Inhaled corticosteroids (ICS) are the first-line therapy in the treatment of persistent asthma. At medium to high doses and prolonged usage, ICS can supresss the hypothalamic-pituitary-adrenal axis. Dehydroepiandrosterone sulphate (DHEA-S) is a corticotropin-dependent adrenal androgen precursor that is supressible in patients treated with ICS.
ObjectivesTo evaluate the adrenal axis in asthmatic children treated with moderate doses of fluticasone propionate and to evaluate the DHEA-S as a possible marker for adrenal axis ın preadrenarchal children.
MethodsTwenty-eight children with persistent asthma with a mean age of 4.4 years (median 4.2; range 2.5-7.1) on long term treatment (mean 6.16; median 6; range 4.5-9 months) with moderate doses (mean 250; median 253; range 158-347 (g/m2/day) of inhaled fluticasone propionate were evaluated with low-dose ACTH stimulation test to assess adrenal function, and DHEA-S levels were compared with the results.
ResultsOne out of 28 patients (3.57%) demonstrated an abnormal cortisol response to low-dose ACTH test. There was no correlation between DHEA-S and peak cortisol, morning cortisol and fasting blood glucose levels. However, mean inhaled corticosteroid dosages were inversely correlated with the DHEA-S.
ConclusıonsIn most of the children with persistent asthma, mild to moderate fluticazone propionate doses supress the hypothalamic-pituitary-adrenal axis rarely. Chronic moderate doses of ICS may suppress adrenal androgen levels without supression of cortisol production. DHEA-S levels may be used as a practical method to follow adrenal functions and may be an earlier indicator of adrenal dysfunction in children.
Inhaled corticosteroids (ICS) are the first-line treatment in the management of persistent asthma.1 Recently there have been reports of symptomatic adrenal insufficiency in children on chronic ICS treatment.2–4 Most of these children were treated with high doses of inhaled fluticasone. For most patients aged <12 years, the recommended dose of fluticasone propionate (FP) is classified as low (88-176μg/day); medium (176-440μg/day); or high (> 440μg/day).1 Fluticasone has the highest receptor binding affinity of any of the commercially available ICS.5 Use of FP has been reported to produce dose-related adrenal suppresion greater than that of other commercially available ICS.6–8 Studies using low or medium doses of FP in adults with mild to moderate asthma have conflicting results.9–12 Children may be even more susceptible to adrenal supression than adults. The number of studies concerning the effects of FP on adrenal axis at low to medium doses in children is limited and results are also conflicting.13–16
For the evaluation of the hypothalamic-pituitary-adrenal (HPA) axis, insulin induced hypoglycaemia and metyrapone are considered the most sensitive tests but they are associated with unpleasant symptoms and significant potential complications.17 Recently, low dose cosyntropin has been found to be a sensitive test for determining adrenal supression.18–20 However, it is an invasive and time-consuming test and not reliable for screening purposes.
Dehydroepiandrosterone sulphate (DHEA-S) is an adrenal androgen precursor secreted by the zona reticularis, under the dominant regulation of corticotropin. Serum levels of DHEA-S are affected by several factors, the most important of which are age, gender, chronic illness and the prior use of glucocorticoids. DHEA-S has a long half-life (10-12hours) and concentrations do not fluctuate throughout the day.21 There are some reports indicating that the adrenal androgen secretion is sensitive and suppressible with the exogenous glucocorticoids.22–24 However, data on the use of DHEA-S in the assessment of adrenal function, especially in preadrenarchal children, are limited.
In the present study, we evaluated the effect of moderate doses of FP on HPA axis in preadrenarchal children and compared the results with DHEA-S levels. Our aim was to document the effects of FP at moderate doses in HPA and determine whether there was a correlation between DHEA-S levels and HPA functions in preadrenarchal children.
Patients and methodsTwenty-eight children with persistent asthma and using moderate doses of fluticasone propionate between doses of 158-347μg/m2/day and for at least 4 months were enrolled in the study. Patients were all at preadrenarchal ages and this was confirmed by a detailed physical examination. None of the children were exposed to systemic corticosteroids more than two courses (each of which at most 5 days and 1mg/kg/day) within the last 6 months. Any patient with exposure of systemic or topical corticosteroids one month before the adrenal function testing or with a history of chronic illnesses other than asthma were excluded from the study. This study was approved by the ethics committee of Dr Sami Ulus Children's Hospital numbered 0016/2007 and informed consent was obtained by the parents of each child who participated in the sudy.
Detailed clinical histories were obtained from the previous medical records, including length of time of exposure and dose of fluticasone propionate. Children were admitted to the Dr Sami Ulus Children's Hospital paediatric allergy and paediatric endocrinology out-patient units with a history of 12hours fasting and they all stopped the ICS usage 24hours prior to admission. First an experienced paediatric endocrinologist made a detailed physical examination and patients with the signs of adrenarche were excluded form the study. Half an hour after inserting an intravenous tube the baseline laboratory measures including morning cortisol (between 9:00 and 9:30 a.m.), fasting blood glucose and DHEA-S levels were taken. Then low-dose ACTH (0.5μg/m2 up to a maximum of 1μg) was administered intravenously, and the cortisol levels were measured at the 10th, 20th, 30th, and 40th minutes. A cortisol level of 19.8μg/dl or greater was considered as passing the test.25
DHEA-S levels were measued by means of chemiluminescence immunoassays using a commercial kit (DHEA-S radioimmunoassay; Diagnostic Systems Laboratories, Inc., Webster, TX, USA); and cortisol levels were measured by means of enzyme immunoassays using the ST AIA PACK CORT system (TOSOH Bioscience, San Francisco, CA, USA).
The data were analysed by using SPSS version 8.0 (SPSS Inc.Chicago, IL, USA). Pearson's correlation coefficient was used to analyse the relationship between DHEA-S and inhaled costicosteroid, morning cortisol, peak cortisol, and fasting blood glucose levels. The level of statistical significance was at 5%.
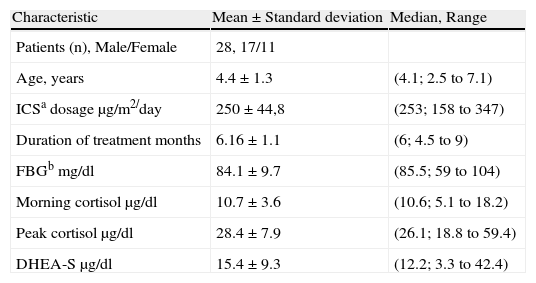
ResultsThe average age of the patients was 4.4 (median 4.2, range 2.5-7.1) and the male-female ratio was 17/11. Demographic data and the other characteristics of patients are shown in Table 1.
Demographic and other characteristics of the patients.
| Characteristic | Mean±Standard deviation | Median, Range |
| Patients (n), Male/Female | 28, 17/11 | |
| Age, years | 4.4±1.3 | (4.1; 2.5 to 7.1) |
| ICSa dosage μg/m2/day | 250±44,8 | (253; 158 to 347) |
| Duration of treatment months | 6.16±1.1 | (6; 4.5 to 9) |
| FBGb mg/dl | 84.1±9.7 | (85.5; 59 to 104) |
| Morning cortisol μg/dl | 10.7±3.6 | (10.6; 5.1 to 18.2) |
| Peak cortisol μg/dl | 28.4±7.9 | (26.1; 18.8 to 59.4) |
| DHEA-S μg/dl | 15.4±9.3 | (12.2; 3.3 to 42.4) |
aICS: Inhaled corticosteroids, bFBG: Fasting blood glucose.
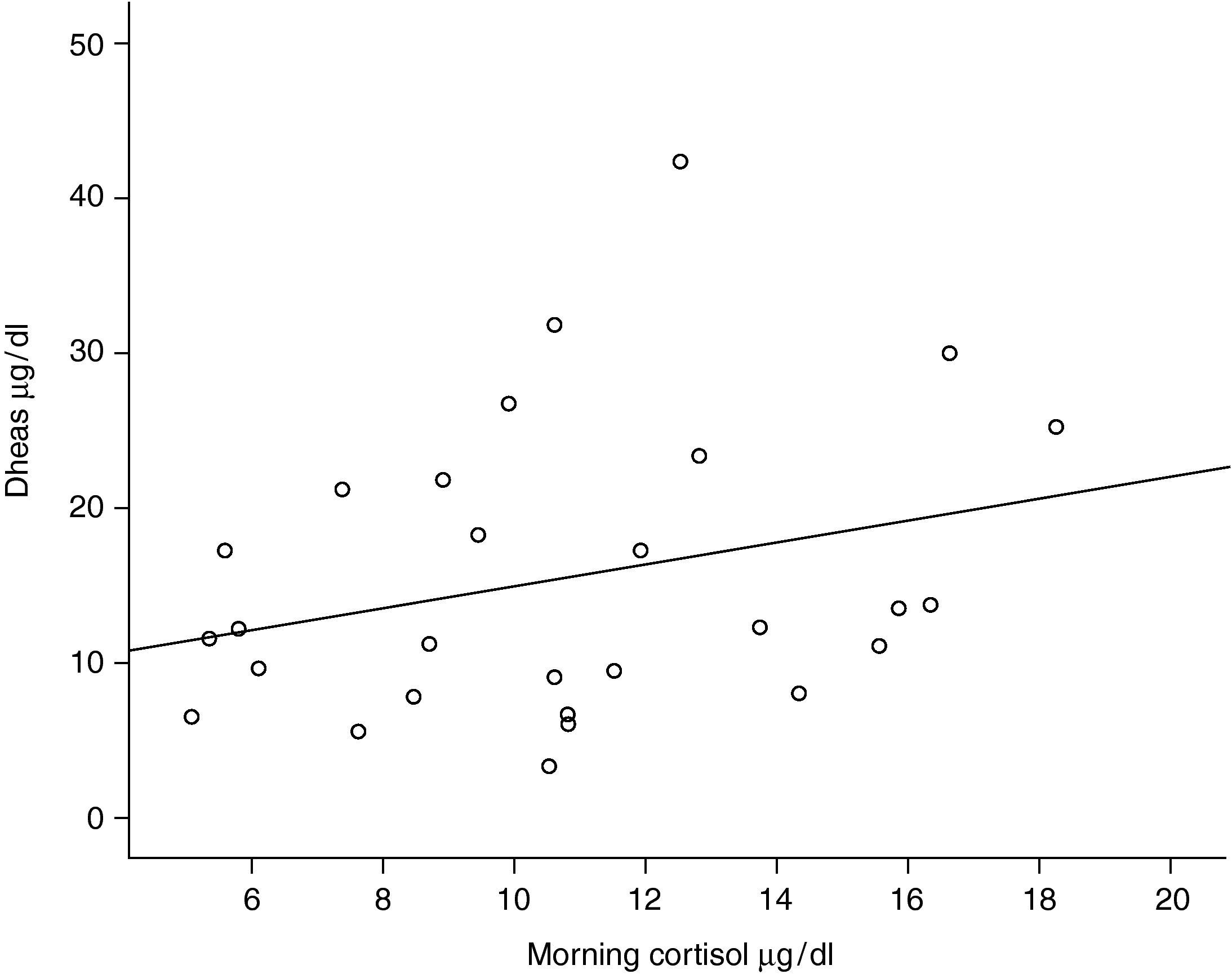
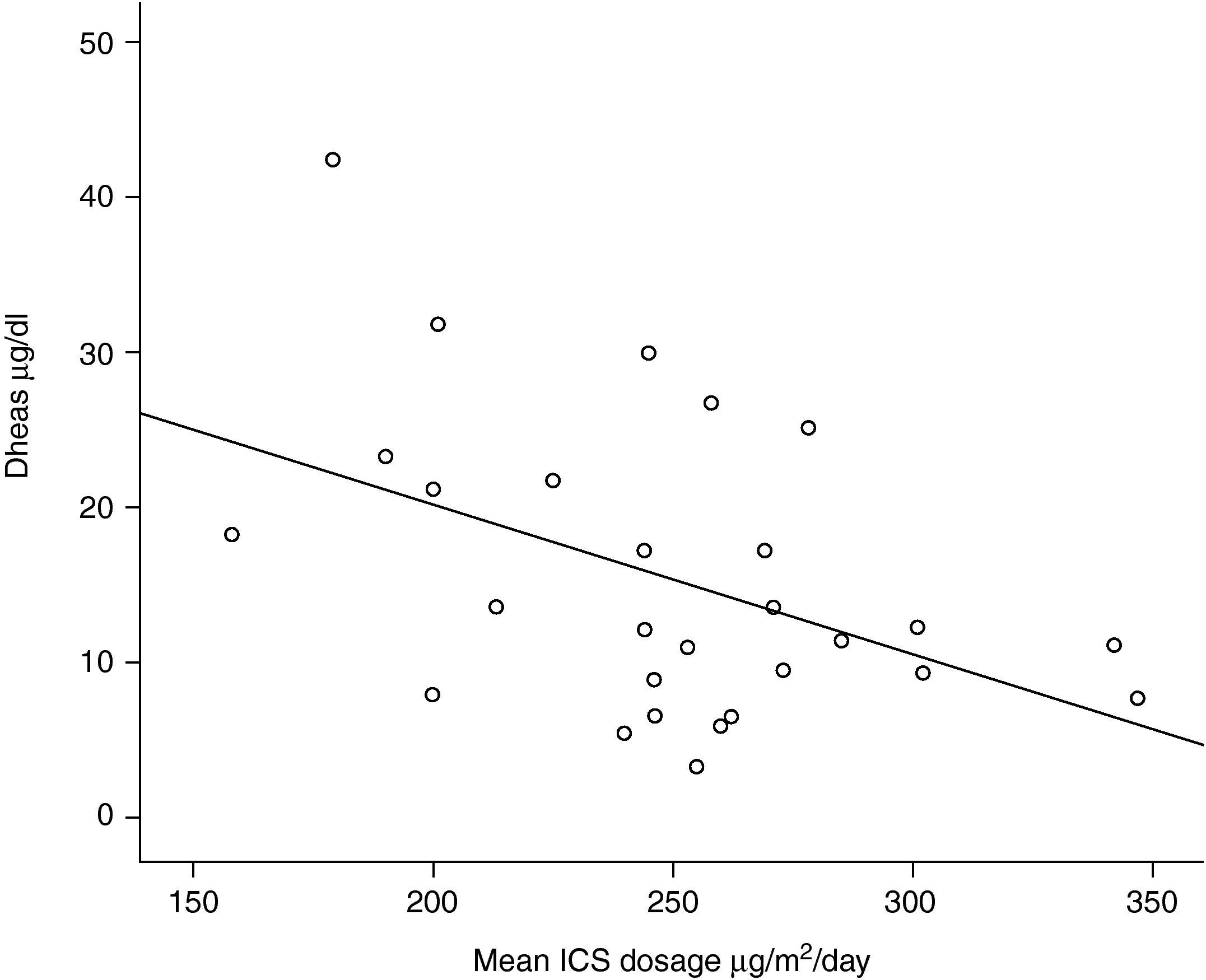
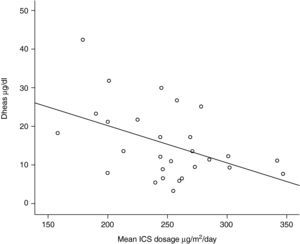
One out of 28 asthmatic children (3.57%) demonstrated biochemical adrenal suppression, that is he had a peak serum cortisol response to low-dose ACTH test <19.8μg/dl. No patient presented symptoms suggestive of adrenal insufficiency. When Pearson's correlation coefficient test was performed no significant correlations were found between DHEA-S and fasting blood glucose levels (r=0.157; p=0.424); DHEA-S and morning cortisol levels (r=0.279; p=0.150) (Figure 1) and between DHEA-S and peak cortisol levels (r=−0.006; p=0.976). There was no correlation between the mean inhaled corticosteroid dose and morning cortisol or peak cortisol levels either (r=−0.140; p=0.478 and r=−0.61; p=0.759 respectively). Mean inhaled corticosteroid dosage of the patients was 250μg/m2/day (median 253, range 158 to 347) and there was an inverse correlation between the mean ICS dosages and DHEA-S levels of the patients (r=−0.440; p=0.019) (Figure 2).
Inhaled corticosteroids (ICS) are the first-line treatment in the management of persistent asthma.1 There have been reports of symptomatic adrenal insufficiency in children who were treated with high doses of inhaled fluticasone.2–4 In adolescent and adult asthmatics most of the therapeutic benefit of FP is achieved at doses of 100-250μg/day and the use of FP in doses up to 500μg/day rarely causes adrenal supression.26
In children, comparable dose response relation for FP has not been formally established for either efficacy or adrenal supression. There have been reports of symptomatic adrenal insufficiency2,27,28 including acute adrenal crisis3,4,29 attributed to FP therapy at doses >500μg/day in asthmatic children. The presented data show that fluticasone propionate at medium doses <400μg/m2/day (or nearly 200-250μg/day at studied age group) does not cause adrenal supression in children. Only one out of 28 children (3.57%) showed biochemical supression of HPA. No symptomatic adrenal insufficiency observed.
Eid et al. made an observational study of 62 children for 8 months and abnormal morning cortisol levels were found in 17% (1/6) of children receiving low-dose (176μg/day), and in 43% (12/28) of children rceiving high-dose (>880μg/day) FP.30 In the study of Peden et al., fluticasone doses up to 200μg/day did not change the 24-hour urinary cortisol levels.13 In another study Verona et al. showed that FP at 400μg/day caused adrenal supression in a small but clinically significant proportion of children, compared with the 200μg/day dose.31 A similar result was found by Kannisto et al. in which 20% of the asthmatic children had an abnormal response at 500μg/day.19 Taken together, these results suggest that fluticasone propionate is unlikely to cause adrenal supression at 200μg/day and adrenal supression occuring in some children at 400 to 500μg/day.13,15,19,30
Since ICS can cause adrenal supression at high doses in asthmatic children, assessment of the adrenal functions during the treatment period is an important issue.2–4 For the evaluation of HPA axis, insulin-induced hypoglycaemia and metyrapone are considered the most sensitive tests but they are associated with significant potential complications.17 Low-dose cosyntropin has been found to be a sensitive test for determining adrenal supression.18–20 However, it is an invasive test requiring intravenous cannula and repeated blood samples and not reliable for screening purposes.
Dehydroepiandrosterone sulhpate (DHEA-S) is an adrenal androgen under the dominant regulation of corticotropin and has a longer half-life (10-12hours) than cortisol (<2hours). Thus, diurnal changes in serum DHEA-S concentrations are much lower than cortisol.21 Levels of DHEA-S have been shown to be decreased in asthmatic patients exposed to high ICS doses.22–24 In the present study, serum DHEA-S levels correlated inversely with the mean inhaled coticosteroid levels and a dose-dependent decrease was found in preadrenarchal asthmatic children. The same correlaton was not found with the peak plasma cortisol levels and low-dose ACTH stimulation test revealed only one patient with the biochemical evidence of adrenal supression. These findings support the studies of Cutler et al. and Rittmaster et al. who found that adrenal androgen secretion is more sensitive than cortisol production to the supressive effect of glucocorticoid therapy.31,32 Kreitzer et al. did not find any decrease in DHEA-S levels in preadrenarchal children after a short one-week course of prednisone, even though the decrease in postadrenarchal children was significant.33 In a preadrenarchal child there may also be alternative ACTH independent regulatory pathways of DHEA-S synthesis. However if the dosage or the duration of the corticosteroids increase as in the present study, DHEA-S levels decrease with the supressive effects of ICS.
Hypoglycaemia was not found in any of the patients and there was not a correlation between DHEA-S levels and fasting blood glucose levels as expected. In adrenal axis supression, hypoglcaemia is a result of loss of gluconeogenic effect of cortisol and we found cortisol function loss in only one out of twenty-eight patients with low-dose ACTH test. That is why we could not find hypoglycaemia in the study group.
In the present study, morning cortisol levels were not correlated with the DHEA-S levels. A morning cortisol level may be practical to assess the adrenal functions. However, it has been shown to be an insensitive measure of adrenal function in different studies.24,34
In conclusion, fluticasone propionate is unlikely to cause adrenal supression at moderate doses (<400μg/m2/day), and conventional doses of inhaled corticosteroids in preadrenarchal children may suppress DHEA-S production. The presence of low DHEA-S levels may be used as a screening test to identify the child who needs further evaluation of adrenal supression.
Conflict of interestThe authors have no conflict of interest to declare.