To develop quality indicators to measure asthma care in primary health care.
MethodA modified RAND was used, which included the systematic review of the literature in Embase, Cochrane and Pubmed Quality Agencies and Database. The work group identified the indicators, translated them into Spanish and resolved any duplicates. Each indicator is composed of several dimensions (access to care, clinical effectiveness, patient-centred quality and patient safety). A multidisciplinary panel of 98 professionals from all over Spain were invited to score each indicator using a Likert scale. After calculating the average and median of each indicator, this information was sent to those who responded (n=38) for a second round and further scoring. The agreement percentage for the group was obtained for each indicator.
ResultsOf the 105 asthma indicators reviewed, we selected 46 that were presented to the panel of experts. In both Delphi phases, 37.1% of the members of the initial panel of experts responded. Of these, 26 were primary care paediatricians, six were pulmonologists, three were nurses, two were pharmacists and one was an allergist.
For 32 indicators, agreement exceeded 70% and seven of those scored highest for the various care aspects for asthmatic children.
ConclusionQuality indicators are presented for the follow-up of asthma and their implementation in primary care, which have undergone a strict selection and agreement process by a multidisciplinary work group.
Asthma is the most prevalent childhood chronic disease. Appropriate primary care follow-up should lead to a decrease in hospitalisations, lower costs and better quality of life for the affected patients.1
The quality of health care was defined by the Institute of Medicine as: “the degree to which the health services increase the probability of desired health results on behalf of individuals and populations and that are coherent with the current professional knowledge”.2
Assessing the quality of asthma care requires good information and useful measurements. We must have process indicators that reflect the quality of the care provided and indicators about the results, which reflect changes in the state of the patient's health.3 These indicators could work to measure and compare the effectiveness and efficiency of the care provided by the various professionals and health services.
Consensus methods that combined the opinion of experts together with the synthesis of the evidence are used to generate indicators. A gold-standard as indicated by the criteria of evidence-based medicine (EBM) is, in many cases, unavailable or fails to provide sufficiently detailed evidence applicable to the wide range of patients in clinical practice. Consequently, we used methods that combine the best available scientific evidence and the opinion of a group of experts.4 One of the techniques used was the RAND/UCLA method, developed by RAND Corporation and the University of California in Los Angeles.5
To generate consensus, the Delphi method was used; initially, this method was developed by industry in the United States to predict objectives. Since then, it has been applied in all scientific fields, as well as research in the medical and health service field. The defining characteristics of the Delphi method are the anonymity among the participants, the repetition and feedback from at least two successive rounds and the explicit statistical analysis of the responses.6
Despite the availability of clinical practice guidelines, processes and integrated plans, there continues to be a significant gap between the best practices accepted for the care of childhood asthma and real patient care. The purpose of this work is to identify the evidence of quality indicators when caring for asthma and generate consensus among the professionals caring for children with asthma, about indicators that could be useful to implement the best practices, and optimise the processes and results of the primary care for children with asthma.7–16
Materials and methodsThe RAND method was used, which combines4,17:
- 1.
Systematic Search Review of the literature to identify quality indicators (QI) in asthma.
- 2.
Nominal group: Evaluation and translation into Spanish of the QI by the authors, and resolution of the duplicates.
- 3.
Assessment of indicators by the panellists according to abbreviated Delphi methodology.
One of the authors of this publication participated in the COSI (Core Set of Indicators/standards for Primary Care in Europe, The European Academy of Paediatricians) project, the objective of which was to develop a group of indicators that would serve to elaborate a primary care, paediatric quality standard that would work as a comparative instrument among countries.
Only Indicators referring to children under the age of 18, included in European paediatric primary care and which could be comparable among countries and that focus on quality measures were included. An information professional searched the Embase and Pubmed databases, limiting publications to those in English, German and Spanish, until August 2011, using specific descriptors. Moreover, a search of indicators at 17 Quality Agencies was also carried out; SEE APPENDIX. An indicator database was created using all indicators identified.
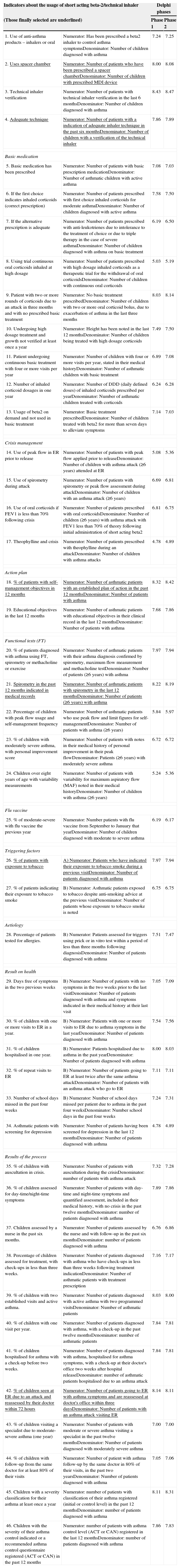
The indicators were selected, eliminating repeated or very similar items. Each indicator was defined with a numerator and a denominator (Table 1). For their evaluation, the scale published by To et al.18 was used, which evaluates each indicator abiding to dimensions of validity, relevance, area of improvement and global, scoring from 1 (worst evaluation) to 9 (best evaluation). The final score for each indicator is the average obtained after tallying all the dimensions making up each indicator. For each indicator, the panellists had to give their opinion about feasibility depending on the possibilities of being obtained by their organisation's registration systems. This qualitative type item was responded by “Yes/No/Don’t know” (Table 2).
Indicators selected for evaluation by the study group.
| Indicators about the usage of short acting beta-2/technical inhaler | Delphi phases | ||
|---|---|---|---|
| (Those finally selected are underlined) | Phase 1 | Phase 2 | |
| 1. Use of anti-asthma products – inhalers or oral | Numerator: Has been prescribed a beta2 inhaler to control asthma symptomsDenominator: Number of children diagnosed with asthma | 7.24 | 7.25 |
| 2. Uses spacer chamber | Numerator: Number of patients who have been prescribed a spacer chamberDenominator: Number of children with prescribed MDI device | 8.00 | 8.08 |
| 3. Technical inhaler verification | Numerator: Number of patients with technical inhaler verification in the last 6 monthsDenominator: Number of children diagnosed with asthma | 8.43 | 8.47 |
| 4. Adequate technique | Numerator: Number of patients with a indication of adequate inhaler technique in the past six monthsDenominator: Number of children with a verification of the technical inhaler | 7.86 | 7.89 |
| Basic medication | |||
| 5. Basic medication has been prescribed | Numerator: Number of patients with basic prescription medicationDenominator: Number of asthmatic children with active asthma | 7.08 | 7.03 |
| 6. If the first choice indicates inhaled corticoids (correct prescription) | Numerator: Number of patients prescribed with first choice inhaled corticoids for moderate asthmaDenominator: Number of children diagnosed with active asthma | 7.58 | 7.50 |
| 7. If the alternative prescription is adequate | Numerator: Number of patients prescribed with anti-leukotrienes due to intolerance to the treatment of choice or due to triple therapy in the case of severe asthmaDenominator: Number of children diagnosed with asthma on basic treatment | 6.19 | 6.50 |
| 8. Using trial continuous oral corticoids inhaled at high dosage | Numerator: Number of patients prescribed with high dosage inhaled corticoids as a therapeutic trial for the withdrawal of oral corticoidsDenominator: Number of children with continuous oral corticoids | 5.03 | 5.19 |
| 9. Patient with two or more rounds of corticoids due to an attack in three months and with no prescribed basic treatment | Numerator: No basic treatment prescribedDenominator: Number of children with two or more oral corticoid bolus, due to exacerbation of asthma in the last three months | 8.03 | 8.14 |
| 10. Undergoing high dosage treatment and growth not verified at least once a year | Numerator: Height has been noted in the last 12 monthsDenominator: Number of children being treated with high dosage corticoids | 7.49 | 7.50 |
| 11. Patient undergoing continuous basic treatment with four or more visits per year | Numerator: Number of children with four or more visits per year, stated in their medical historyDenominator: Number of asthmatic children with basic treatment | 6.89 | 7.08 |
| 12. Number of inhaled corticoid dosages in one year | Numerator: Number of DDD (daily defined doses) of inhaled corticoids prescribed per yearDenominator: Number of asthmatic children treated with corticoids | 6.24 | 6.28 |
| 13. Usage of beta2 on demand and not used in basic treatment | Numerator: Basic treatment prescribedDenominator: Number of children treated with beta2 for more than seven days to alleviate symptoms | 7.14 | 7.03 |
| Crisis management | |||
| 14. Use of peak flow in ER prior to release | Numerator: Number of patients with peak flow applied prior to releaseDenominator: Number of children with asthma attack (≥6 years) attended at ER | 5.08 | 5.36 |
| 15. Use of spirometry during attack | Numerator: Number of patients with spirometry or peak flow assessment during attackDenominator: Number of children with an asthma attack (≥6 years) | 6.69 | 6.81 |
| 16. Use of oral corticoids if FEV1 is less than 70% following crisis | Numerator: Number of patients prescribed with oral corticoidsDenominator: Number of children (≥6 years) with asthma attack with FEV1 less than 70% of theory following initial administration of short acting beta2 | 6.81 | 6.75 |
| 17. Theophylline and crisis | Numerator: Number of patients prescribed with theophylline during an attackDenominator: Number of children with asthma attacks | 4.78 | 4.89 |
| Action plan | |||
| 18. % of patients with self-management objectives in 12 months | Numerator: Number of asthmatic patients with an established plan of action in the past 12 monthsDenominator: Number of patients with asthma | 8.32 | 8.42 |
| 19. Educational objectives in the last 12 months | Numerator: Number of asthmatic patients with educational objectives in their clinical record in the last 12 monthsDenominator: Number of patients with asthma | 7.68 | 7.86 |
| Functional tests (FT) | |||
| 20. % of patients diagnosed with asthma using FT, spirometry or methacholine or exercise | Numerator: Number of asthmatic patients with their asthma diagnosis confirmed by spirometry, maximum flow measurement and methacholine testDenominator: Number of patients (≥6 years) with asthma | 7.97 | 7.94 |
| 21. Spirometry in the past 12 months indicated in medical records | Numerator: Number of asthmatic patients with spirometry in the last 12 monthsDenominator: Number of patients (≥6 years) with asthma | 8.22 | 8.19 |
| 22. Percentage of children with peak flow usage and self-management frequency | Numerator: Number of asthmatic patients who use peak flow and limit figures for self-managementDenominator: Number of patients with asthma (≥6 years) | 5.84 | 5.97 |
| 23. % of children with moderately severe asthma, with personal improvement score | Numerator: Number of patients with notes in their medical history of personal improvement in their peak flowDenominator: Patients (≥6 years) with moderately severe asthma | 6.72 | 6.72 |
| 24. Children over eight years of age with variability measurements | Numerator: Number of patients with variability for maximum aspiratory flow (MAF) noted in their medical historyDenominator: Number of children with asthma (≥6 years) | 5.24 | 5.36 |
| Flu vaccine | |||
| 25. % of moderate-severe with flu vaccine the previous year | Numerator: Number patients with flu vaccine from September to January that yearDenominator: Number of children diagnosed with moderate to severe asthma | 6.19 | 6.17 |
| Triggering factors | |||
| 26. % of patients with exposure to tobacco | A) Numerator: Patients who have indicated their exposure to tobacco smoke during a previous visitDenominator: Number of patients diagnosed with asthma | 7.97 | 7.94 |
| 27. % of patients indicating their exposure to tobacco smoke | B) Numerator: Asthmatic patients exposed to tobacco despite anti-smoking advice at the previous visitDenominator: Number of patients whose exposure to tobacco smoke is noted | 6.75 | 6.75 |
| Aetiology | |||
| 28. Percentage of patients tested for allergies. | B) Numerator: Patients assessed for triggers using prick or in vitro test within a period of less than three months following diagnosisDenominator: Number of patients diagnosed with asthma | 7.51 | 7.47 |
| Result on health | |||
| 29. Days free of symptoms in the two previous weeks | B) Numerator: Number of patients with no symptoms in the two weeks prior to the last visitDenominator: Number of patients diagnosed with asthma and symptoms indicated in their medical history at their last visit | 7.05 | 7.09 |
| 30. % of children with one or more visits to ER in a year. | B) Numerator: Patients with one or more visits to ER due to asthma symptoms in the last yearDenominator: Number of patients diagnosed with asthma | 7.54 | 7.56 |
| 31. % of children hospitalised in one year. | B) Numerator: Patients hospitalised due to asthma in the past yearDenominator: Number of patients diagnosed with asthma | 8.00 | 8.03 |
| 32. % of repeat visits to ER | B) Numerator: Number of patients going to ER at least twice after the same asthma attackDenominator: Number of patients with an asthma attack who go to ER | 7.11 | 7.11 |
| 33. Number of school days missed in the past four weeks | B) Numerator: Number of school days missed per patient due to asthma in the past four weeksDenominator: Number school days in the past four weeks | 7.24 | 7.31 |
| 34. Asthmatic patients with screening for depression | Numerator: Number of patients having been screened for depression in the last 12 monthsDenominator: Number of patients diagnosed with asthma | 4.78 | 4.89 |
| Results of the process | |||
| 35. % of children with auscultation in crisis. | Numerator: Number of patients with auscultation during the crisisDenominator: number of patients with asthma attack | 7.32 | 7.28 |
| 36. % of children assessed for day-time/night-time symptoms | Numerator: Number of patients with day-time and night-time symptoms and quantified assessment, included in their medical history, with no crisis in the past twelve monthsDenominator: number of patients diagnosed with asthma | 7.89 | 7.86 |
| 37. Children assessed by a nurse in the past six months. | Numerator: Number of patients assessed by the nurse and with follow-up in the past six monthsDenominator: number of patients diagnosed with asthma | 6.76 | 6.86 |
| 38. Percentage of children assessed for treatment, with check-ups in less than three weeks. | Numerator: Number of patients diagnosed with asthma who have check-ups in less than three weeks following treatment indicationDenominator: Number of asthmatic patients with treatment prescription | 7.16 | 7.17 |
| 39. % of children with two established visits and active asthma. | Numerator: Number of patients diagnosed with active asthma with two programmed visitsDenominator: Number of asthmatic patients | 8.03 | 8.00 |
| 40. % of children with one visit per year. | Numerator: Number of patients diagnosed with asthma, with a check-up in the past twelve monthsDenominator: number of asthmatic patients | 7.84 | 7.81 |
| 41. % of children hospitalised for asthma with a check-up before two weeks. | Numerator: Number of patients diagnosed with asthma, hospitalised for asthma symptoms, with a check-up at their doctor's office two weeks after hospital releaseDenominator: number of asthmatic patients hospitalised due to an asthma attack | 7.84 | 7.81 |
| 42. % of children seen at ER due to an attack and reassessed by their doctor within 72 hours | Numerator: Number of patients going to ER with asthma symptoms and are reassessed at doctor's office within three daysDenominator: Number of patients with an asthma attack visiting ER | 8.14 | 8.11 |
| 43. % of children visiting a specialist due to moderate- severe asthma (one year) | Numerator: Number of patients with moderate or severe asthma visiting a specialist in the past twelve monthsDenominator: Number of patients diagnosed with moderately severe asthma | 7.00 | 7.00 |
| 44. % of children with follow-up from the same doctor for at least 80% of their visits | Numerator: Number of patient with asthma follow-up by the same doctor in 80% of their visits, in the past two yearsDenominator: Number of patients diagnosed with asthma | 7.05 | 7.06 |
| 45. Children with a severity classification for their asthma at least once a year | Numerator: number of patients with classification of their asthma registered (initial or control level) in the past 12 monthsDenominator: number of patients diagnosed with asthma | 8.11 | 8.31 |
| 46. Children with the severity of their asthma control indicated or a recommended asthma control questionnaire registered (ACT or CAN) in the past 12 months | Numerator: number of patients with asthma control level (ACT or CAN) registered in the last 12 monthsDenominator: number of patients diagnosed with asthma | 7.86 | 7.83 |
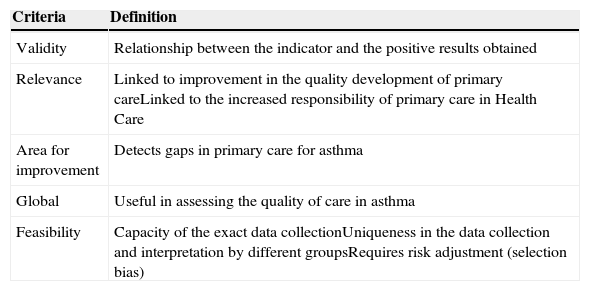
Indicator evaluation criteria and definition.
| Criteria | Definition |
|---|---|
| Validity | Relationship between the indicator and the positive results obtained |
| Relevance | Linked to improvement in the quality development of primary careLinked to the increased responsibility of primary care in Health Care |
| Area for improvement | Detects gaps in primary care for asthma |
| Global | Useful in assessing the quality of care in asthma |
| Feasibility | Capacity of the exact data collectionUniqueness in the data collection and interpretation by different groupsRequires risk adjustment (selection bias) |
The indicators were detailed in an Excel table questionnaire in which each participant had to perform his/her evaluation.
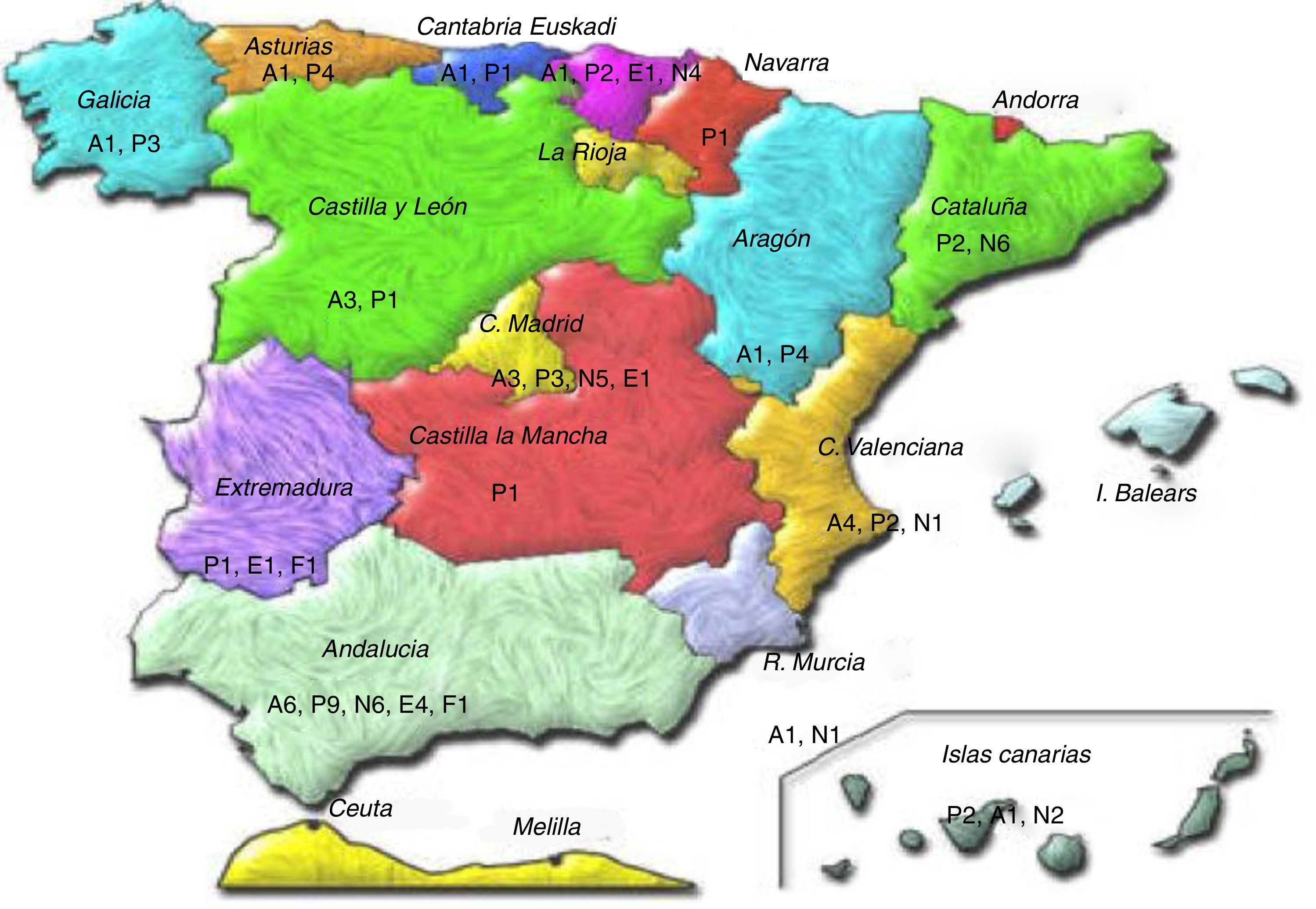
Ninety-eight primary and hospital healthcare professionals (Fig. 1) were e-mailed and invited to participate (30 allergy specialists, 27 pulmonologists, 32 primary care paediatricians, 7 nurses and 2 pharmacists). If there was no response within two weeks, the questionnaire was re-sent, requesting their collaboration for a second time. One month following the reminder, a descriptive statistical study was performed with the scores of those who had responded. For the second round, the same questionnaire was sent again in a personalised e-mail, with information about the average score for each indicator and the panellist's evaluation for the first round. Once again, a reminder was sent out two weeks following this second e-mail. There was a time lapse of approximately four months between the first e-mail and the reception of questionnaires following the second phase. In this study, contact between participants and researchers was by e-mail only. There were no personal meetings to avoid dominating personalities.
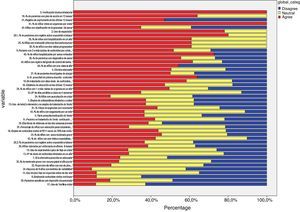
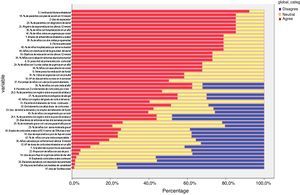
The mean and median of the values for each indicator sent by the respondents were calculated. An agreement percentage was obtained for each; agreement among panellists was scored using a Disagreement Index, which was calculated with an inter-percentile range 30–70 divided by the inter-percentile range adjusted for symmetry as defined by the RAND working Group (IPRAS). This disagreement index describes the dispersion of the results. Scores of 7–9 were categorised as agreement, from 1 to 3 as disagreement and from 4 to 6 as neutral. In the RAND method, an index score >1 indicates disagreement.4
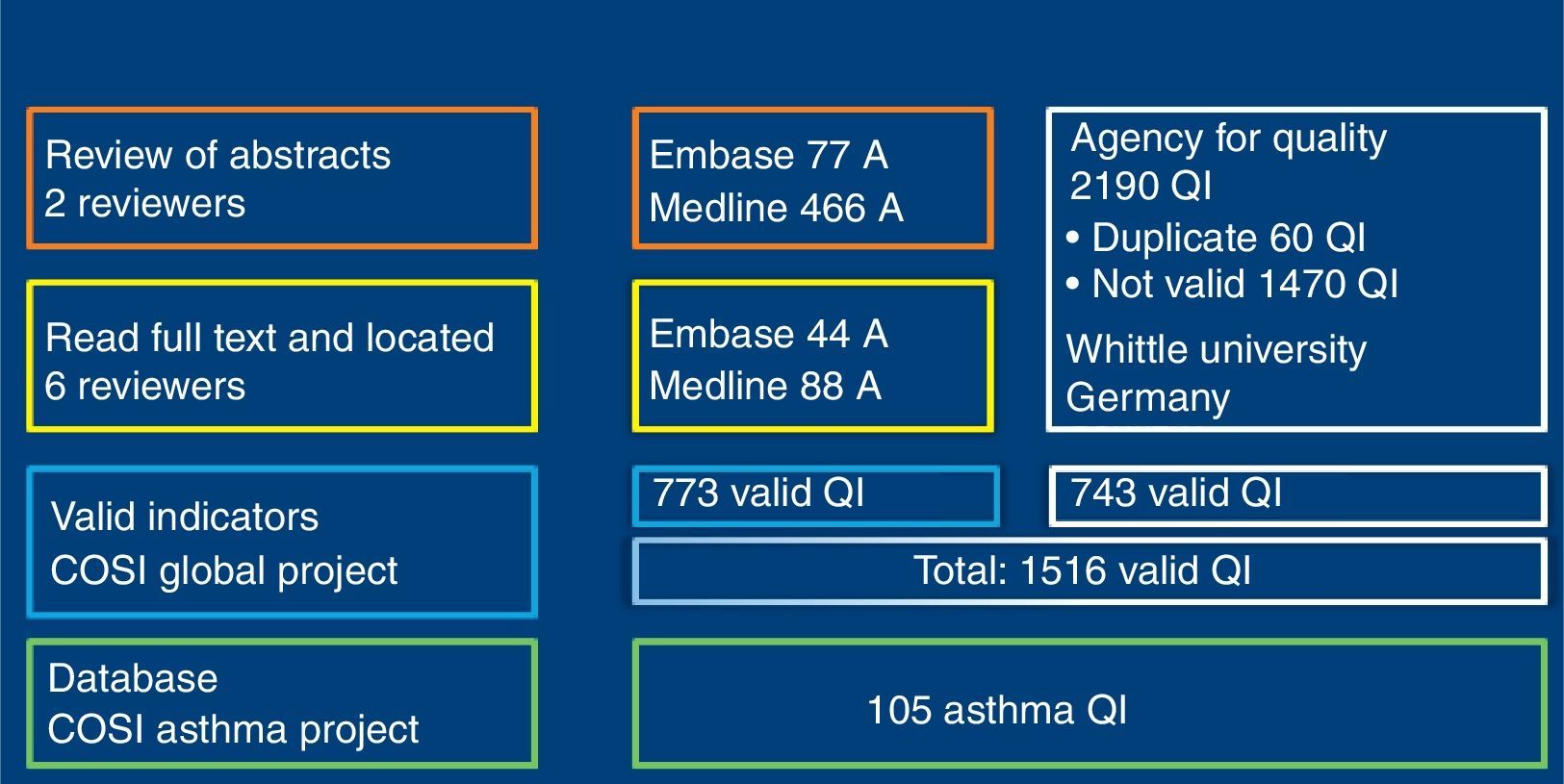
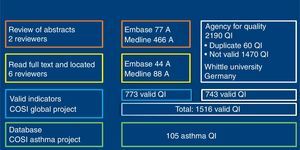
ResultsSearchOne hundred and sixty-seven (167) articles supplied by QI were selected. Of these, 1516 QI were obtained for paediatric primary care, of which 773 were extracted from magazine articles and 743 from the Quality Agency databases (Fig. 2). Of all the indicators, 105 were related with bronchial asthma. Using nominal group, we selected 46 of the 105 asthma indicators, as listed in Table 1.
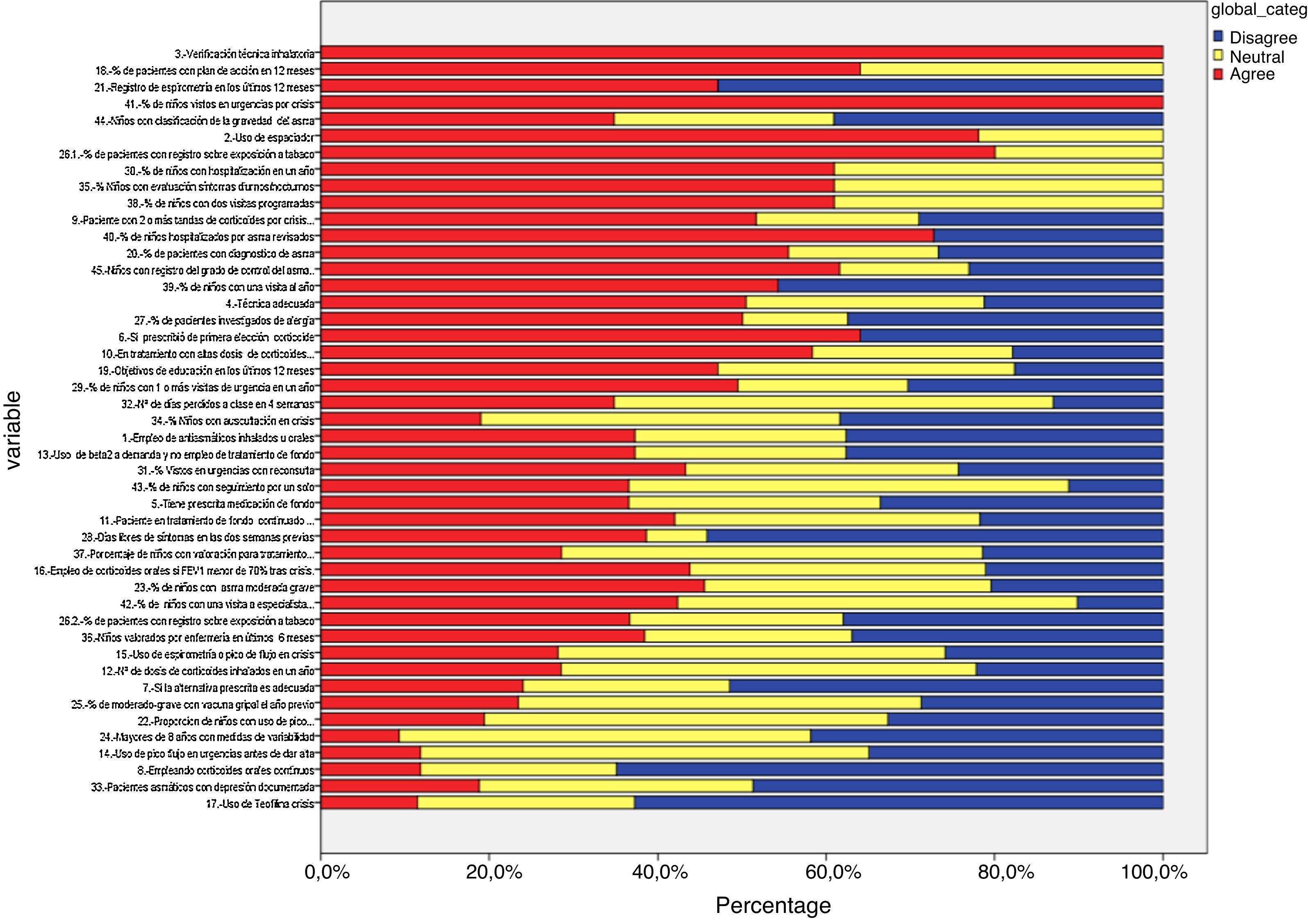
Delphi phase oneIn the first phase, 38 responded (39.71% of the panel), of these, 26 were primary care paediatricians, 6 pulmonologists, 3 nurses, and 1 an allergy specialist (Table 1 and Fig. 3). IPRAS showed participant agreement for all indicators that exceeded a score of 7; there was only disagreement regarding the use of continuous oral corticoids and the use of theophylline during an asthma attack.
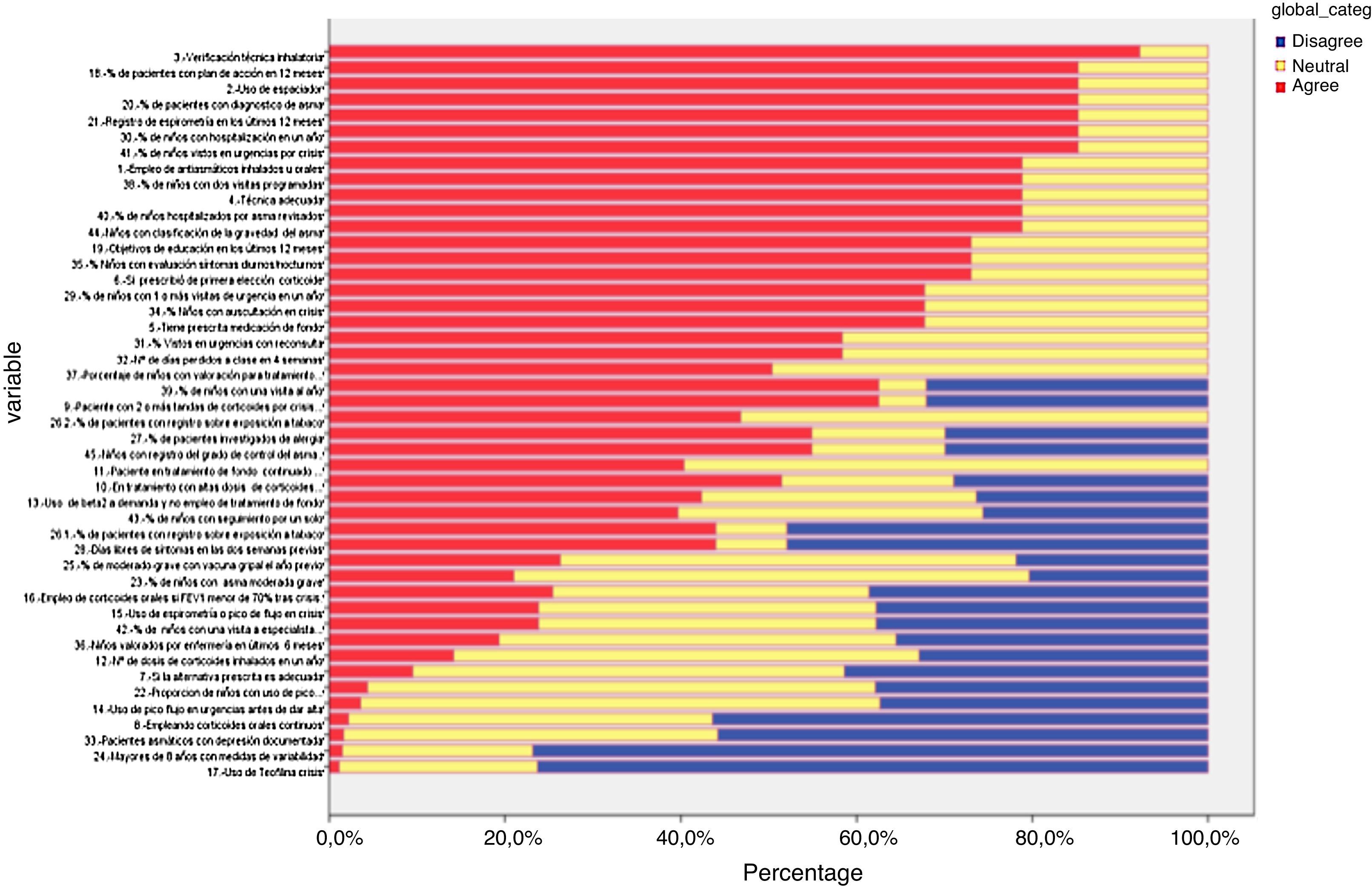
Delphi phase twoAll those who responded during the first phase participated in the second phase; responses from six new panellists who had not sent their evaluation in the first phase were also received, but these were discarded (Table 1 and Fig. 4). In the second phase of Delphi, there were no scores of less than 3 and IPRAS showed an agreement on behalf of the panel for all the indicators.
DiscussionAsthma is a prevalent disease among children; in Spain, it is the largest chronic condition.3 A wide variety of measures have proven useful to improve the healthcare process of children with asthma. Steuten19 found that educational programmes reduce visits to the emergency room (ER), hospitalisations, unplanned visits to primary care centres and school absenteeism. Also, by increasing programmed medical visits, adherence and self-control improves, as does patient lifestyle by reducing the environmental triggers in the home. These aspects also facilitate improved healthcare organisation, which leads to a better accessibility, coordination and continuity of the services. Indicators are included in most plans or guidelines. Verification of the inhaler technique,7,12 quality of life, carrying out action plans,7,16 emergency consultations, hospitalisation8 and the use of spirometry20 are mentioned most.
Our study used a modified Delphi methodology to generate indicators based on evidence and the opinions of experts. The group of indicators covers several aspects of primary care for asthma, including prevention, promotion, care and collaboration with all parties involved in asthma care.
In other studies published in Spain, indicators were developed based on the opinion of experts and printed guidelines.21,22 The two main strengths of our study lie in the comprehensive systematic review of the literature and the participation of all those professionals involved in the care of paediatric asthma patients. For the most part, these professionals come from within the various regions of Spain and have different professional profiles, ranging from primary care to hospitals and pharmacies. We have a broad range of indicators that cover the estimation for prevalence, the care process, education and the health results. There was major consensus among members of the panel and no disagreement was found among the panellists regarding the most valued indicators.
Another strength of this study is that it is based on an opportunity, since the authors of the project are part of a European child health indicator project, the idea of which is to promote a better understanding of children's needs and facilitate comparisons among the European countries.23 An extensive search of quality indicators in the bibliography and health agencies was carried out, which translates into the project being founded on the scientific evidence.
The main difficulty in assessing the quality of primary care is the lack of data. For example, there are no data, except for local studies, on an evaluation of the seriousness of asthma, the correct use of inhaler techniques and an evaluation of education. Based on the direct observation of backgrounds, the traditional audit system should no longer be used because it is tedious and incomplete. The new, electronic clinical history systems in Spain's National Healthcare System should integrate indicators that are crucial, both in the implementation and the evaluation. Mangione comments—based on medical register documentation—that there are deficiencies in the healthcare provided during childhood (for which the global adherence rate was 46.5%). These deficits could give rise to the inadequate handling of the disease with potentially avoidable adverse health results. This author mentions that only 44.0% of the children with asthma using β2-agonists at least three times a day were prescribed an anti-inflammatory.24
The general quality improvement models are based on the “Plan-Do-Check-Act”. This system seeks to transmit the importance of the following points:
- •
Plan: Identify the problem and the potential solution.
- •
Do: Try the proposed real life solutions.
- •
Check (study indicators): to see if the solution worked.
- •
Act: implementation of the successful solution.3
Two key characteristics of this model are the measurability and continuity of the process to close a permanent quality circuit. The indicators contributed by this study have a high degree of consensus to allow appropriate and standardised information to be provided in all the populations within the scope of primary care. This model could be incorporated into the regional and national healthcare systems to monitor the effectiveness and the impact of the healthcare policies to work as the standard to asses programmes. Systematic reviews and clinical trials supply evidence that the circles of quality improve the clinical practice in asthma and adherence to the guidelines provided.25,26 The data collected allow us to help establish reference points and identify areas for improvement in primary care.18
WeaknessesIt is possible that another panel studying this same topic could reach different conclusions depending on the characteristics of their experience. However, although our panel is comprehensive, it is always advisable to bear in mind the profiles of the panel to control possible confounding factors.
This study has limited participation of specialists from hospitals. Nevertheless, the consensus of those participating in the study was included in the battery of indicators focusing on asthma management within the area of primary care, which was the objective of this study.
ConclusionsThe multidisciplinary work panel has considered the following as asthma care quality indicators with a high score and maximum level of agreement: chosen indicators.
Process indicators: Percentage of patients with an action plan stating objectives, written in the past 12 months (8.42); Spirometry Registration in the past 12 months (8.19); use of spacer chamber (8.03); percentage of patients registered as having exposure to tobacco (7.94); correct inhaler technique (7.89); register of the degree of asthma control (7.83).
Indicators of results on health: Percentage of children seen at ER for an asthma attack, who were re-evaluated by their doctor within 72h (8.11).
These indicators could be used by the healthcare services of the entire country given the methodology used to obtain this information.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingSpain's Ministerio de Ciencia e Innovación, thanks to a grant from the Fondo de Investigación Sanitario del Instituto de la Salud Carlos III (PI10/01244). Funding by Andalusia's Consejería de Salud through the 2010 summons for Health Research grants (PI-0177-2010).
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank the following for their participation on the panel of experts (in alphabetical order):
Ane Aldosoro-Ruiz (e, 13); Arantxa Aranguez (f, 8); Maite Asensi-Monzó (p, 14); Luis Bamonde-Rodríguez (p, 9); Alberto Bercedo-Sanz (p, 5); Javier E Blanco-González (p, 6); Manuel Boquete-Paris (a, 9); Maite Callén-Blecua (p, 13); Carmen Candela-Fuster (e, 1); Alfredo Cano-Carcinuño (p, 6); Ignacio Carvajal-Urueña (p, 3); José Antonio Castillo-Laita (p); Olga Cortés-Rico (p, 10); María Elena De Jaime-Revuelta (p, 1); Begoña Domínguez-Aurrecoechea (p, 3); Fernando Echavarri-Olavarría (n, 10); José Javier Elorz-Lambarri (n, 13); Amparo Escribano-Montaner (n, 14); María Luisa García-Gestoso (p, 1); Agueda García-Merino (p, 3); Arantxa Garmendia-Iglesias (p, 13); Marcel Ibero-Iborra (a, 7); Antonio Jiménez-Cortés (p, 3); Javier Korta-Murúa (n, 13); Ángel López-Silvarrey (p, 9); Alfonsa Lora-Espinosa (p, 1); Ana Martínez-Cañavate-Burgos (a, 1); José Luis Montón-Álvarez (p, 10); Isabel Mora-Gandarillas (p, 3); Juan José Morell-Bernabé (p, 8); Isabel Moneo-Hernández (p, 2); Antonio Moreno-Galdó (n, 7); Carlos Pardos-Martínez (p, 2); Manuel Praena-Crespo (p, 1); Carmen Rosa-Rodríguez-Fernández-Oliva (p, 4); Juan Rodríguez-Sánchez (f, 1); Juan Ruiz-Canela-Cáceres (p, 1); Santiago Rueda-Esteban (n, 10); Adulfo Sánchez-Marenco (n, 1); Concepción Segovia-Ferrera (p, 1); María Isabel Toscano (e, 1); Isabel Úbeda-Sansano (p, 14); José Valverde-Molina (n, 11); Francisca Verdugo-Romero (p, 1).
Description notes: a: allergy treatment; e: nursing; f: primary care pharmacy; n: pulmonology; p: primary care paediatrician; 1: Andalusia; 2: Aragon; 3: Asturias; 4: Canary Island; 5: Cantabria; 6: Castilla y León; 7: Catalonia; 8: Extremadura; 9 Galicia; 10: Madrid; 11: Murcia; 13 Basque Country; 14 Valencia.
Gottfried Huss coordinator, who allowed us to use the COSI project database, and his help with the in the bibliographic) search. Antonio Romero Tabares information specialist at the Agencia de Evaluación de Tecnología de Andalucía for his help in the bibliographic search.