 2288 "Ancho" => 3160 "Tamanyo" => 379645 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "al0010" "detalle" => "Fig. " "rol" => "short" ] ] "descripcion" => array:1 [ "en" => "<p id="sp0010" class="elsevierStyleSimplePara elsevierViewall">Proposal of essential concepts in types of vaccine presentation (own production).</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Silvia Domínguez-Fernández, Jose Antonio Forcada-Segarra, Inmaculada Cuesta-Esteve, Silvia Fernández-Fernández, Rosario Cáceres Fernández-Bolaños, Guadalupe Fontán-Vinagre, Roberto Guerrero-Menéndez" "autores" => array:7 [ 0 => array:2 [ "nombre" => "Silvia" "apellidos" => "Domínguez-Fernández" ] 1 => array:2 [ "nombre" => "Jose Antonio" "apellidos" => "Forcada-Segarra" ] 2 => array:2 [ "nombre" => "Inmaculada" "apellidos" => "Cuesta-Esteve" ] 3 => array:2 [ "nombre" => "Silvia" "apellidos" => "Fernández-Fernández" ] 4 => array:2 [ "nombre" => "Rosario" "apellidos" => "Cáceres Fernández-Bolaños" ] 5 => array:2 [ "nombre" => "Guadalupe" "apellidos" => "Fontán-Vinagre" ] 6 => array:2 [ "nombre" => "Roberto" "apellidos" => "Guerrero-Menéndez" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S1576988724000487" "doi" => "10.1016/j.vacun.2024.06.004" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S1576988724000487?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2445146024000724?idApp=UINPBA00004N" "url" => "/24451460/unassign/S2445146024000724/v1_202410300908/en/main.assets" ] "en" => array:14 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Editorial</span>" "titulo" => "Next-generation oral polio vaccine: The solution to reach eradication?" "tieneTextoCompleto" => true "autores" => array:1 [ 0 => array:3 [ "autoresLista" => "Nuria Torner" "autores" => array:1 [ 0 => array:3 [ "nombre" => "Nuria" "apellidos" => "Torner" "email" => array:1 [ 0 => "nuriatorner@ateneu.ub.edu" ] ] ] "afiliaciones" => array:1 [ 0 => array:2 [ "entidad" => "CIBER Epidemiología y Salud Pública CIBERESP, Unidad de Medicina Preventiva y Salud Pública, Departamento de Medicina, Universitat de Barcelona, Barcelona, Spain" "identificador" => "af0005" ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Vacuna polio oral de nueva generación: ¿solución para la erradicación?" ] ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:8 [ "identificador" => "f0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 2173 "Ancho" => 3188 "Tamanyo" => 489342 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "al0005" "detalle" => "Fig. " "rol" => "short" ] ] "descripcion" => array:1 [ "en" => "<p id="sp0005" class="elsevierStyleSimplePara elsevierViewall">Milestones for stopping cVDPV transmission in outbreaks and at-risk countries from 2021 to 2030 and beyond.</p>" ] ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="p0005" class="elsevierStylePara elsevierViewall">The aim of the Global Polio Eradication Initiative (GPEI) is to ensure that no child is ever paralysed again, either by wild polioviruses or vaccine-derived polioviruses. A secondary important objective consists of ensuring that the outcome achieved can be sustained for future generations and that the infrastructure developed to eradicate polio serves to obtain public health benefits in emergency situations after the global disappearance of the disease.<a class="elsevierStyleCrossRef" href="#bb0005"><span class="elsevierStyleSup">1</span></a></p><p id="p0010" class="elsevierStylePara elsevierViewall">The foundation of this global certification of the eradication of wild polioviruses has been achieved for 2 of the 3 types that cause polio, wild poliovirus type 2 (PV2) in 2015, and wild poliovirus type 3 (PV3) in 2019. Certification of elimination of wild poliovirus type 1 (PV1) will mark the beginning of the post-eradication era and its associated strategies. It is vital to ensure that appropriate post-eradication vaccination plans are in place to minimise the risk and consequences of a possible re-emergence of poliovirus in a post-eradication world. One of the main risks of a possible reintroduction of polio in the post-eradication period is the continued use of oral polio vaccines (OPV) in routine vaccination programmes, due to their potential to produce outbreaks of infectious vaccine-derived poliovirus variants. The OPV that has brought wild poliovirus to the brink of eradication clearly has many benefits, as the live attenuated (weakened) virus in the vaccine provides enhanced immunity in the gut, where it replicates. However, the vaccine virus is shedded in faeces and therefore in communities with poor sanitation this translates into the potential for person-to-person transmission if antigenic drift of the virus were to occur. In communities with low vaccination rates, as the virus is transmitted from one unvaccinated child to another over a long period of time (often over the course of 12–18 months), it can mutate into a form that causes paralysis in the same way as wild poliovirus does.<a class="elsevierStyleCrossRef" href="#bb0010"><span class="elsevierStyleSup">2</span></a> The mutated poliovirus can then spread in communities, giving rise to circulating vaccine-derived polioviruses (cVDPV). The inadvertent release of poliovirus from a research or diagnostic laboratory or vaccine manufacturing facility could also occur. A third risk is associated with shedding by immunocompromised carriers (iVDPV), which is currently a theoretical risk as secondary spread leading to outbreaks caused by iVDPV has never been clearly established. However, this theoretical risk also needs to be addressed. The most prevalent cVDPV is cVDPV type 2 (cVDPV2), with 723 cases reported worldwide in 2023, compared with 241 cases of cVDPV1 and no cases of cVDPV3.<a class="elsevierStyleCrossRef" href="#bb0015"><span class="elsevierStyleSup">3</span></a></p><p id="p0015" class="elsevierStylePara elsevierViewall"><a class="elsevierStyleCrossRef" href="#f0005">Fig. 1</a> shows the timeline for stopping the cVDPV transmission in outbreaks and in at-risk countries, as are the milestones for monitoring progress towards goal achievement in being completely free from cVDPV.<a class="elsevierStyleCrossRef" href="#bb0015"><span class="elsevierStyleSup">3</span></a></p><elsevierMultimedia ident="f0005"></elsevierMultimedia><p id="p0020" class="elsevierStylePara elsevierViewall">The World Health Organisation (WHO) prequalified the novel OPV type 2 (nOPV2) in early 2024.<a class="elsevierStyleCrossRef" href="#bb0020"><span class="elsevierStyleSup">4</span></a> In late December 2023, WHO issued its first prequalification approval for a vaccine already in use under the Emergency Use Listing Procedure (EUL) regulatory pathway: the novel OPV type 2 (nOPV2). Since the rollout of this new-generation vaccine began in March 2021, the Global Polio Eradication Initiative has administered nearly 1 billion doses of nOPV2 in 35 countries, protecting millions of children from polio. Prequalification enables easier access to the vaccine for a more sustainable response to outbreaks of cVDPV2. The WHO Emergency Procedure (EUL) pathway is reserved for the use of vaccines, medicines, and diagnostic tools that have not yet been authorised during public health emergencies. Following a rigorous assessment of existing data on quality, safety, and efficacy from completed clinical trials, the pathway allows for accelerated availability of products in settings affected by these emergencies.<a class="elsevierStyleCrossRef" href="#bb0025"><span class="elsevierStyleSup">5</span></a></p><p id="p0025" class="elsevierStylePara elsevierViewall">OPV is widely used in campaigns in lower-resource countries because it is easy to store and transport and easy to administer. However, the attenuated virus in the oral vaccine can mutate and become virulent again, especially in settings where vaccine uptake is low. To date, nOPV2 has been used in 35 countries under the EUL framework, mainly in the African region, which is the most affected by cVDPV2 outbreaks. The first nOPV2 campaigns were conducted in March 2021 in response to cVDPV2 outbreaks in Nigeria and Liberia, and other countries soon followed suit using nOPV2.<a class="elsevierStyleCrossRef" href="#bb0030"><span class="elsevierStyleSup">6</span></a><span class="elsevierStyleSup">,</span><a class="elsevierStyleCrossRef" href="#bb0035"><span class="elsevierStyleSup">7</span></a> Throughout its clinical development and community use, nOPV2 has proven to be as safe to use and effective in stopping outbreaks as its predecessor, the monovalent oral vaccine type 2. But more importantly, this new vaccine is genetically more stable, being 80% less likely to trigger new outbreaks due to variants.<a class="elsevierStyleCrossRef" href="#bb0040"><span class="elsevierStyleSup">8</span></a><span class="elsevierStyleSup">,</span><a class="elsevierStyleCrossRef" href="#bb0045"><span class="elsevierStyleSup">9</span></a> According to GPEI data, the new vaccine has helped achieve an 85% reduction in cVDPV2 cases in Nigeria, where it has been in use since 2021.</p><p id="p0030" class="elsevierStylePara elsevierViewall">The development of the vaccine began in 2011 through a consortium of experts led by the Bill and Melinda Gates Foundation, including the UK's National Institute for Biological Standards and Controls, the US Centers for Disease Control and Prevention, the US Food and Drug Administration, and the University of California, San Francisco.</p><p id="p0035" class="elsevierStylePara elsevierViewall">While nOPV2 has played a key role in reducing cVDPV2 cases, its success, like that of any polio vaccine, depends on the ability to rapidly implement immunisation campaigns that reach all children.</p><p id="p0040" class="elsevierStylePara elsevierViewall">According to this week's GPEI report, a circulating case of cVDPV2 was identified in Gaza earlier this month, the first case in 25 years (Polio This Week – GPEI [<a href="http://polioeradication.org">polioeradication.org</a>]). A confirmed case in Gaza has thus led to a 2-round vaccination campaign to secure receipt of 2 doses of vaccine and targeting 640 000 children that began in late August in this war-torn region.<a class="elsevierStyleCrossRef" href="#bb0050"><span class="elsevierStyleSup">10</span></a></p><p id="p0045" class="elsevierStylePara elsevierViewall">To overcome the final challenges remaining in polio eradication, GPEI is looking for new ways to reach children living in hard-to-reach areas or in regions with war-torn conflict to promote community uptake of vaccines and improve early detection and response to outbreaks.</p><span id="s0005" class="elsevierStyleSection elsevierViewall"><span class="elsevierStyleSectionTitle" id="st0010">Funding</span><p id="p0050" class="elsevierStylePara elsevierViewall">This study did not receive any specific funding from public sector agencies, the business sector or not-for-profit entities.</p></span></span>" "textoCompletoSecciones" => array:1 [ "secciones" => array:2 [ 0 => array:2 [ "identificador" => "s0005" "titulo" => "Funding" ] 1 => array:1 [ "titulo" => "References" ] ] ] "pdfFichero" => "main.pdf" "tienePdf" => true "NotaPie" => array:1 [ 0 => array:2 [ "etiqueta" => "☆" "nota" => "<p class="elsevierStyleNotepara" id="np4005">Please cite this article as: Torner N. Vacuna polio oral de nueva generación: ¿solución para la erradicación? Vacunas. 2024. <span class="elsevierStyleInterRef" id="ir3005" href="https://doi.org/10.1016/j.vacun.2024.09.003">https://doi.org/10.1016/j.vacun.2024.09.003</span>.</p>" ] ] "multimedia" => array:1 [ 0 => array:8 [ "identificador" => "f0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 2173 "Ancho" => 3188 "Tamanyo" => 489342 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "al0005" "detalle" => "Fig. " "rol" => "short" ] ] "descripcion" => array:1 [ "en" => "<p id="sp0005" class="elsevierStyleSimplePara elsevierViewall">Milestones for stopping cVDPV transmission in outbreaks and at-risk countries from 2021 to 2030 and beyond.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bs0005" "bibliografiaReferencia" => array:10 [ 0 => array:3 [ "identificador" => "bb0005" "etiqueta" => "1." "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "World Health Organization RO for S-EA, Fifth Regional Workshop to Strengthen Surveillance and Outbreak Preparedness and Response for Priority Vaccine Preventable Diseases Including Measles, Rubella, and Polio, New Delhhi, 2024. [consultado 09 sept 2024]. Disponible en: <a target="_blank" href="https://iris.who.int/handle/10665/376905">https://iris.who.int/handle/10665/376905</a>" ] ] ] 1 => array:3 [ "identificador" => "bb0010" "etiqueta" => "2." "referencia" => array:1 [ 0 => array:1 [ "host" => array:2 [ 0 => array:1 [ "Libro" => array:2 [ "titulo" => "Plan de acción en España para la erradicación de la poliomielitis 2024-2028, Consejo Interterritorial del Sistema Nacional de Salud Ministerio de Sanidad" "fecha" => "2024" ] ] 1 => array:1 [ "WWW" => array:1 [ "link" => "https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/polio/docs/Plan_erradicacion_poliomielitis.pdf" ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bb0015" "etiqueta" => "3." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Global Circulating Vaccine- Derived Poliovirus (cVDPV),2021.2024" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "Global Polio Eradication Initiative GPEI" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "WWW" => array:2 [ "link" => "https://polioeradication.org/circulating-vaccine-derived-poliovirus-count/" "fecha" => "2024" ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bb0020" "etiqueta" => "4." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Comunicado de Prensa de la GPEI Sobre la Precalificación de la nOPV2" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "Global Polio Eradication Initiative" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "WWW" => array:2 [ "link" => "https://polioeradication.org/news/gpei-press-release-on-nopv2-prequalification/" "fecha" => "2024" ] ] ] ] ] ] 4 => array:3 [ "identificador" => "bb0025" "etiqueta" => "5." "referencia" => array:1 [ 0 => array:1 [ "host" => array:1 [ 0 => array:1 [ "Libro" => array:5 [ "titulo" => "Emergency Use Listing Procedure" "fecha" => "2022" "paginaInicial" => "1" "paginaFinal" => "62" "editorial" => "World Health Organization" ] ] ] ] ] ] 5 => array:3 [ "identificador" => "bb0030" "etiqueta" => "6." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Exploring public perceptions of vaccine-derived poliovirus and a novel oral polio vaccine in the Democratic Republic of the Congo, Kenya, and Nigeria" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "L. Lorenzetti" 1 => "R. Haydarov" 2 => "E. Namey" 3 => "A. Lawton" 4 => "H. Nam" 5 => "M.R. Hasan" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.vaccine.2022.05.020" "Revista" => array:6 [ "tituloSerie" => "Vaccine" "fecha" => "2023" "volumen" => "41" "paginaInicial" => "A128" "paginaFinal" => "A135" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/35871107" "web" => "Medline" ] ] ] ] ] ] ] ] 6 => array:3 [ "identificador" => "bb0035" "etiqueta" => "7." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Poliovirus antibodies following two rounds of campaigns with a type 2 novel oral poliovirus vaccine in Liberia: a clustered, population-based seroprevalence survey" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "S.B. Kennedy" 1 => "G.R. Macklin" 2 => "G. Mason Ross" 3 => "R. Lopez Cavestany" 4 => "R.A. Moukom" 5 => "K.A.V. Jones" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S2214-109X(23)00116-X" "Revista" => array:6 [ "tituloSerie" => "Lancet Glob Health" "fecha" => "2023" "volumen" => "11" "paginaInicial" => "e917" "paginaFinal" => "e923" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/37202026" "web" => "Medline" ] ] ] ] ] ] ] ] 7 => array:3 [ "identificador" => "bb0040" "etiqueta" => "8." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Engineering the live-attenuated polio vaccine to prevent reversion to virulence" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "Yeh M. Te" 1 => "E. Bujaki" 2 => "P.T. Dolan" 3 => "M. Smith" 4 => "R. Wahid" 5 => "J. Konz" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:3 [ "tituloSerie" => "Cell Host Microbe" "fecha" => "2020" "volumen" => "27" ] ] ] ] ] ] 8 => array:3 [ "identificador" => "bb0045" "etiqueta" => "9." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Assessment of genetic changes and neurovirulence of shed Sabin and novel type 2 oral polio vaccine viruses" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "R. Wahid" 1 => "L. Mercer" 2 => "A. Macadam" 3 => "S. Carlyle" 4 => "L. Stephens" 5 => "J. Martin" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1038/s41541-021-00355-y" "Revista" => array:5 [ "tituloSerie" => "Npj Vaccines" "fecha" => "2021" "volumen" => "6" "paginaInicial" => "94" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/34326330" "web" => "Medline" ] ] ] ] ] ] ] ] 9 => array:3 [ "identificador" => "bb0050" "etiqueta" => "10." "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Newsdesk Polio vaccination campaign in Gaza" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "T. Burki" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Lancet Infect Dis" "fecha" => "2024" "volumen" => "3099" "paginaInicial" => "1" "paginaFinal" => "2" ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/24451460/unassign/S244514602400075X/v1_202410300909/en/main.assets" "Apartado" => null "PDF" => "https://static.elsevier.es/multimedia/24451460/unassign/S244514602400075X/v1_202410300909/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S244514602400075X?idApp=UINPBA00004N" ]
Journal Information
Share
Download PDF
More article options
Editorial
Available online 30 October 2024
Next-generation oral polio vaccine: The solution to reach eradication?
Vacuna polio oral de nueva generación: ¿solución para la erradicación?
Nuria Torner
CIBER Epidemiología y Salud Pública CIBERESP, Unidad de Medicina Preventiva y Salud Pública, Departamento de Medicina, Universitat de Barcelona, Barcelona, Spain
Article information
These are the options to access the full texts of the publication Vacunas (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail