Cirrhosis and liver cancer are currently common causes of death worldwide. The global epidemic of obesity has increased the incidence of nonalcoholic fatty liver disease (NAFLD) and cirrhosis in recent years. Advanced fibrosis increases the morbimortality rate in NAFLD. The Mexican population has one of the highest prevalence of obesity and diabetes mellitus (DM) worldwide.
AimTo determine the prevalence of advanced liver fibrosis in Mexican general population.
MethodsAdult individuals, without a history of liver disease nor heavy alcohol consumption were randomly sampled from 20,919 participants of a health and nutrition survey applied to the general population. Clinical and laboratory evaluations were performed to calculate the NAFLD fibrosis score (NFS) (an extensively validated non-invasive method). Two cut-off points were used. Advanced fibrosis was defined as a result >0.676.
ResultsIn total 695 individuals were included. The mean age was 47.8±16.4. The majority were between 20 and 50 years (59%), 70.2% were female, 35.5% showed obesity and 15.8% DM. The 93% had normal serum ALT. Based on the NFS results, 56 individuals (8.1%) had a high probability of fibrosis. Most patients from this subgroup showed normal serum ALT (92.9%), 89.3% were >45yr. old, 52% were obese and 27% suffered from DM.
ConclusionsBased on these results, 8.1% of Mexican general population without a history of liver disease is at high risk of having advanced liver fibrosis and complications and death derived from cardiovascular disease and cirrhosis. Most of them showed normal ALT serum levels.
Cirrhosis and liver cancer are currently the 11th and the 16th most common cause of death globally. In Latin America, cirrhosis is the 9th cause of death, representing 2.7% of the total deaths. Cirrhosis has an incidence of 15.6 per 100,000 inhabitants. It is within the top 20 causes of disability-adjusted life years and years of life lost, accounting for 1.6% and 2.1% of the worldwide burden. Excessive alcohol consumption is the most frequent cause of cirrhosis in the world; other causes are viruses, medication intake, and autoimmunity. Recently, the global epidemic of obesity and diabetes mellitus which affects 2 billion and 400 million subjects worldwide respectively, have significantly increased the incidence of non-alcoholic fatty liver disease (NAFLD) and related liver disease (cirrhosis and hepatocellular carcinoma) [1].
NAFLD is a metabolic disorder characterized by macro vesicular steatosis in more than 5% of hepatocytes in individuals without significant alcohol consumption, drug intake, or other known causes of steatosis [2]. It is closely associated with the metabolic syndrome (MS) which is a constellation of abnormalities, such as visceral obesity, insulin resistance, diabetes mellitus (DM), hypertriglyceridemia, and arterial hypertension. It is the hepatic manifestation of a disequilibrium in the metabolic status (MS, intestinal dysbiosis, adipokine changes, vitamin D deficiency, hypercortisolism) in which the central factor is insulin resistance [3]
NAFLD affects a third of the world's population and its incidence continues to increase. The risk of progression to cirrhosis in NAFLD seems to be low, with an incidence of 3.1% over a mean 7.6-year follow-up [4]. However, these figures can be underestimated, since in about 25–30% of patients, NAFLD is diagnosed in the inflammatory phase called non-alcoholic steatohepatitis (NASH), characterized by hepatocellular damage. Currently 30–40% of patients with NASH-cirrhosis succumb to a liver related death within 10 years and hospitalizations have increased 97% between 2000 and 2012 which have significantly increased the burden of this condition [5].
Advanced age, diabetes mellitus, and visceral obesity are the most important factors involved in the development of liver fibrosis in NAFLD. The stages of liver fibrosis are classified as F0 (without fibrosis), F4 (cirrhosis) and >F2 advanced or significant fibrosis [6]. Advanced fibrosis, (and not inflammation) is independently associated with a significant increase in the mortality rate from cardiovascular disease (CVD) and liver disease [7–9]. The progression rate from NASH to cirrhosis and hepatocellular carcinoma is from 5 to 18% when fibrosis is absent and of 40% when is present [10–12]. For this reason, the detection of advanced fibrosis is crucial for therapeutic decisions [13]. Previously, liver biopsy was the only available method to evaluate liver fibrosis. However, it is an invasive and expensive procedure, subjected to sampling errors and to severe complications. In recent years, several non-invasive methods, based on serological biomarkers and radiological methods have been developed [14].
The improvement of socioeconomic conditions, the migration to urban areas and the changes in habits and diet of the Mexican population in recent decades have resulted in a significant increase in the prevalence of obesity, CVD and DM [15,16]. According to the Ministry of Health of Mexico, the number one leading cause of mortality in 2008 was DM (14%), followed by CVD (11%) and stroke (5.6%) [17]. The International Diabetes Federation estimates that Mexico has the highest prevalence of DM of adults in OECD countries (15%) [18]. All the above discussed led us to speculate that screening for liver fibrosis in the general population of Mexico might detect naïf individuals affected by chronic liver disease in whom the application of therapeutic measures might reduce mortality, as well as the financial burden derived from cirrhosis and hepatocellular carcinoma.
2AimsIn a first step, we determined the prevalence of advanced liver fibrosis in Mexican general population; in subjects without excessive alcohol consumption or a history of known liver disease using an inexpensive, readily available and extensively validated non-invasive method.
3Patients and methods3.1Design and patientsThe subjects included in this research were randomly selected from a big sample of 20,919 individuals (of all ages and both sexes) of the general population who previously participated in the Health and Nutrition Survey applied by the Ministry of Health of the Government of the State of Nuevo León, in 2015. The survey was applied to gather information on the socio-demographic profile, health, and dietary and nutritional status of the population.
In an observational cross sectional and prospective survey, clinical and laboratory data were collected to select individuals. Laboratory evaluation included routine liver tests (AST and ALT, total bilirubin, total proteins, albumin, alkaline phosphatase, and γ-glutamyl transpeptidase (GGT)), and complete blood count, fasting serum glucose, total cholesterol, HDL cholesterol, and serum triglyceride levels. Additionally, (systolic and diastolic) blood pressure, body weight, height and waist circumference were measured. The socioeconomic level of individuals was also classified according to socioeconomic parameters established by the Instituto Nacional de Estadistica y Geografia (INEGI), an institution in charge of performing national population and economic censuses. These parameters were: housing characteristics, family and individual economic income, level of schooling, working conditions, etc.
The body mass index (BMI) was calculated using the formula: body weight (in kg)/height (in m2). Waist circumference was measured in the midway between the lowest rib edge and the iliac crest. Visceral obesity was defined as a waist circumference >102cm in men and >88cm in women. The presence of DM was established by fasting serum glucose >126mg/dL or treatment with antidiabetic drugs. Overweight and obesity were defined by BMI values from 25 to 29.9kg/m2 and >30kg/m2 respectively.
For the final analysis, the subjects under 20 years old, those with a history of known liver disease and those who recorded alcohol consumption greater than 30g/day in men and 20g/day in women for a period of more than 5 years were excluded.
The NAFLD fibrosis score (NFS) was calculated based on the previously published formula that can be found on http://nafldscore.com/ −1.675+0.037×age (years)+0.094×BMI (kg/m2)+1.13×impaired fasting glycemia or diabetes (yes=1, no=0)+0.99×AST/ALT ratio−0.013×platelet (×109/L)−0.66×albuming/dL [19].
Two cut-off points were used to categorize the subjects into three groups:
- (a)
Low probability of advanced fibrosis: value <−1.455
- (b)
High probability of advanced fibrosis: value >0.676
- (c)
Indeterminate probability: value from −1.455 to 0.676
The Ethics Committee of the Faculty of Medicine of the Autonomous University of Nuevo León approved this study, and all individuals gave their consent for participation into the study.
3.2Statistical analysisCategorical variables were expressed as medians and ranges and were statistically analyzed using Chi2 tests. Continuous variables were expressed as means and standard deviations and were analyzed by Student's t-test, Mann–Whitney U-test, ANOVA, and Kruskal–Wallis test. Two-sided values of alpha <0.05 were considered statistically significant. Statistical software (Version 25.0, SPSS, and Chicago, IL) was used for the statistical analysis.
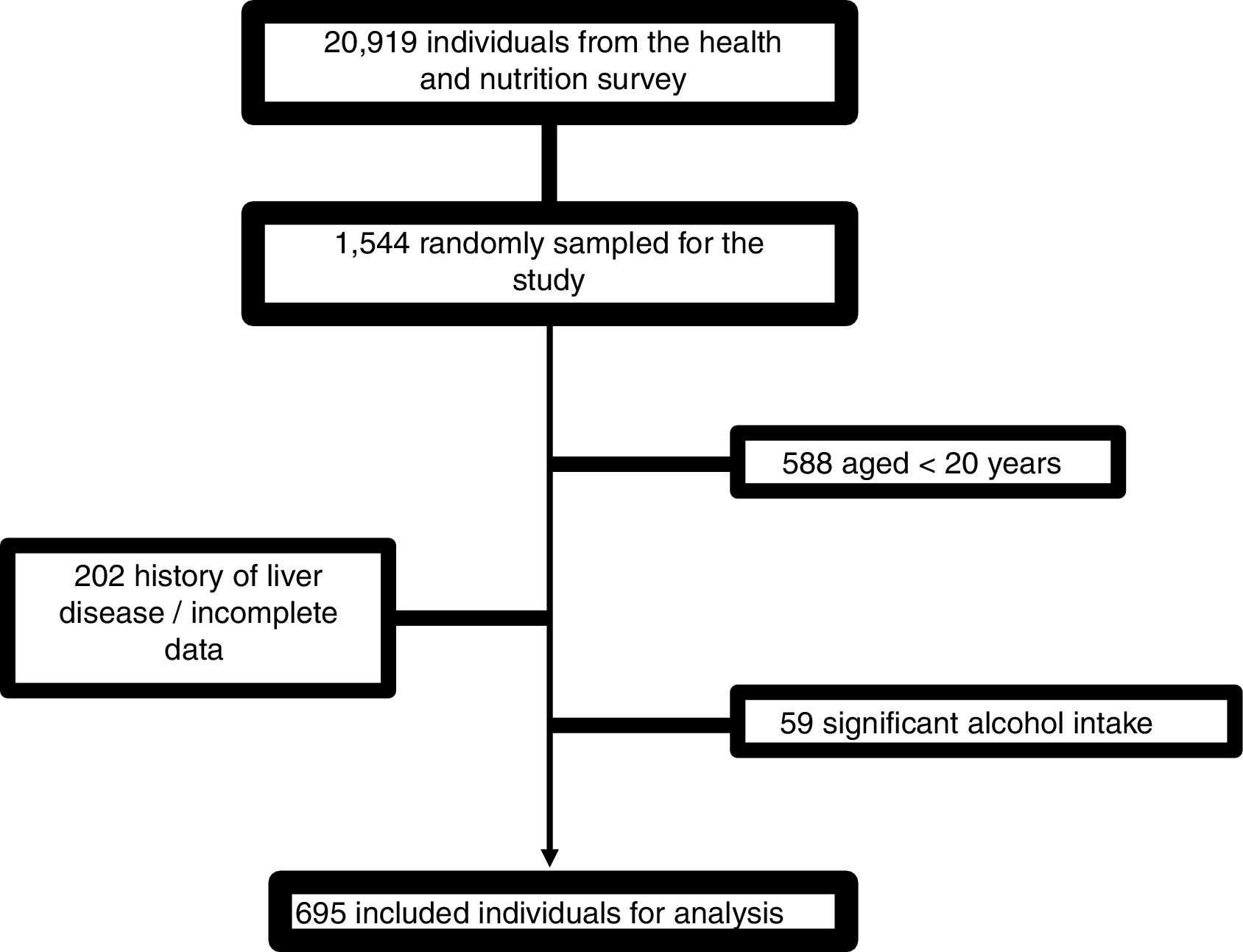
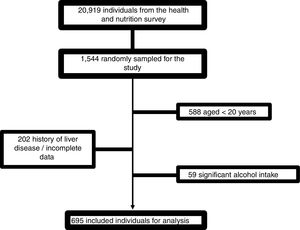
4Results4.1Patient characteristicsFrom 1544 subjects evaluated, 695 were finally included. The causes of elimination were age <20 years: 588; incomplete data or history of liver disease: 202; and excessive alcohol consumption: 59 (Fig. 1).
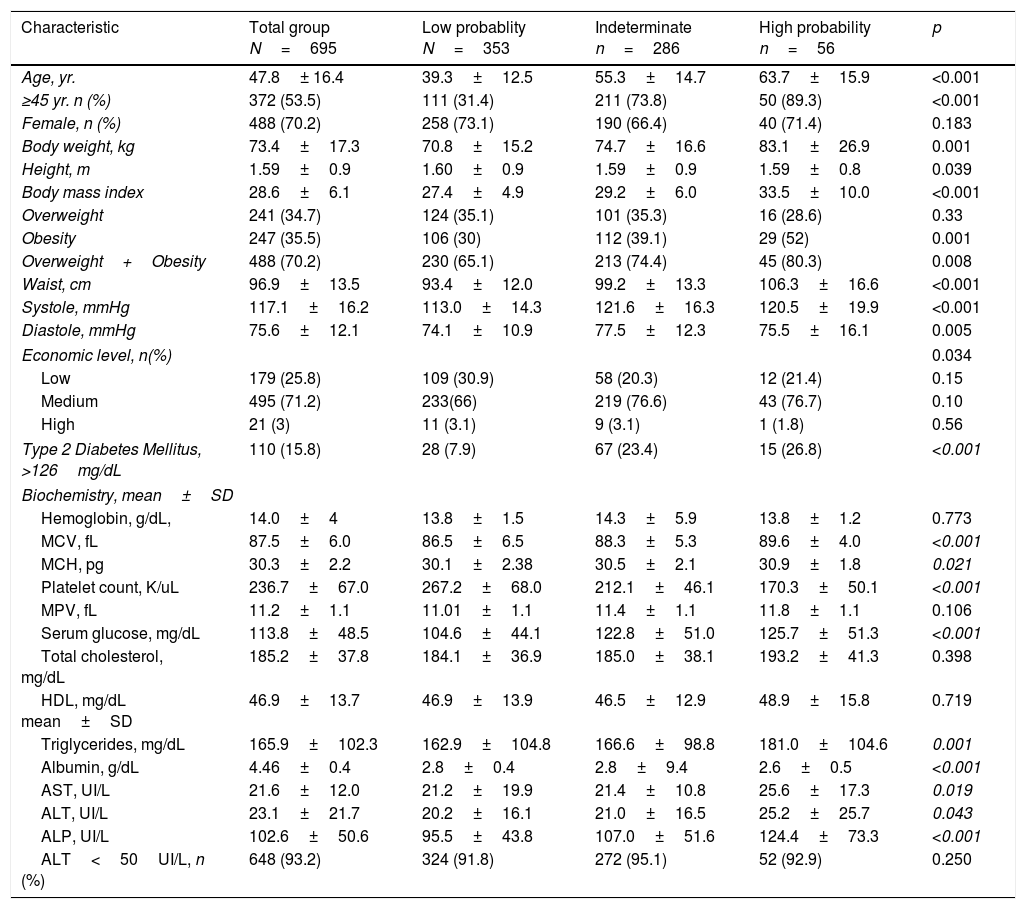
The demographic, clinical and laboratory characteristics of the total number of subjects are shown in Table 1. The average age of the population was 47.8±16.4 years. The majority of subjects were between 20 and 50 years old (59%). Likewise, 38% of the subjects were aged between 20 and 40 years. The majority were female (70.2%); 35.5% had obesity; 70.2% of the cases showed overweight or obesity and the average waist circumference was 97±13.5cm. Similarly, 71.2% of the subjects belonged to the medium socioeconomic strata. Finally, 15.8% of the subjects had DM and 93.2% showed normal ALT blood levels.
Demographic, clinical and biochemical characteristics of 695 individuals included into the study and comparison intergroup according the NAFLD fibrosis score results.
| Characteristic | Total group N=695 | Low probablity N=353 | Indeterminate n=286 | High probability n=56 | p |
|---|---|---|---|---|---|
| Age, yr. | 47.8± 16.4 | 39.3±12.5 | 55.3±14.7 | 63.7±15.9 | <0.001 |
| ≥45 yr. n (%) | 372 (53.5) | 111 (31.4) | 211 (73.8) | 50 (89.3) | <0.001 |
| Female, n (%) | 488 (70.2) | 258 (73.1) | 190 (66.4) | 40 (71.4) | 0.183 |
| Body weight, kg | 73.4±17.3 | 70.8±15.2 | 74.7±16.6 | 83.1±26.9 | 0.001 |
| Height, m | 1.59±0.9 | 1.60±0.9 | 1.59±0.9 | 1.59±0.8 | 0.039 |
| Body mass index | 28.6±6.1 | 27.4±4.9 | 29.2±6.0 | 33.5±10.0 | <0.001 |
| Overweight | 241 (34.7) | 124 (35.1) | 101 (35.3) | 16 (28.6) | 0.33 |
| Obesity | 247 (35.5) | 106 (30) | 112 (39.1) | 29 (52) | 0.001 |
| Overweight+Obesity | 488 (70.2) | 230 (65.1) | 213 (74.4) | 45 (80.3) | 0.008 |
| Waist, cm | 96.9±13.5 | 93.4±12.0 | 99.2±13.3 | 106.3±16.6 | <0.001 |
| Systole, mmHg | 117.1±16.2 | 113.0±14.3 | 121.6±16.3 | 120.5±19.9 | <0.001 |
| Diastole, mmHg | 75.6±12.1 | 74.1±10.9 | 77.5±12.3 | 75.5±16.1 | 0.005 |
| Economic level, n(%) | 0.034 | ||||
| Low | 179 (25.8) | 109 (30.9) | 58 (20.3) | 12 (21.4) | 0.15 |
| Medium | 495 (71.2) | 233(66) | 219 (76.6) | 43 (76.7) | 0.10 |
| High | 21 (3) | 11 (3.1) | 9 (3.1) | 1 (1.8) | 0.56 |
| Type 2 Diabetes Mellitus, >126mg/dL | 110 (15.8) | 28 (7.9) | 67 (23.4) | 15 (26.8) | <0.001 |
| Biochemistry, mean±SD | |||||
| Hemoglobin, g/dL, | 14.0±4 | 13.8±1.5 | 14.3±5.9 | 13.8±1.2 | 0.773 |
| MCV, fL | 87.5±6.0 | 86.5±6.5 | 88.3±5.3 | 89.6±4.0 | <0.001 |
| MCH, pg | 30.3±2.2 | 30.1±2.38 | 30.5±2.1 | 30.9±1.8 | 0.021 |
| Platelet count, K/uL | 236.7±67.0 | 267.2±68.0 | 212.1±46.1 | 170.3±50.1 | <0.001 |
| MPV, fL | 11.2±1.1 | 11.01±1.1 | 11.4±1.1 | 11.8±1.1 | 0.106 |
| Serum glucose, mg/dL | 113.8±48.5 | 104.6±44.1 | 122.8±51.0 | 125.7±51.3 | <0.001 |
| Total cholesterol, mg/dL | 185.2±37.8 | 184.1±36.9 | 185.0±38.1 | 193.2±41.3 | 0.398 |
| HDL, mg/dL mean±SD | 46.9±13.7 | 46.9±13.9 | 46.5±12.9 | 48.9±15.8 | 0.719 |
| Triglycerides, mg/dL | 165.9±102.3 | 162.9±104.8 | 166.6±98.8 | 181.0±104.6 | 0.001 |
| Albumin, g/dL | 4.46±0.4 | 2.8±0.4 | 2.8±9.4 | 2.6±0.5 | <0.001 |
| AST, UI/L | 21.6±12.0 | 21.2±19.9 | 21.4±10.8 | 25.6±17.3 | 0.019 |
| ALT, UI/L | 23.1±21.7 | 20.2±16.1 | 21.0±16.5 | 25.2±25.7 | 0.043 |
| ALP, UI/L | 102.6±50.6 | 95.5±43.8 | 107.0±51.6 | 124.4±73.3 | <0.001 |
| ALT<50UI/L, n (%) | 648 (93.2) | 324 (91.8) | 272 (95.1) | 52 (92.9) | 0.250 |
SD: standard deviation; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MPV: mean platelet volume; HDL: high-density lipoprotein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase.
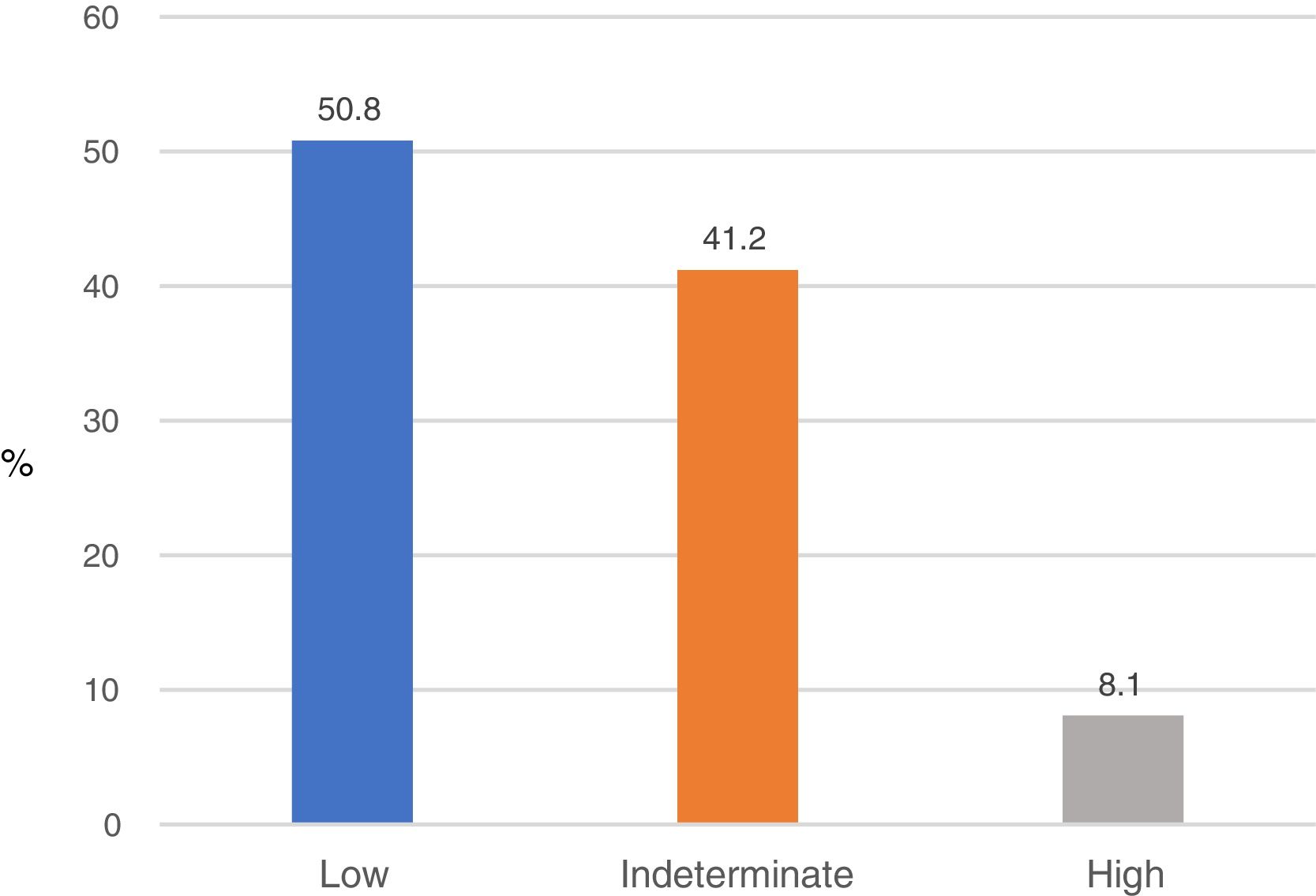
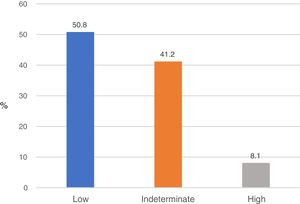
Based on the results of the NFS, 353 subjects (50.8%) had a low probability of fibrosis (NFS<−1.544); 56 (8.1%) had a high probability of fibrosis (NFS>0.676), and 286 (41.2%) showed an indeterminate probability of fibrosis (NFS from −1.544 to 0.676) (Fig. 2).
4.3Differences among groupsDemographic, clinical and laboratory characteristics of the groups showing low, high and indeterminate probability of fibrosis are shown in Table 1. The subjects in the high-risk group were significantly older than those of the other groups. In this high-risk group, 89.3% were aged >45 years, 52% had any grade of obesity, and 80.3% were overweighed or obese and showed the highest values of waist circumference and blood pressure. The medium socioeconomic level was predominantly in the high-risk group. The prevalence of DM in the high-risk group was the highest compared to that observed in the indeterminate and low-risk groups: 27%, 23%, and 8% respectively.
Finally, high-risk subjects showed significantly lower values in blood platelet count, albumin levels, and higher values in total triglyceride, AST, ALT, and alkaline phosphatase blood levels as compared to subjects from the other groups. 92.9% of the patients from the high-risk group showed normal ALT serum levels.
5DiscussionAlthough liver cirrhosis is a major health problem worldwide because of its high mortality and heavy financial burden, it has not received the required attention by policy-makers, public opinion, nor physicians.
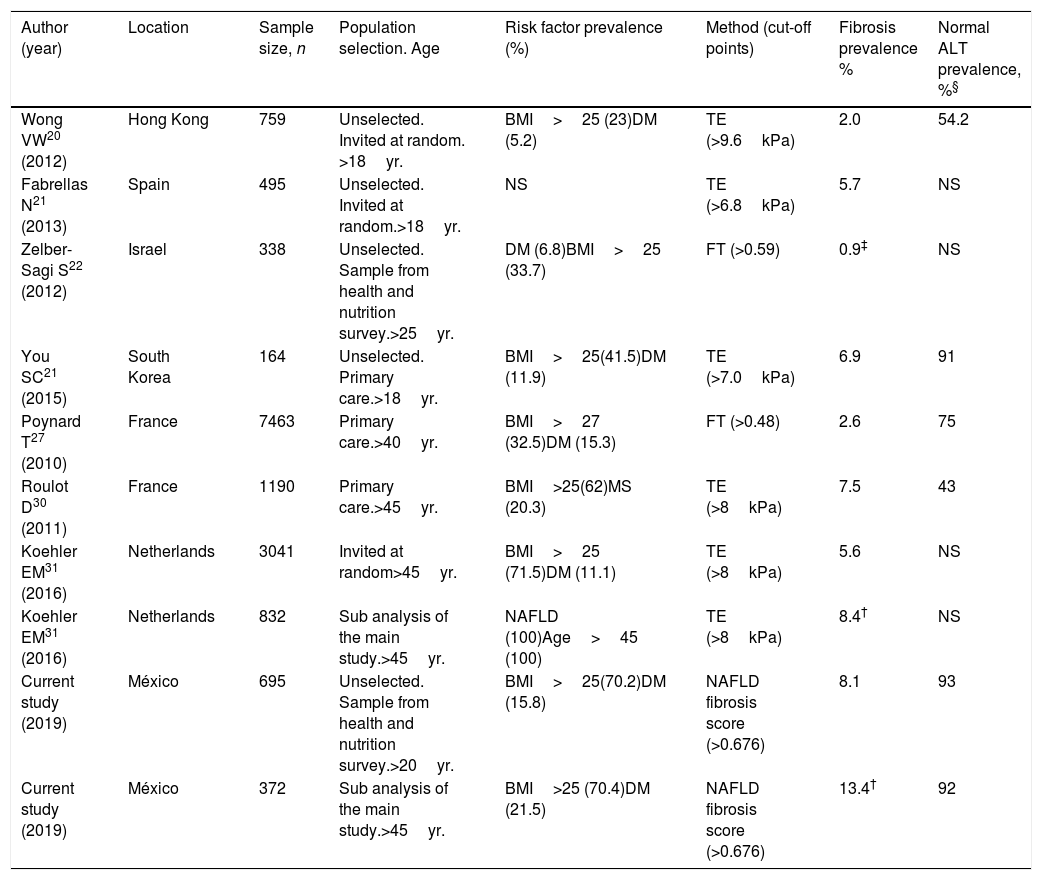
Until now, most published screening studies for liver fibrosis have been performed in targeted population based on the presence of risk factors of NASH (DM, metabolic syndrome, visceral obesity). Only few screening studies in unselected population have been published worldwide [20–23] (Table 2). Fibro Test (FT) and transition elastography (TE) were used as screening tests. FT is a biomarker that includes α2 macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, and GGT [24]. TE involves measuring liver stiffness as a reflection of fibrosis [25]. Both methods have been extensively validated with histological confirmation of fibrosis of any etiology [26–28]. The prevalence of liver fibrosis obtained in unselected population ranged from 0.9 to 6.9% [20–23]. The studies showing a higher prevalence of fibrosis included individuals with a higher prevalence of DM, visceral obesity, metabolic syndrome or used lower cut-off points of the screening method. In the study with the lowest prevalence (0.9%), a high cut-off point of FT was used (>0.59) [22].
Published studies screening for significant/advanced liver fibrosis (F>2) in general population.
| Author (year) | Location | Sample size, n | Population selection. Age | Risk factor prevalence (%) | Method (cut-off points) | Fibrosis prevalence % | Normal ALT prevalence, %§ |
|---|---|---|---|---|---|---|---|
| Wong VW20 (2012) | Hong Kong | 759 | Unselected. Invited at random. >18yr. | BMI>25 (23)DM (5.2) | TE (>9.6kPa) | 2.0 | 54.2 |
| Fabrellas N21 (2013) | Spain | 495 | Unselected. Invited at random.>18yr. | NS | TE (>6.8kPa) | 5.7 | NS |
| Zelber-Sagi S22 (2012) | Israel | 338 | Unselected. Sample from health and nutrition survey.>25yr. | DM (6.8)BMI>25 (33.7) | FT (>0.59) | 0.9‡ | NS |
| You SC21 (2015) | South Korea | 164 | Unselected. Primary care.>18yr. | BMI>25(41.5)DM (11.9) | TE (>7.0kPa) | 6.9 | 91 |
| Poynard T27 (2010) | France | 7463 | Primary care.>40yr. | BMI>27 (32.5)DM (15.3) | FT (>0.48) | 2.6 | 75 |
| Roulot D30 (2011) | France | 1190 | Primary care.>45yr. | BMI>25(62)MS (20.3) | TE (>8kPa) | 7.5 | 43 |
| Koehler EM31 (2016) | Netherlands | 3041 | Invited at random>45yr. | BMI>25 (71.5)DM (11.1) | TE (>8kPa) | 5.6 | NS |
| Koehler EM31 (2016) | Netherlands | 832 | Sub analysis of the main study.>45yr. | NAFLD (100)Age>45 (100) | TE (>8kPa) | 8.4† | NS |
| Current study (2019) | México | 695 | Unselected. Sample from health and nutrition survey.>20yr. | BMI>25(70.2)DM (15.8) | NAFLD fibrosis score (>0.676) | 8.1 | 93 |
| Current study (2019) | México | 372 | Sub analysis of the main study.>45yr. | BMI>25 (70.4)DM (21.5) | NAFLD fibrosis score (>0.676) | 13.4† | 92 |
BMI: body mass index, DM: diabetes mellitus, TE: transient elastography, FT: fibro test, NS: non-specified.
In other three studies that stratified members of the general population according to an age cutoff >40 and 45 years [29–31], the overall prevalence of fibrosis ranged from 2.6 to 7.5% (Table 2). The lowest estimate used a two-step approach with only half of the patients re-attending for the second test [29].
Finally, in a sub-analysis of one of these studies involving 832 patients with NAFLD (after excluding excessive alcohol consumption, viral infection and drug toxicity) the prevalence of fibrosis was higher than that observed in the total group (8.4 vs. 5.6%) (Table 2).
Our study is the first screening study to detect liver fibrosis in an unselected general population in Latin America. The epidemiological characteristics (high prevalence of obesity and DM) of the Mexican population made us to carry out this research. Besides, Mexican population have higher genetic susceptibility, than other ethnic groups, to develop NAFLD, since the PNPLA3 gene (which is closely related to the development of steatosis, NASH, and liver cirrhosis), has been found in 70% in Mexican population [32,33].
The prevalence of fibrosis obtained in our study (8.1%) was higher than that observed in other unselected population studies [20–23] probably due to the higher prevalence of DM and obesity of our population. This prevalence was also higher than that observed in the studies stratified according to an age >40 and 45 years [29,30] and similar to that observed in the sub-analysis of NAFLD individuals stratified by age >45 years [31]. Notwithstanding, our work included subjects aged between 20 and 75 years. In a sub-analysis of our study, with 372 individuals aged >45 years, the prevalence of fibrosis increased to 13.4% that was higher compared to all the above-reviewed screening studies (Table 2).
The FT (Fibro Test®) is a patented method and the TE (Fibroscan®) is a radiological procedure requiring specialized equipment and trained personnel, so they are expensive and do not have wide availability in many centers. Due to this, their use as screening tools for large populations may not be cost-effective.
In our study, we decided to use NAFLD fibrosis score due to its following characteristics:
- a.
It uses an algorithm that includes clinical and biochemical parameters that can be easily obtained in the doctors’ office at a very low cost. In Mexico, the approximate calculated cost per test of NFS is $ 34 dollars. In contrast, the costs of Fibrotest® and Fibroscan® are $ 260 and $ 215 dollars respectively.
- b.
It was designed for subjects with NAFLD [19].
- c.
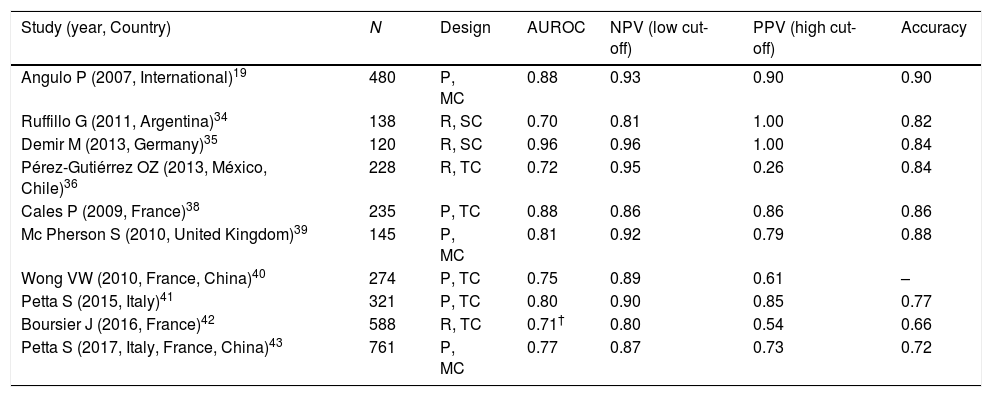
It has been extensively validated with liver biopsy showing AUROC values from 0.70 to 0.96, negative predictive values (NPV) from 81 to 96%, positive predictive values (PPV) of 90% and an accuracy from 66 to 90%. Therefore, it is considered as an excellent method to rule out fibrosis [19,34–43], so that the American Association for the Study of Liver Diseases (AASLD) recommends it as a useful tool for screening fibrosis in patients with NASH [44] (Table 3).
Table 3.Results of studies of the NAFLD fibrosis score for diagnosis of advanced liver fibrosis (F>2) determined by biopsy in patients with NAFLD.
Study (year, Country) N Design AUROC NPV (low cut-off) PPV (high cut-off) Accuracy Angulo P (2007, International)19 480 P, MC 0.88 0.93 0.90 0.90 Ruffillo G (2011, Argentina)34 138 R, SC 0.70 0.81 1.00 0.82 Demir M (2013, Germany)35 120 R, SC 0.96 0.96 1.00 0.84 Pérez-Gutiérrez OZ (2013, México, Chile)36 228 R, TC 0.72 0.95 0.26 0.84 Cales P (2009, France)38 235 P, TC 0.88 0.86 0.86 0.86 Mc Pherson S (2010, United Kingdom)39 145 P, MC 0.81 0.92 0.79 0.88 Wong VW (2010, France, China)40 274 P, TC 0.75 0.89 0.61 – Petta S (2015, Italy)41 321 P, TC 0.80 0.90 0.85 0.77 Boursier J (2016, France)42 588 R, TC 0.71† 0.80 0.54 0.66 Petta S (2017, Italy, France, China)43 761 P, MC 0.77 0.87 0.73 0.72 - d.
It has a diagnostic performance mildly lower than the TE, which is currently the best noninvasive method for the diagnosis of fibrosis [45]. Besides, most published studies have reported that NFS has higher performance than other non-invasive biomarkers, such as APRI, FIB-4, BARD, AST/ALT ratio [46–49].
- e.
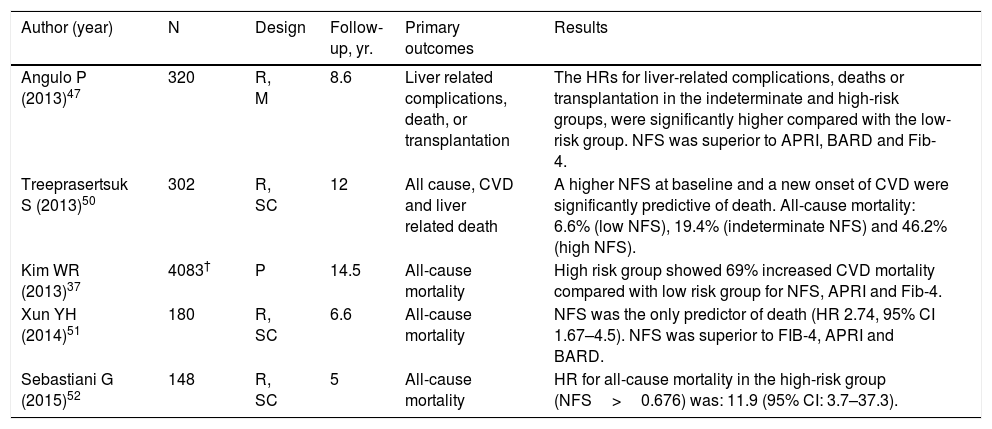
It has outcome-predictive capacity in NAFLD patients. In studies with a follow-up from 5 to 14.5 years, patients with a high and indeterminate probability of fibrosis had significantly higher rate of death from any cause, due to CVD and liver disease compared to patients at low risk [37,47,50–52] (Table 4).
Table 4.Results of studies of NAFLD fibrosis score for prediction of morbidity and mortality rates among patients with NAFLD.
Author (year) N Design Follow-up, yr. Primary outcomes Results Angulo P (2013)47 320 R, M 8.6 Liver related complications, death, or transplantation The HRs for liver-related complications, deaths or transplantation in the indeterminate and high-risk groups, were significantly higher compared with the low-risk group. NFS was superior to APRI, BARD and Fib-4. Treeprasertsuk S (2013)50 302 R, SC 12 All cause, CVD and liver related death A higher NFS at baseline and a new onset of CVD were significantly predictive of death. All-cause mortality: 6.6% (low NFS), 19.4% (indeterminate NFS) and 46.2% (high NFS). Kim WR (2013)37 4083† P 14.5 All-cause mortality High risk group showed 69% increased CVD mortality compared with low risk group for NFS, APRI and Fib-4. Xun YH (2014)51 180 R, SC 6.6 All-cause mortality NFS was the only predictor of death (HR 2.74, 95% CI 1.67–4.5). NFS was superior to FIB-4, APRI and BARD. Sebastiani G (2015)52 148 R, SC 5 All-cause mortality HR for all-cause mortality in the high-risk group (NFS>0.676) was: 11.9 (95% CI: 3.7–37.3). P: prospective; R: retrospective; SC: single center; HR: hazard ratio; CI: confidence interval; CVD: cardiovascular disease.
The individuals detected here as having high probability of fibrosis, represent a relevant proportion of the general population which may be at risk of suffering a chronic liver disease, and with a well-documented probability of having complications and death from CVD, cirrhosis and hepatocellular carcinoma. It is important to highlight that 93% of these individuals had normal ALT blood levels. A similar prevalence of normal liver enzymes in high-risk subjects (43 to 91%) has been reported in other screening studies [20,23,29,30]. Considering that Mexico's population is 120 million inhabitants, of which 63.6% are over 20 years old (76, 320,000 inhabitants) [53], extrapolating the results of this study, it can be estimated that around 6,181,920 subjects (8.1%) may be at risk.
Our study has some limitations: (a) No reinvestigations in order to confirm advanced fibrosis were performed; (b) No follow-up to determine morbidity and mortality rates, in high-risk subjects was done; and (c) We did not take into account subjects with an indeterminate probability of fibrosis. The proportion of fibrosis in this group is highly variable (from 35 to 60%) [19,34–36]. However, these limitations were the result of the nature of our research conducted in the open population.
The results of this and other similar studies may support the need to perform screening for liver fibrosis in general populations as part of public health policies [54,55], particularly in those communities with a high risk of liver disease. Screening should be done ideally with a non-invasive and low-cost test, with adequate reliability for fibrosis detection, easy to apply and widely available. Whether or not one of the currently available tests may be able to gather these properties is unknown.
The justification for screening for liver fibrosis in general populations is because subjects with early stages of fibrosis are completely asymptomatic, most of them have normal transaminase blood levels and the fibrosis progression is quietly slow (7.7 years for each stage of fibrosis in NASH) [56]. In most cases, liver fibrosis manifests clinically in advanced stage, as decompensated cirrhosis, particularly in diseases in which serological markers for early detection are lacking (such as NAFLD). The detection of patients in the pre-cirrhotic phase of the process, would allow the application of preventive or corrective measures to reduce the progression of the disease and consequently, the mortality rate and the financial burden derived from the care of complications. Although the FDA has not approved any drug for the treatment of NASH, the substantial loss of body weight by bariatric surgery or lifestyle changes, reduces liver fibrosis and inflammation in patients with NASH and possibly improves the prognosis [57–59]. Besides, new drugs that promise to have important therapeutic effects (Obeticholic acid, selonsertif, elafibranor, cenicriviroc) are under intensive evaluation currently [60,61].
6ConclusionsAccording to the results of our study in the adult Mexican general population, the prevalence of advanced liver fibrosis is 8.1%. In the future, studies that confirm or rule out fibrosis through complementary studies, and that include long-term follow up with the application of preventive and corrective measures and the analysis of the cost-benefit relationship will be decisive to determine the validity of systematic screening for liver fibrosis in general populations.
EthicsThe study was approved by the Ethics Committee of the Faculty of Medicine of the Autonomous University of Nuevo León. Informed consent and assent were obtained from participants in the survey.
FundingThis study was funded by the Ministry of Health of the Government of the State of Nuevo León, Mexico and the Universidad Autonoma de Nuevo Leon, Mexico.
Conflict of interestThe authors have no conflicts of interest to declare.