Asthma is a heterogeneous disease characterised by chronic airway inflammation. One of the most devastating consequences of this inflammatory process is the generation of reactive oxygen and nitrogen species responsible for oxidative stress. The aim of this study is to analyse the efficiency of treatment with human bone marrow-derived mesenchymal stromal cells (hMSC) in maintaining the oxidative balance in a murine model of allergic asthma by quantifying nitrotyrosine in lung tissues. After confirmation of asthma in the experimental model, samples of lung parenchyma were submitted to immunohistochemical assessment. Intravenous administration of hMSC reduced the levels of nitrotyrosine in the ASTHMA-hMSC group compared to those in the ASTHMA-SAL group. In conclusion, therapeutic administration of hMSC had a beneficial effect on oxidative stress, reducing the levels of nitrotyrosine in lung tissues in a model of allergic asthma.
Asthma is a serious public health problem that affects an estimated 300 million individuals worldwide.1–4 Approximately 5–10% of those affected do not respond to conventional treatments and have a poor quality of life as a result.5,6
Asthma is defined as a chronic inflammatory disease that predominantly affects the large airways where multiple cells, both inflammatory and structural, play roles in its pathogenesis.7,4 It is a multifactorial disease resulting from the interaction of genetic and environmental factors and involving an imbalance of oxidants and antioxidants factors.
Reactive oxygen species (ROS) are produced in response to many physiological conditions during the normal functioning of the human body. However, the production of ROS can have negative effects, as a consequence, the body has an antioxidant system. When there is an imbalance between the oxidant and antioxidant systems and oxidants predominate, oxidative stress occurs.8
There is strong evidence that the oxidative state in asthma is marked by an imbalance in the antioxidant and oxidant systems. ROS and reactive nitrogen species (RNS) play a role in inflammation of the airways and are determinants of disease severity.9
Oxidative stress can harm lung functioning causing hyperplasia of goblet cells with mucus hypersecretion, hypertrophy of smooth muscles of the airways and subepithelial fibrosis, vascular exudation, as well as other changes that exacerbate inflammation and consequently remodel airways.10
Stem cells are a possible treatment for asthma. Cell therapies can be described as a set of technological approaches that use stem cells for the treatment of diseases. Cell therapies have shown surprising results in different conditions and diseases related to airways.11–15
Oxidative stress plays a role in the pathophysiology of asthma, and it seems that it can be minimised with the use of stem cells.16 One method to quantify oxidative damage is the measurement of nitrotyrosine, a marker of nitration of proteins by peroxynitrite.17–19
Thus, the aim of this work was to evaluate the efficacy of treatment with human bone marrow-derived mesenchymal stromal cells (hMSC) in maintaining the oxidative balance in an experimental model of allergic asthma by quantifying nitrotyrosine in lung tissues.
MethodsThis study was approved by the Ethics Committee of the Carlos Chagas Filho Institute of Biophysics, Health Sciences Centre, Federal University of Rio de Janeiro. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the U.S. National Academy of Sciences.
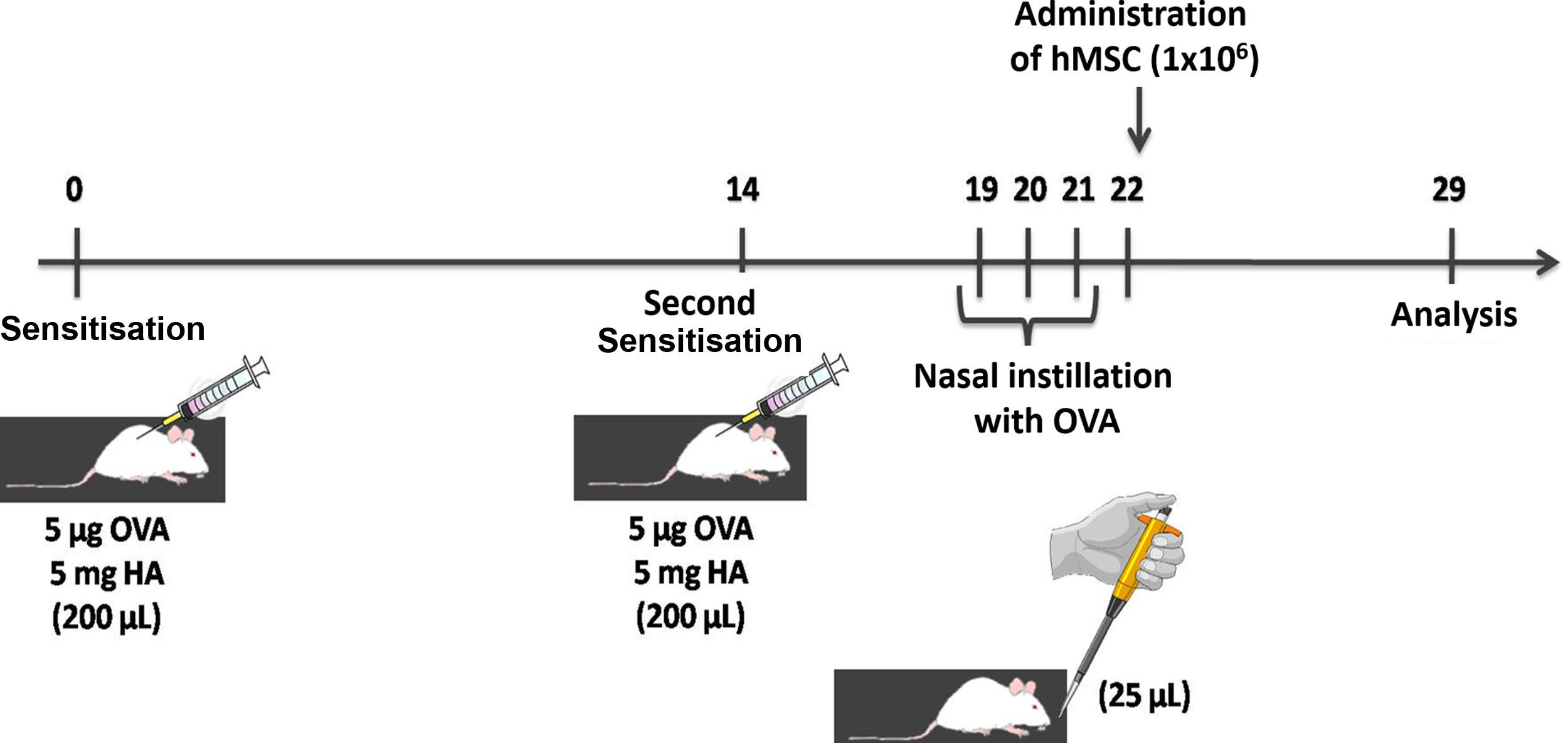
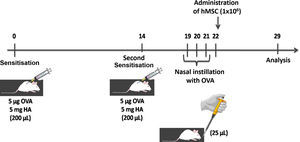
Animal preparation and experimental protocolBALB/c mice from the laboratory of the Pontifical Catholic University of Paraná were used in experiments.
Thirty-two adult female BALB/c mice, weighing 24–34g were randomly divided into the following groups:
- •
CTRL-SAL: The animals were sensitised and challenged with saline solution, and then received a saline solution treatment through the tail vein 24h after the last allergenic challenge (n=8).
- •
CTRL-hMSC: The animals were sensitised and challenged with saline solution, and then received an injection of hMSC (1×106 cells in 50μL of IMDM) through the tail vein 24h after the last allergenic challenge (n=8).
- •
ASTHMA-SAL: Animals were sensitised by two subcutaneous injections containing 5μg of ovalbumin (OVA) and 5mg of aluminium hydroxide, and then challenged with nasal instillation of 25μg of OVA diluted in 25μL of sterile saline solution. The saline solution was administered through the tail vein 24h after the last allergenic challenge (n=8) (Fig. 1).
- •
ASTHMA-hMSC: The animals were sensitised by two subcutaneous injections containing 5μg of OVA and 5mg of aluminium hydroxide, and then challenged with nasal instillation of 25μg of OVA diluted in 25μL of sterile saline solution. hMSC (1×106 cells in 50μL of IMDM) were injected into the tail vein 24h after the last allergenic challenge (n=8).
Mononuclear cells isolated from human bone marrow were used to obtain hMSC.20 IMDM (Gibco, Invitrogen, NY, USA) supplemented with 15% foetal bovine serum (Gibco, Invitrogen, NY, USA) and 1% of antibiotic solution with penicillin (Gibco Invitrogen, NY, USA) and streptomycin (Gibco Invitrogen, NY, USA).
Asthma confirmationPulmonary mechanicsSeven days after treatment, the animals were sedated (diazepam, 1mg IP), anaesthetised (sodium thiopental 20mg/kg intraperitoneal), tracheotomised, paralysed (vecuronium bromide, 0.005mg/kg intravenous), and ventilated with a ventilator for small animals (Samay VR15, Universidad de la Republica, Montevideo, Uruguay) with the following parameters: frequency of 100cycles/min, tidal volume (TV) of 0.2mL, and fraction of inspired oxygen of 0.21. The anterior chest wall was surgically removed and a positive end-expiratory pressure of 2cmH2O was applied. Pulmonary mechanics were measured using the end-inspiratory airway occlusion method.21 In animals with open chests, tracheal pressure reflected the transpulmonary pressure (PL). After airway occlusion, there was a rapid fall in the PL (ΔP1) corresponding to maximum pressure by subtracting the inflection point, followed by a new slow decrease (ΔP2) in PL, represented by the lung elastic recoil pressure (Pel). Static compliance (Est) was determined by dividing Pel by TV. Measurements of lung mechanics were performed 10 times in each animal. All data were analysed using the software ANADAT (RHT-InfoData, Montreal, Canada).
Histological and morphometric analysisAfter the analysis of pulmonary mechanics, samples of lung parenchyma were fixed in 10% formaldehyde and embedded in paraffin (PFFE) for morphology and morphometry studies. Sections, 5-μm thick, were stained with haematoxylin and eosin (HE), periodic acid-Schiff with Alcian blue and PicroSirius Red and analysed by light microscopy (BX51, Olympus Latin America Inc., Miami, FL, USA).
Index of bronchoconstriction: The index of bronchoconstriction was analysed on slides stained with HE, using the conventional point-counting technique.22 The inner diameters of airways were computed at 400× magnification, counting the number of points that fell in the lumen of airways and the inner layers of airways from epithelium to smooth muscle. The perimeters of airways were estimated by counting the number of lines that intercepted the basal epithelial membrane. This procedure was evaluated in at least four airways. The areas of smooth muscle and airway epithelium were corrected by the perimeter of the airways, dividing by the number of lines that intersected the basal membrane of the epithelium of the corresponding airway. Since the number of lines that intersect the basal epithelial membrane (NI) is proportional to the perimeter of the airway, and the number of points that fall inside the airway (NP) is proportional to the area of the airway, the intensity of bronchoconstriction was computed by the following equation: [index of bronchoconstriction (IB)]=NI/NP.23
Collapse: To estimate lung collapse, the fraction of the area occupied by the collapsed pulmonary parenchyma was demarcated, quantified, and divided by the total area of the lung parenchyma in a high-power field (HPF) on slides stained with HE, using Image Pro Plus 5.1 for Windows software (Media Cybernetics, Silver Spring, MD, USA) and a computer connected to a digital camera and optical microscope.24
Mucus and collagen fibres: The slides were stained using specific methods to quantify mucus (periodic acid-Schiff with Alcian blue) and collagen fibres (PicroSirius Red) and then analysed with 20× amplification evaluating ten scanned images of random and unmatched fields per event. The quantification of collagen fibres and mucus was calculated as the area occupied divided by the total area examined.24 Blood vessels were excluded from the measures. The results are expressed in percentages.
Smooth muscle α-actin: Immunohistochemistry was used to evaluate the expression of smooth muscle α-actin. Sections, 5-μm thick, were stained with monoclonal anti-smooth muscle α-actin antibody (Dako, Carpenteria, CA, USA) at a dilution of 1:500. Ten random and unmatched fields were evaluated per slide, and antibody positivity was observed as brown-stained cytoplasm.
Tissue expression of nitrotyrosineImmunohistochemistry was performed to analyse the expression of nitrotyrosine in lung tissue, which indirectly indicates oxidative stress in lung tissue.
Immunoperoxidase assay was used for immunohistochemistry with modifications as reported by Chong et al. Briefly, specific Mouse monoclonal [HM11] anti-nitrotyrosine antibody from Santa Cruz Biotechnology, Inc. (Dallas, USA) was used in the assay.
The conventional technique, omitting the primary antibody and using RAM11 antibody (Dako North America, Inc., Carpinteria, USA) as negative primary antibody, was the negative control.
Results were revealed with a mouse on mouse Polymer IHC Kit ab127055 (Abcam, Cambridge, UK). Counterstaining was performed with Harris haematoxylin.
The immunostained slides were photographed with an Axio Scan.Z1 Digitizer, and images were analysed with the program Image Pro Plus 4. For each sample, approximately 600 images were obtained using a 20× lens. Of these, about 500 images were excluded, resulting in approximately 100 satisfactory images per animal. The positive control was used as a “mask” displaying sufficient levels of tissue expression. The mask was then superimposed on images of the samples. Based on the ideal immunoexpression indicated by the mask, an image analysis was performed to identify the positive areas in samples, and these results were transformed to measures of positive expression by square micrometre (μm2). The area in μm2, obtained by this method, was divided by the total area of the field observed to generate a percentage value for each image.
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics v.20.0 software. The results obtained are presented as means, medians, minimum values, maximum values, and standard deviations. A one-way analysis of variance (ANOVA) was used to compare nitrotyrosine levels in the various groups. The multiple comparisons (post hoc) were performed using the least significant difference (LSD) test, and normality of the data was assessed using the Kolmogorov–Smirnov test. p values <0.05 were considered statistically significant.
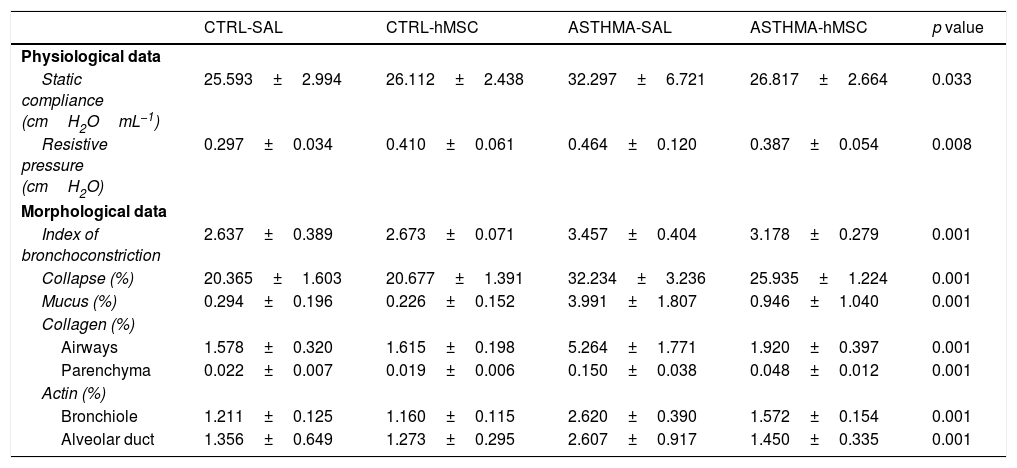
ResultsEvidence of asthmaThe ASTHMA group displayed a statistically-significant increase in static compliance and resistive pressure in comparison with those of the CTRL group (Table 1).
Statistical values of the attestation of asthma in the experimental model.
| CTRL-SAL | CTRL-hMSC | ASTHMA-SAL | ASTHMA-hMSC | p value | |
|---|---|---|---|---|---|
| Physiological data | |||||
| Static compliance (cmH2OmL−1) | 25.593±2.994 | 26.112±2.438 | 32.297±6.721 | 26.817±2.664 | 0.033 |
| Resistive pressure (cmH2O) | 0.297±0.034 | 0.410±0.061 | 0.464±0.120 | 0.387±0.054 | 0.008 |
| Morphological data | |||||
| Index of bronchoconstriction | 2.637±0.389 | 2.673±0.071 | 3.457±0.404 | 3.178±0.279 | 0.001 |
| Collapse (%) | 20.365±1.603 | 20.677±1.391 | 32.234±3.236 | 25.935±1.224 | 0.001 |
| Mucus (%) | 0.294±0.196 | 0.226±0.152 | 3.991±1.807 | 0.946±1.040 | 0.001 |
| Collagen (%) | |||||
| Airways | 1.578±0.320 | 1.615±0.198 | 5.264±1.771 | 1.920±0.397 | 0.001 |
| Parenchyma | 0.022±0.007 | 0.019±0.006 | 0.150±0.038 | 0.048±0.012 | 0.001 |
| Actin (%) | |||||
| Bronchiole | 1.211±0.125 | 1.160±0.115 | 2.620±0.390 | 1.572±0.154 | 0.001 |
| Alveolar duct | 1.356±0.649 | 1.273±0.295 | 2.607±0.917 | 1.450±0.335 | 0.001 |
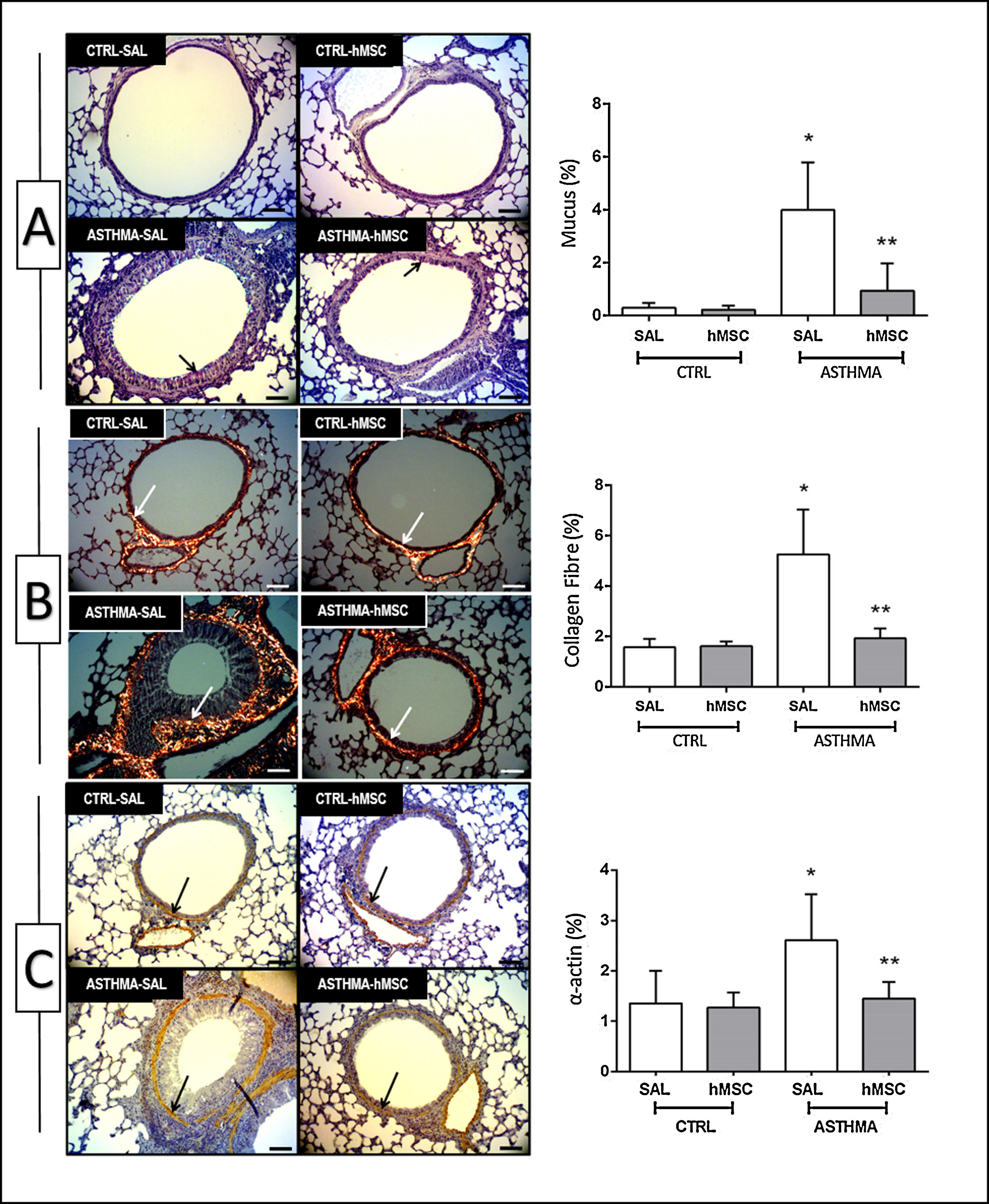
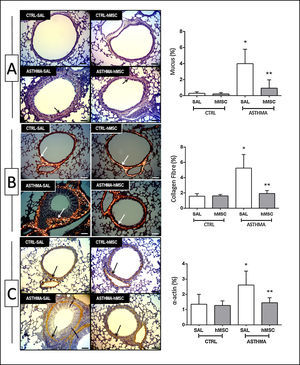
The animals in the ASTHMA group displayed changes in airways and lung parenchyma, with evidence of epithelial injury, subepithelial fibrosis, hypertrophy, and hyperplasia of smooth muscle cells and goblet cells. Moreover, interrupted respiratory epithelium, collapsed alveoli, infiltration of inflammatory cells, deposition of mucus, and increased collagen fibre content were found (Table 1 and Fig. 2).
Photomicrograph of lung parenchyma and immunohistochemical identification and quantification of mucus (A), collagen fibre (B) and α-actin (C). CTRL: animals sensitised and challenged with saline solution. ASTHMA: animals sensitised with a solution containing ovalbumin and aluminium hydroxide as an adjuvant and challenged with three intranasal instillations of ovalbumin. hMSC: animals treated intravenously with 1×106 mesenchymal cells 24h after the third challenge. SAL: animals treated with saline solution under the same protocol. The arrows indicate positive staining for mucus (A), collagen fibre (B) and α-actin (C). Original amplification: 200×. Scale bars: 10μm. Bars represent the mean±SD of eight animals/group. *Significantly different from the control (p<0.05). **Significantly different from the ASTHMA-SAL group (p<0.05).
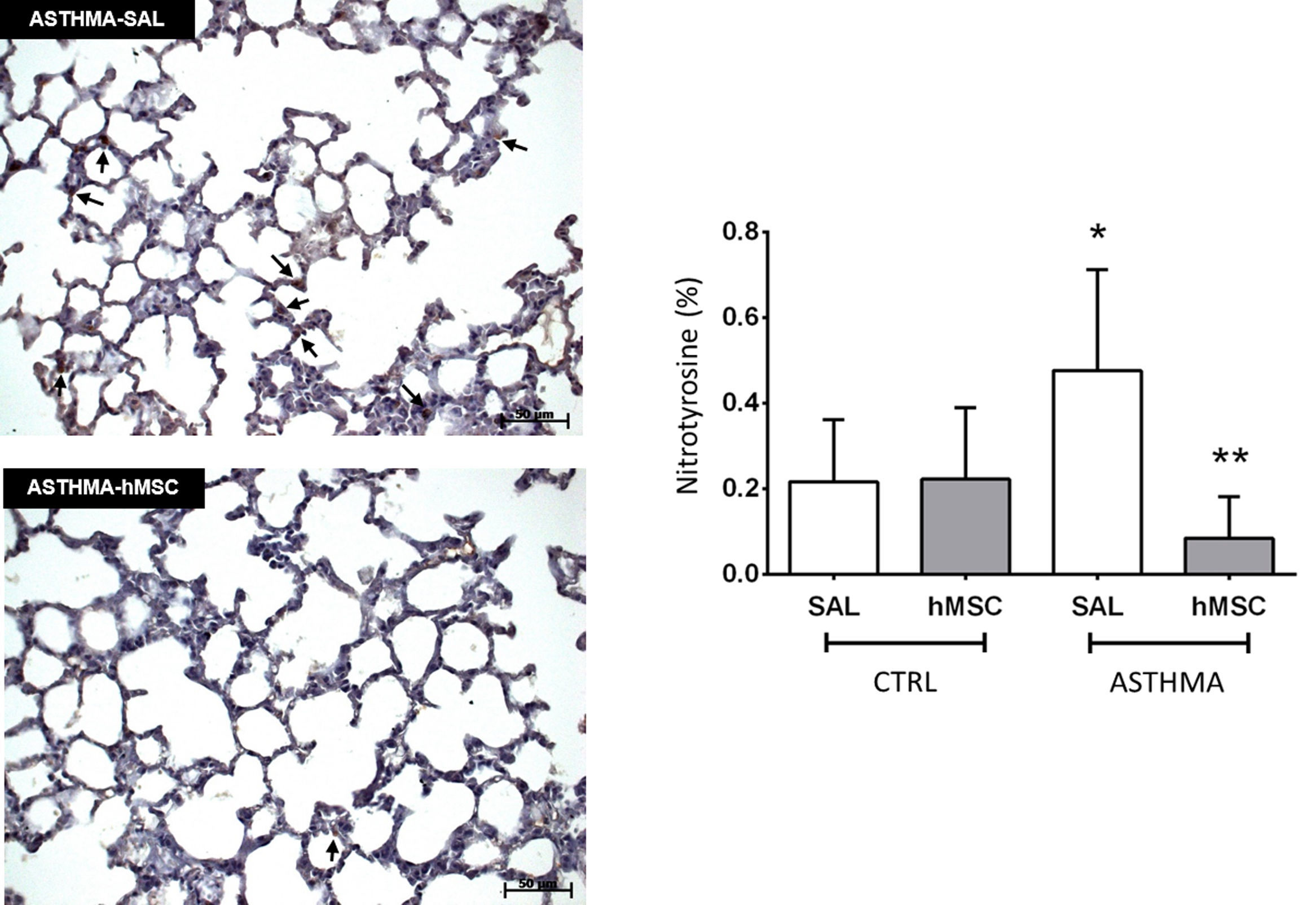
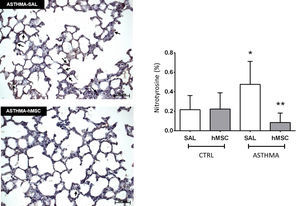
The ASTHMA-SAL group displayed an increase in levels of nitrotyrosine in the lung parenchyma when compared to that of the CTRL-SAL group (0.477±0.236% vs. 0.217±0.146%, p<0.001). Intravenous administration of hMSC derived from bone marrow reduced the levels of this substance in the ASTHMA-hMSC group compared to that of the ASTHMA-SAL group (0.085±0.097% vs. 0.477±0.236%, p<0.001) (Fig. 3).
Photomicrograph of lung parenchyma and immunohistochemical identification and quantification of nitrotyrosine. CTRL: animals sensitised and challenged with saline solution. ASTHMA: animals sensitised with a solution containing ovalbumin and aluminium hydroxide as an adjuvant and challenged with three intranasal instillations of ovalbumin. hMSC: animals treated intravenously with 1×106 mesenchymal cells 24h after the third challenge. SAL: animals treated with saline solution under the same protocol. The arrows indicate positive staining for nitrotyrosine. Original amplification: 200×. Scale bars: 50μm. Bars represent the mean±SD of eight animals/group. *Significantly different from the control (p<0.05). **Significantly different from the ASTHMA-SAL group (p<0.05).
This study developed an experimental model of allergic asthma in adult mice with histological features and ultrastructural characteristics suggestive of remodelling of the airways and lung parenchyma, indicating epithelial injury, subepithelial fibrosis, eosinophilic infiltration, and hypertrophy and hyperplasia of smooth muscle cells and goblet cells, respectively. Such changes resulted in an increase in lung mechanic parameters (static compliance of the lung and resistive pressure) in vivo. The thickness of the epithelium, basal lamina, and subepithelial smooth muscle was greater in animals of the ASTHMA group than of those in the CTRL group. Interruption in the respiratory epithelium, infiltration of inflammatory cells, and accumulation of mucus in the airways, as well as increased levels of α-actin, were found in the ASTHMA group. These results indicate the successful establishment of an experimental model of allergic asthma, enabling a study on the oxidant/antioxidant imbalance in asthma.
There is evidence that an imbalance between antioxidant and oxidant systems favours the oxidative state observed in asthma, corroborated in our model by the increase of nitrotyrosine in this group (see Fig. 3, p<0.05). The high concentration of oxygen in the pulmonary environment makes lungs susceptible to oxidative stress reactions, including the generation of ROS and RNS, which play a role in inflammation of airway and are determinants of disease severity.9 The major ROS and RNS include superoxide anions, hydrogen peroxide, hydroxyl radicals, perchloric acid, nitric oxide (NO), and peroxynitrite.
Thus, ROS are formed by metabolic reactions such as the transport of electrons that occurs in mitochondria during cellular respiration processes25 and are produced by both inflammatory cells (eosinophils, neutrophils, monocytes, and macrophages) and resident cells (epithelial and smooth muscle cells).26,27
Antioxidant defences are needed to contain the damage caused by excess ROS, but changes in antioxidants are also expected, either as a result of their depletion or inactivation, resulting in insufficient protection and leading to lung and systemic inflammation in asthmatic individuals.10
Thus, antioxidant defence mechanisms in blood and lungs are lost with asthma. The main antioxidant defence factors of the body, both enzymatic or non-enzymatic, include: glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), carotenoids, and vitamins A, D, and E.28
An increase in ROS and RNS leads to structural and functional protein modifications that trigger and maintain the inflammatory process. These changes include a reduction in the activity of GPx, SOD, and CAT. Consequently, ROS and RNS are produced in high concentrations, able to overcome the antioxidant mechanisms in the body, leading to changes such as airway hyperresponsiveness, hypersecretion of mucus, increased vascular permeability, contraction of airway smooth muscles, secretion of neuropeptides, and impairment of beta-adrenergic receptor responsiveness.10
Activated alveolar macrophages secrete both NO and superoxide anions in the alveolar coating fluid, and they react rapidly to form peroxynitrite, a powerful oxidising agent capable of damaging lipids and proteins in biological membranes.29,17,30–33 This powerful oxidising agent appears to be largely responsible for the adverse effects of excess NO production, causing tissue damage, lipid peroxidation, and nitrosylation of tyrosine residues. Most of the effects of high concentrations of NO are mediated by peroxynitrite. This substance adds a nitro group to the hydroxyl group of tyrosine, forming 3-nitrotyrosine, a stable product.17 For these reasons, nitrotyrosine was selected for measurement in the present study and was increased in the asthmatic group (see Fig. 3, p<0.05). Previous studies have demonstrated the role of nitrotyrosine in oxidative stress, a major step in the pathophysiology of asthma.9 There is some difficulty in determining the quantity of substances that result from oxidative stress in tissues because of their volatile nature. Therefore, stable final products of the oxidation or nitrosylation pathways, such as nitrotyrosine, can be used as tissue markers of oxidative stress.9 Thus, when assessing the tissue concentrations of nitrotyrosine, we were indirectly defining the ability of peroxynitrite to produce NO, a primary factor in the maintenance of inflammation in asthma.
In a previous study,17 a significant increase in the expression of nitrotyrosine in neutrophils, eosinophils, and macrophages was observed in the airways of asthmatic patients. In a study by Saleh,17 an inverse correlation between the presence of 3-nitrotyrosine and pulmonary functions measured by spirometry, such as FEV1 (forced expiratory volume in one second) and FVC (forced vital capacity), was also found. These results suggested that the formation of peroxynitrite in the airways of patients with asthma can serve as an important marker and mediator of oxidative stress in airway diseases.17
A reduction in oxidative stress would be the solution to many health problems. With this objective, we used hMSC as a therapy and evaluated their effectiveness by quantifying nitrotyrosine in lung tissues of an experimental model of allergic asthma.
Mesenchymal stromal cells (MSC) are known for their adaptability, as well as their anti-proliferative and anti-inflammatory properties. By reducing inflammation, the production of substances responsible for oxidative stress is also reduced.
In this study, hMSC acted positively on tissues, reducing oxidative stress. There was a reduction in nitrotyrosine of 0.392% in the ASTHMA-hMSC group compared to the ASTHMA-SAL group (see Fig. 3, p<0.001). Therefore, by reducing the percentage nitrotyrosine in the lung parenchyma in this model, hMSC were shown to be effective in reducing oxidative stress, because nitrotyrosine is a stable marker of this stress.
Therapies with MSC have been the focus of research related to asthma. Recently, one study investigated the efficacy of MSC in relation to remodelling and inflammation in the airways of a murine model of chronic asthma. In this study, intravenous administration of these cells significantly reduced the histopathological changes observed in disease, including changes in structures such as the basal membrane, epithelium, smooth muscle tissue, and goblet cell hyperplasia (p<0.001).34 Another study35 followed, for eight months, the changes that occurred in a model of feline asthma after administration of MSC, and significant beneficial effects, such as lung attenuation and reduction in the thickness of bronchial walls, were observed in two of the computed tomography images used to analyse patients with asthma.
In this study, MSC may be working in a paracrine manner in disease recovery. The effectiveness of MSC is believed to result from the favourable microenvironment for restoration of the activities previously impaired by disease. Along with this, oxidative stress would be reduced, which is represented by a decrease in the percentage nitrotyrosine in lung tissue.
Saleh et al. evaluated treatment with inhaled corticosteroids, the gold standard for patients with asthma, and noticed a significant reduction in nitrotyrosine in the airway epithelium, lung parenchyma, and inflammatory cells of patients with asthma.17 As quoted earlier, similar results were obtained in the present study by using hMSC. In order to achieve effects similar to those obtained with conventional therapy, MSC are a therapeutic option that should be explored further for future use in the treatment of asthma.
ConclusionhMSC administered therapeutically showed a beneficial effect on oxidative stress, reducing the levels of nitrotyrosine in the lung tissue of an allergic asthma model.
FundingThis study was supported by Araucaria Foundation for the Support of Scientific and Technological Development of Paraná (FA); and Coordination for the Improvement of Higher Education Personnel (Capes).
Conflict of interestThe authors declare that they have no conflict of interest.
The authors express their gratitude to Ms. Ana Paula Martins for her help with microscopy, and Ms. Marina Louise Viola Azevedo for her assistance with Immunohistochemistry.