Polycystic ovary syndrome (PCOS) is the most common endocrinology disorder in women of reproductive age; these patients have a higher risk of suffering from non-alcoholic fatty liver disease (NAFLD). We determine the frequency of NAFLD in Mexican patients with PCOS and matched-controls.
Patients and methodsCross-sectional study, with 98 women of 18–44 years old. Rotterdam 2003 criteria integrated PCOS diagnosis. Those with significant alcohol consumption, chronic liver disease, use of steatogenic drugs, and pharmacological PCOS treatment or fertility protocol were excluded. Controls were matched in a 1:1 ratio by age and body mass index (BMI). The presence of NAFLD was determined by transient elastography performed by a single experienced operator.
ResultsA total of 98 female volunteers at reproductive age were recruited. NAFLD denoted markedly higher in patients with than without PCOS at 69.3% vs. 34.6%, respectively. Compared to controls, PCOS patients had a significantly higher risk of NAFLD (OR=4.26, 95% CI 1.83–9.93). Severe steatosis was the most frequent NAFLD stage between women with PCOS and NAFLD. Patients with hyperandrogenism have a significantly higher mean CAP 277.83dB/m than controls without hyperandrogenism 191.57dB/m. NAFLD prevalence was 84.3% in PCOS patients with phenotype A, while in another phenotype, it was 41.1%.
ConclusionsPCOS is an independent risk factor for NAFLD development. NAFLD screening needs to be considered in all PCOS patients independently of BMI, except in PCOS patients without hyperandrogenism and BMI<25.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age [1], with a prevalence between 8 and 13% according to the population studied and the definitions used. In Mexican, the prevalence is 6% [2] and the etiology remains unclear; nevertheless, it is strongly associated with obesity, metabolic syndrome, insulin resistance (IR) with compensatory hyperinsulinemia, type 2 diabetes mellitus, endometrial carcinoma and possibly cardiovascular disease [3,4]. PCOS comprises a broad spectrum of clinical presentations and some chronic inflammation degree [1,5]; central obesity and IR frequently occur in PCOS and seem to play a notable role in its pathogenesis [6].
PCOS diagnosis can be made using the 1990 definition of the United States National Institute of Health, the criteria of the Androgen Excess Society or the 2003 Rotterdam criteria [2]. To diagnose PCOS with Rotterdam criteria, the presence of at least two of the following three parameters is required: (a) oligo-anovulation, (b) clinical or biochemical hyperandrogenism (HA), and (c) polycystic ovarian morphology (PCOM) [7]. Furthermore, there are different PCOS phenotypes, the first one refers to “A” or complete phenotype, which comprises HA, oligo-anovulation, and PCOM, “B” phenotype includes HA, oligo-anovulation, without PCOM, the phenotype “C” called ovulatory because it includes HA and PCOM without oligo-anovulation, and finally the “D” phenotype called non-hyperandrogenic as it only presents oligo-anovulation and PCOM [8].
PCOS has been identified as a significant risk factor for non-alcoholic fatty liver disease (NAFLD) development [3,9], that is defined as fat accumulation in at least 5% of the hepatic parenchyma, which turns out to be the most frequent chronic liver disease with a global prevalence of 25% [10,11]. In a variable way, it presents an inflammatory response that damages the parenchyma, marking the progression of the disease to non-alcoholic steatohepatitis, which conditions the development of fibrosis that eventually progresses to cirrhosis or directly to hepatocellular carcinoma. Clinically, there is a high burden of metabolic comorbidities associated with NAFLD, where obesity is present in 51% of these people and 82% of patients have NASH; in addition, more than 90% of patients with severe obesity who undergo bariatric surgery have NAFLD and 76% of type 2 diabetics also have it [10,12]. IR and type 2 diabetes mellitus are among the most important predictors of progression to fibrosis and cirrhosis [10,13], and IR is detected in 70–80% of NAFLD cases [14]. On the other hand, death related to the heart is one of the main death causes for patients with NAFLD, also many patients had hypertension, hypertriglyceridemia and dyslipidemia, which are all risk factors for progression to NASH, which creates implications for the clinical management of the disease. This is why NAFLD is increasingly recognized as the liver disease component of metabolic syndrome [10].
The NAFLD diagnosis is defined by the excessive liver fat accumulation demonstrated by imaging or histopathology, ruling out the most common alternative causes of liver steatosis, among them significant alcohol consumption, hepatitis C virus, steatogenic medication, parenteral nutrition, Wilson's disease, and severe malnutrition [13,15]. NAFLD diagnosis is made using different diagnostic methods, liver biopsy is the gold standard for the diagnosis and staging of the disease [5]; due to its high prevalence, non-invasive tests such as transient elastography (Fibroscan®) should be used as first-line tools to evaluate patients [16], which allows determining two important values, the controlled attenuation parameter (CAP) measured in decibels per meter (dB/m) and the liver stiffness measurement (LSM) reported in kilopascals (kPa) [17,18]. CAP values have been developed based on ultrasonic signals properties and can detect and quantify hepatic steatosis [17,19,20].
Both PCOS and NAFLD share crucial characteristics of the metabolic syndrome that include visceral obesity, hypertension, dyslipidemia, and IR [9]. NAFLD pathophysiology is multifactorial; however, obesity and IR appear to be fundamental contributing factors [1]. PCOS prevalence studies show contradictory results; some suggest that there are different manifestations in selected populations, while others describe a similar prevalence in different ethnic groups. Such controversy might be due to the use of different diagnostic criteria and the variability in the manifestations of PCOS among different ethnic groups [21]. In general, ethnicity is a contributing factor to the presence of metabolic alterations in women who present PCOS [22], regardless, prevalence studies are lacking in Latin-American patients [9]. The objective of this study was to determine the frequency of NAFLD development and severity in Mexican patients with PCOS and matched-controls by age and body mass index (BMI).
2Material and methods2.1PatientsA cross-sectional study was conducted to determine the relevance of PCOS as a risk factor for NAFLD in Mexican women from the gynecology services of the Manuel Gea González and Medica Sur hospitals in Mexico City. Reproductive age patients between 18 and 44 years old who attended from November 1, 2018 to July 31, 2019, were included.
PCOS diagnosis was made according to the 2003 Rotterdam criteria, defined by the presence of at least 2 of the following 3 criteria: Oligo or anovulation, clinical HA, and PCOM defined by the presence of at least 12 follicles of 2–9mm in diameter or an ovarian volume greater than or equal to 10 cubic centimeters. We consider oligo-anovulation by the duration of the cycles of 35 days or more. Clinical HA was defined in the presence of hirsutism, acne, androgenic alopecia, or virilization, and was considered in those with a score>8 on the modified Ferriman-Gallwey scale, obtained in the initial evaluation.
Those patients who had established any of the following diagnoses were excluded from the study: hyperprolactinemia, pregnancy, dyslipidemia, thyroid or adrenal function alterations, diabetes mellitus, adrenal hyperplasia, Cushing syndrome, active or latent viral infection hepatitis C, hepatitis B virus, or human immunodeficiency. Similarly, women who were in pharmacological management at the time of the study or in the three months before the study, with hormonal contraceptives, anti-androgens, insulin receptor sensitizers, glucagon-like peptide analogs, and clomiphene citrate or infertility treatment protocol. Finally, patients with known chronic liver disease or significant alcohol consumption defined as>7 drinks per week were not included in the statistical analysis.
The study protocol conforms to the ethical guidelines of the 1975 declaration of Helsinki (6th revision, 2008), as reflected in a priori approval by the institution's human research committee. Each patient included in the study signed the informed consent.
2.2Clinic evaluationAnthropometric data, such as body weight and height, were collected before transient elastography evaluation, while the patient wore a light gown, without shoes. The BMI was calculated with the weight in kilograms (kg) and height in meters (m), using the formula: BMI=kg/m2. At the same time, the PCOS phenotype presented by the patient was identified. The patient with HA, oligo-anovulation, and ultrasound polycystic ovary morphology was defined as phenotype A.
2.3Determination of the controlled attenuation parameterTransient elastography was performed with the Fibroscan® Touch 502 model after a minimum of 4h fasting, by a single experienced operator initially using the M probe. The patient was placed supine with the right arm adducted and the hand under the head; the rest of the limbs extended. Next, an imaginary line was drawn between the xiphoid apophysis and the mid-clavicular line, on which an optimal intercostal space was sought, and the transducer was placed. When the device indicated it, the XL probe was used and the measurements were made again. The study was completed when the following characteristics were met: at least ten valid measurements, 60% success (valid measurements/invalid measurements), and the interquartile/median range was 30%. The median CAP in decibels per meter dB/m, the median liver stiffness in kPa, and the interquartile range were obtained.
Steatosis degree according to the CAP was determined, in agreement to the Shen et al. [23] cuts where S0: <232dB/m, S1: 232–256dB/m, S2: 257–290dB/m, S3: ≥290dB/m, and hepatic fibrosis degree was determined according to LSM, where F2 ≥7.0kPa, F3: ≥8.7kPa, F4: ≥10.3kPa [23].
2.4Statistical analysisVariable distributions were analyzed for normality using the Kolmogorov–Smirnov test. Continuous variables were reported as mean values and standard deviations. Categorical variables were presented as frequency and percentage. Comparisons between study groups were made with Student-t. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to reflect the effects of PCOS on NAFLD. Data analysis was performed using SPSS statistics desktop version 25.0 media pack software (IBM, Armonk, NY, USA). To detect the potential risk factor, a multivariate logistic regression analysis was conducted with NAFLD as the dependent variable and was categorized by BMI<25 and BMI≥25.
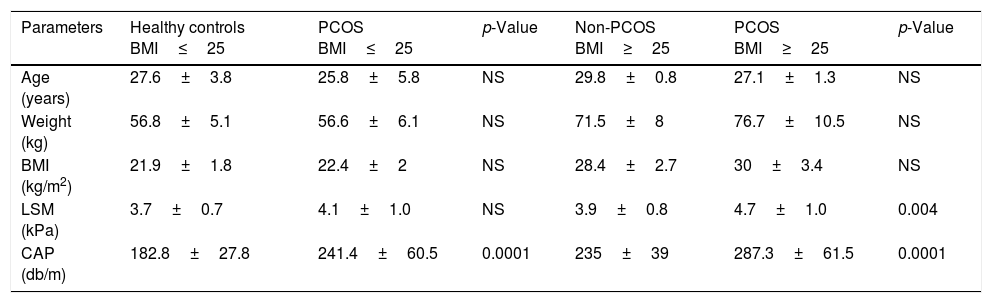
3Results3.1Patient featuresA total of 98 female volunteers at reproductive age (49 patients with PCOS, and 49 healthy women) were recruited. The group of patients with PCOS and healthy controls were matched by age and BMI, divided according to a BMI higher or lower than 25. PCOS diagnosis was defined by the presence of Rotterdam guideline, which includes at least two of the following criteria, clinical or biochemical HA, oligo-ovulation, and PCOM. The mean age in the healthy controls BMI<25 was 27.6 years, in the PCOS BMI<25 was 25.8 years, in the controls without PCOS BMI≥25 was 29.8 years, and the PCOS BMI≥25 was 27.1 years without difference; while the mean BMI in the healthy-controls BMI<25 was 21.9kg/m2, in the PCOS-BMI<25 was 22.4kg/m2, in the controls without PCOS-BMI≥25 was 28.4kg/m2, and the PCOS-BMI≥25 was 30.3kg/m2, the mean BMI was different between women with and without PCOS (Table 1).
Clinical characteristics of polycystic ovary syndrome and healthy women with body mass index ≥25 and ≤25.
| Parameters | Healthy controls BMI≤25 | PCOS BMI≤25 | p-Value | Non-PCOS BMI≥25 | PCOS BMI≥25 | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 27.6±3.8 | 25.8±5.8 | NS | 29.8±0.8 | 27.1±1.3 | NS |
| Weight (kg) | 56.8±5.1 | 56.6±6.1 | NS | 71.5±8 | 76.7±10.5 | NS |
| BMI (kg/m2) | 21.9±1.8 | 22.4±2 | NS | 28.4±2.7 | 30±3.4 | NS |
| LSM (kPa) | 3.7±0.7 | 4.1±1.0 | NS | 3.9±0.8 | 4.7±1.0 | 0.004 |
| CAP (db/m) | 182.8±27.8 | 241.4±60.5 | 0.0001 | 235±39 | 287.3±61.5 | 0.0001 |
Body mass index (BMI), polycystic ovary syndrome (PCOS), controlled attenuation parameter (CAP), liver stiffness measurement (LSM), No significance (NS).
Clinical HA was present in 86.9% of the PCOS-BMI<25 women and 84.6% of the PCOS-BMI≥25. Patients with PCOS-BMI<25 had oligo-anovulation in 91.3%, and all of the PCOS-BMI≥25 presented this characteristic. PCOM was present in 82.6% and 88.5% of the PCOS patients with BMI<25 and ≥25, respectively. Classic phenotype A was the most frequent presentation of PCOS in both groups, with a frequency of 60.8% in BMI<25 and 69.2% in BMI≥25 (Supp. Table 1).
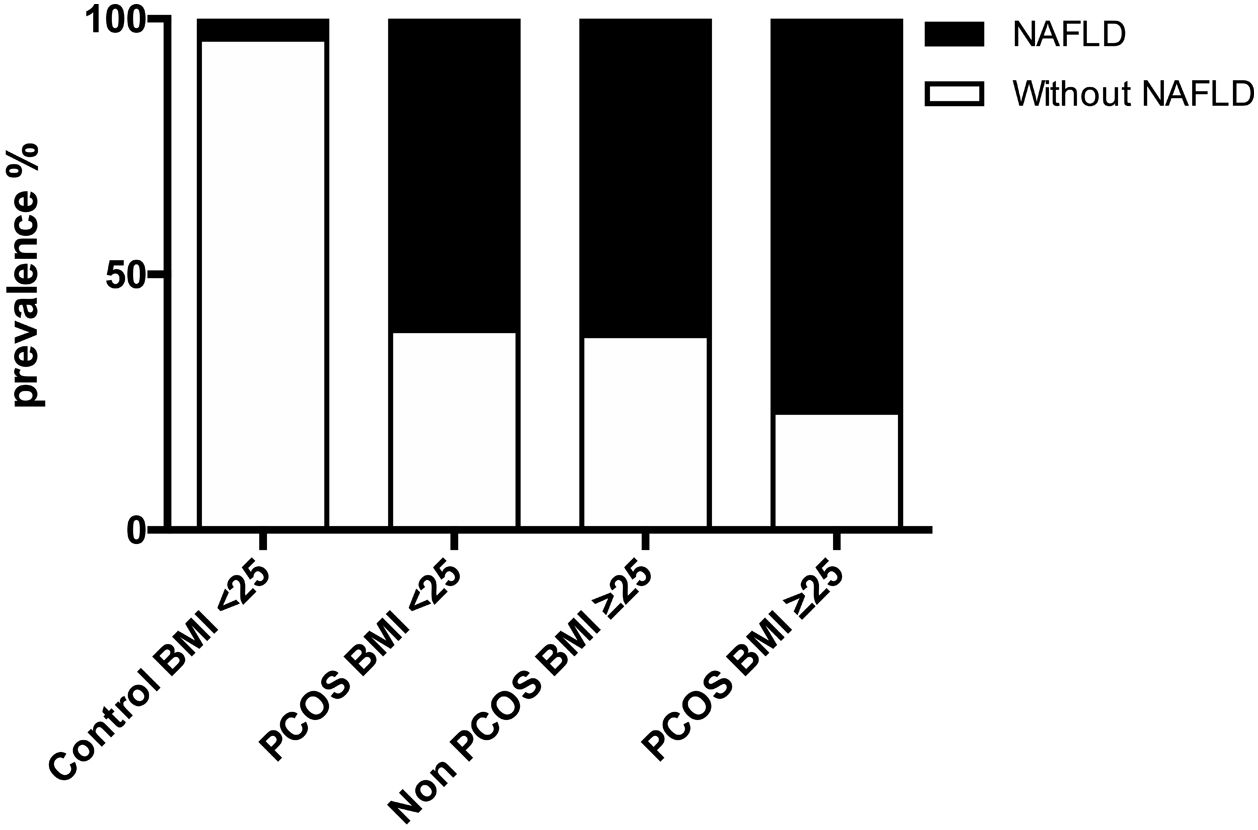
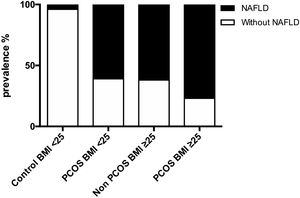
3.2NAFLD prevalence in women with PCOS and controlsNAFLD was present in the majority of women with PCOS, which was markedly higher than in women without PCOS at 69.3% (95% CI 56.48–82.29%) vs. 34.6%, respectively, p<0.001. The prevalence of NAFLD was 76.9% for PCOS-BMI≥25, 61.5% for control-BMI≥25, and 60.9% for PCOS-BMI<25, while in control-BMI<25 were just 4.3%. Compared to controls, PCOS patients had a significantly higher risk of NAFLD (OR=4.26, 95% CI 1.83–9.93) (Fig. 1).
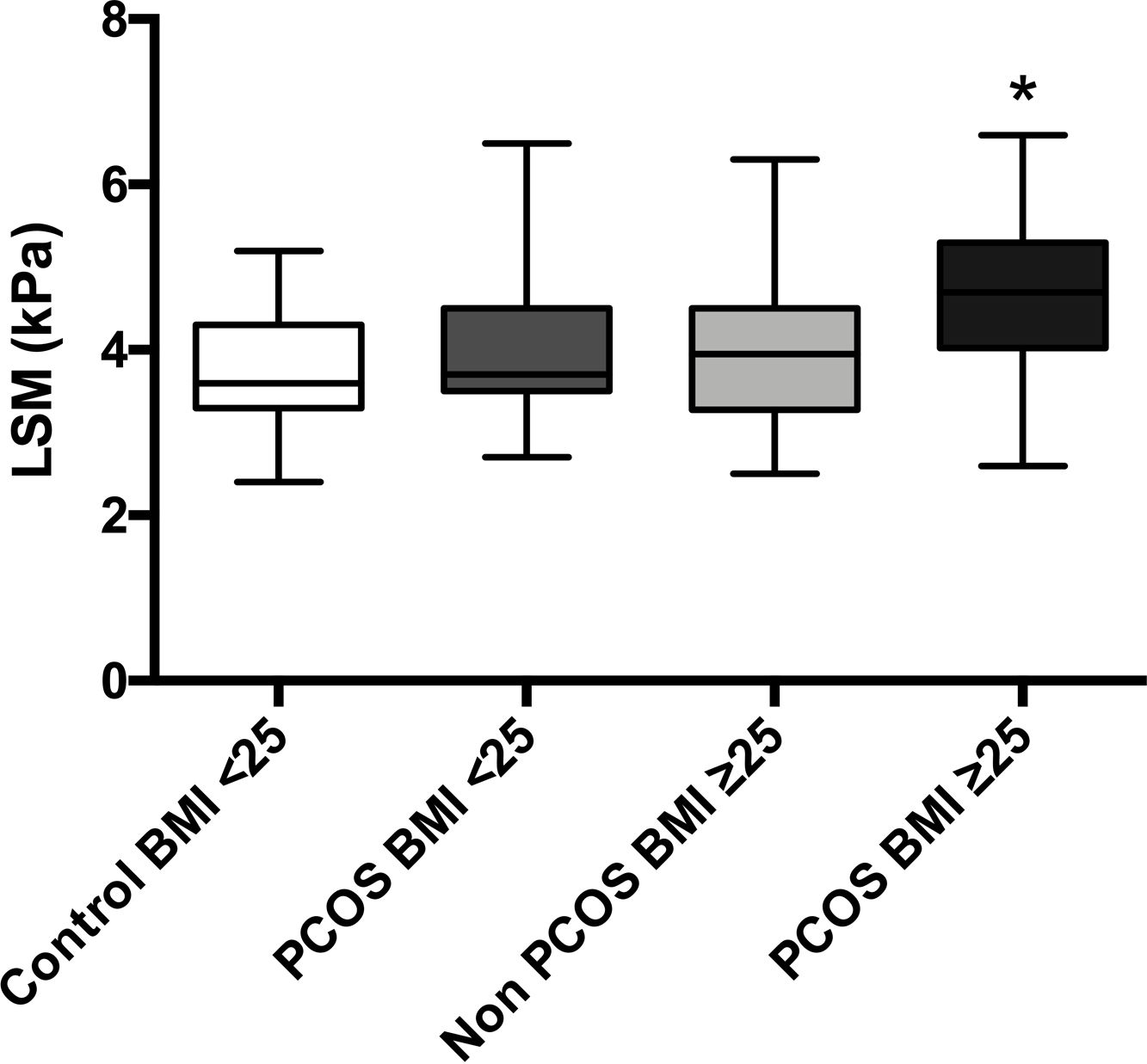
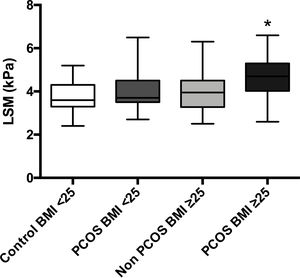
The mean LSM showed no differences between patients with BMI<25 with and without PCOS (4.1 vs. 3.7kPa, p=0.15); nevertheless, patients with PCOS-BMI≥25 showed slightly higher LSM 4.6kPa than controls 3.9kPa (p=0.01); even so, these values reflect no liver fibrosis for the four groups (Supp. Fig. 1).
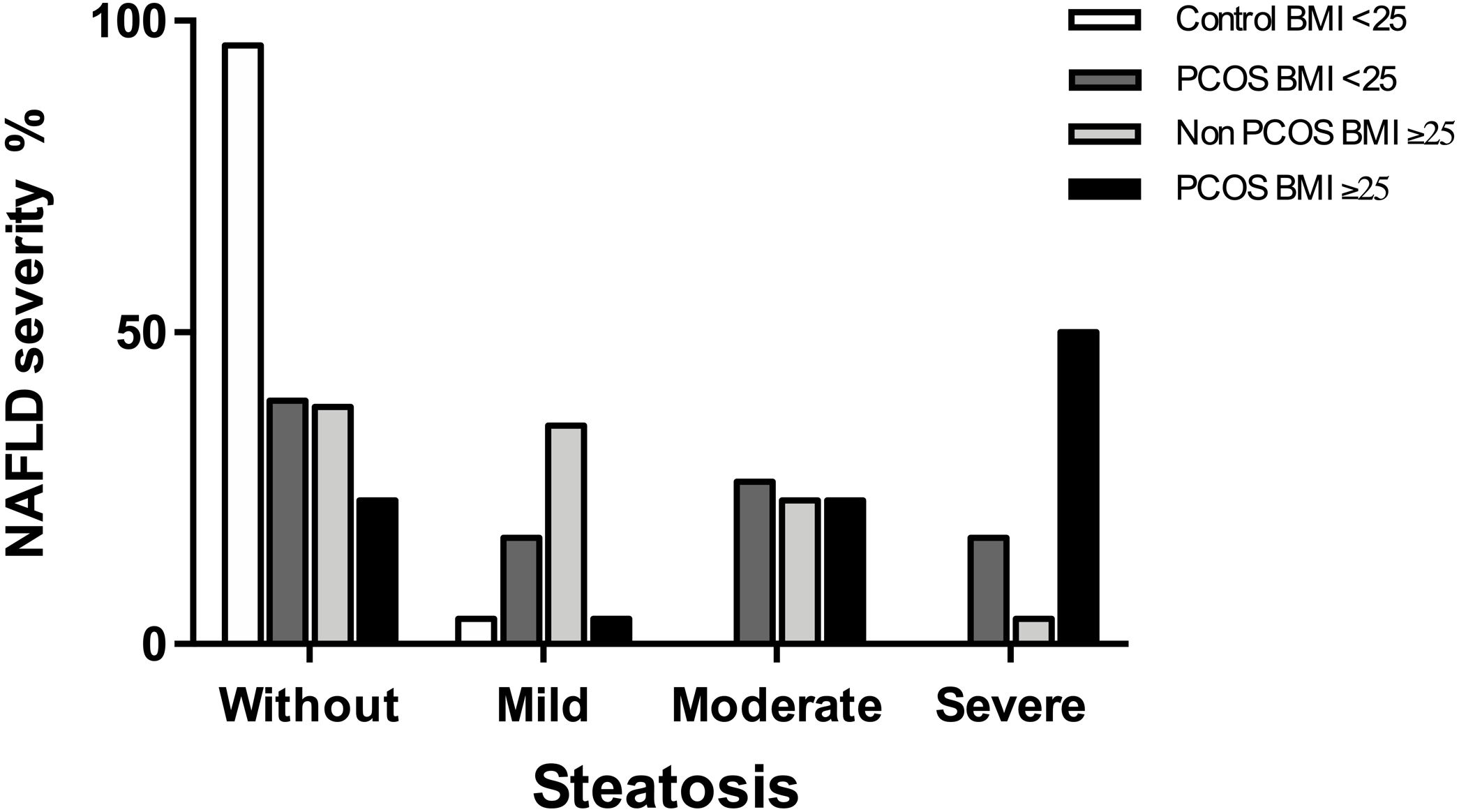
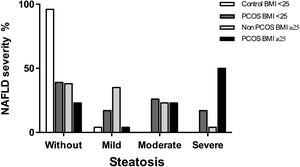
3.3NAFLD severity among women with PCOSThe most frequent stage of hepatic steatosis between women with PCOS and NAFLD was severe, observed in 50% of the patients. When analyzed the patient's subgroups, the frequency for each steatosis stage was as following: In controls-BMI<25 was 95.6% for patients without steatosis and 4.3% for mild steatosis while in PCOS-BMI<25 was 39.1% for women without steatosis, 17.4% for mild steatosis, 26.1% for moderate steatosis, and 17.4% for severe steatosis. On the other hand, the frequency of steatosis stages in controls-BMI≥25 were 38.5% for women without steatosis, 34.6% for mild steatosis, 23.1% for moderate steatosis and 3.8% for severe steatosis, while in PCOS-BMI≥25 were 23.1% for women without steatosis, 3.8% for mild steatosis, 23.1% for moderate steatosis, and 50% for severe steatosis (Fig. 2).
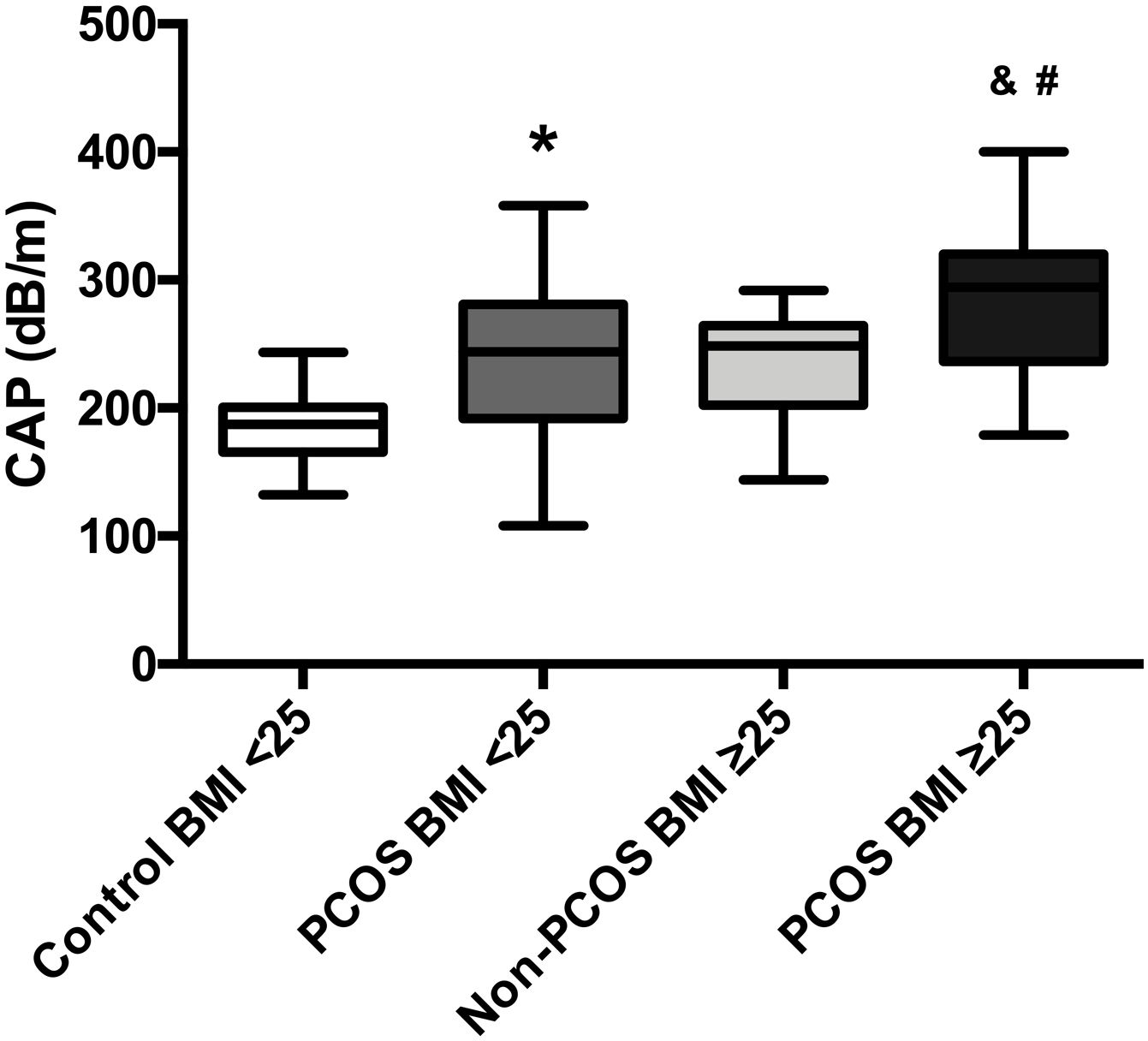
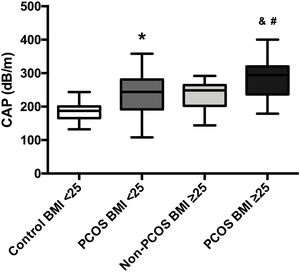
The mean CAP between the four groups was also compared; patients with PCOS-BMI<25 (241.4dB/m) had a significant difference (p=0.0001) in contrast to healthy controls (182.8dB/m). Moreover, women with PCOS-BMI≥25 (287.31dB/m) had also a significant difference (p=0.0006) against overweight patients without PCOS (235.1dB/m), besides we found differences between PCOS-BMI<25 and PCOS-BMI≥25 when we compared mean CAP, 241.4dB/m vs. 287.31dB/m, respectively (p=0.01). No differences were found in the mean CAP between PCOS-BMI<25 and controls without PCOS-BMI≥25 (Fig. 3).
Mean CAP in PCOS patients compared to age-BMI matched controls. Data represent the mean±standard deviation of the mean. * Refers compared to the control-BMI<25 group, p=0.0001, and refers compared to the Non-PCOS-BMI≥25 group, p=0.0006, # refers compared to the PCOS BMI<25 group, p=0.01. Polycystic ovary syndrome (PCOS), body mass index (BMI), controlled attenuation parameter (CAP).
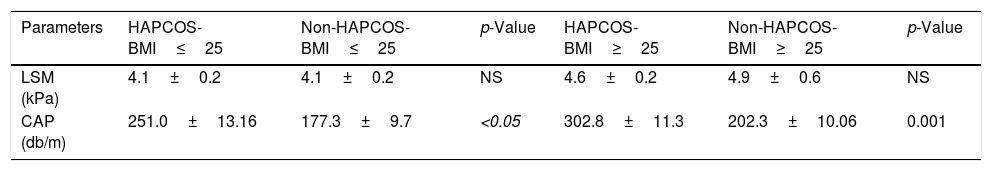
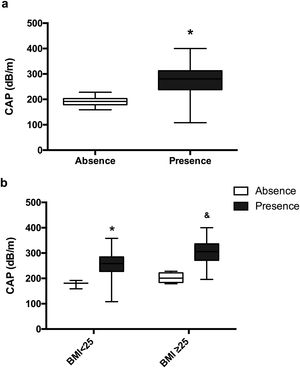
NAFLD prevalence estimates among PCOS patients were also stratified by BMI and presence or absence of HA. The prevalence of NAFLD was 90.4% for HA-BMI≥25, 0% for Non-HA-BMI≥25, while it was 70% for HA-BMI<25, and 0% for Non-HA-BMI<25. The highest prevalence of NAFLD was reported from women with HA-PCOS, which was markedly higher than in PCOS women without HA, 80.9% vs. 0%, respectively.
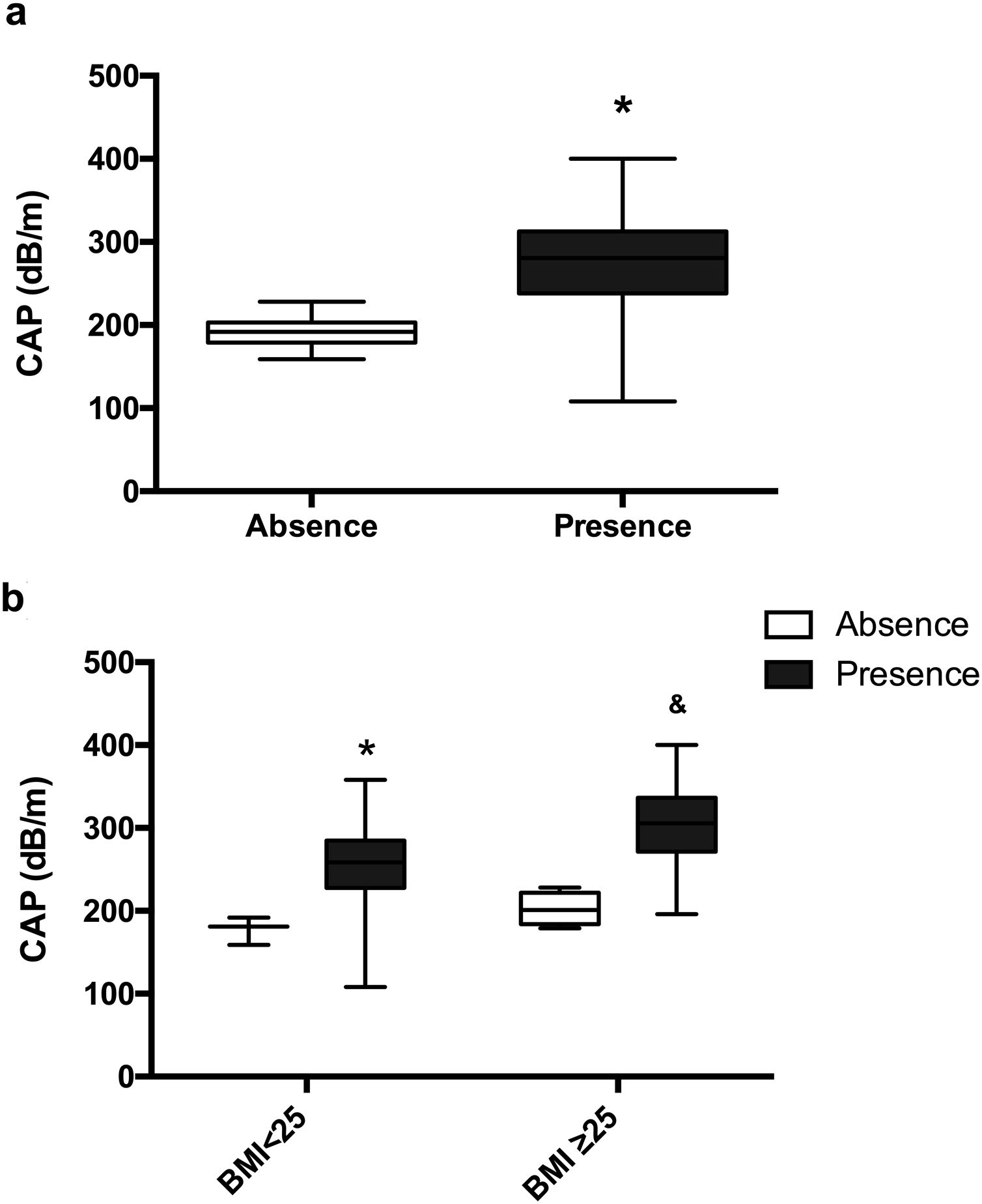
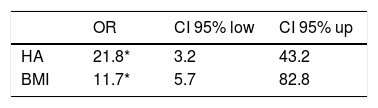
Patients with HA have a significantly higher CAP 277.83dB/m than controls without HA 191.57dB/m (p=0.0006) (Fig. 4a). The mean CAP between PCOS with and without HA in different BMI groups was also compared (Table 2), and differences were found in the CAP mean between PCOS-BMI<25 and PCOS≥25 (p=0.005) (Fig. 4b). The multivariate logistic regression model show that the HA (OR 21.8) and the BMI (OR 11.7) had a significant effect as a risk factor for developing NAFLD in patients with PCOS (Table 3).
Mean CAP, according to clinical hyperandrogenism. (a) Mean CAP in PCOS women with clinical HA (black) and without clinical HA (white). Data represent the mean±standard deviation. * Refers compared to the absence of clinical HA, p=0.0006. (b) Mean CAP according to the presence or absence of clinical HA in different BMI groups. Data represent the mean±standard deviation. * Refers compared to the absence of clinical HA and BMI<25 group, p<0.05 and refers compared to the non-clinical HA and BMI≥25 group, p=0.001. Body mass index (BMI), controlled attenuation parameter (CAP).
Transient elastography values according to clinical hyperandrogenism and BMI.
| Parameters | HAPCOS-BMI≤25 | Non-HAPCOS-BMI≤25 | p-Value | HAPCOS-BMI≥25 | Non-HAPCOS-BMI≥25 | p-Value |
|---|---|---|---|---|---|---|
| LSM (kPa) | 4.1±0.2 | 4.1±0.2 | NS | 4.6±0.2 | 4.9±0.6 | NS |
| CAP (db/m) | 251.0±13.16 | 177.3±9.7 | <0.05 | 302.8±11.3 | 202.3±10.06 | 0.001 |
Hyperandrogenism (HA), polycystic ovary syndrome (PCOS), body Mass Index (BMI), controlled attenuation parameter (CAP), liver stiffness measurement (LSM), no significance (NS).
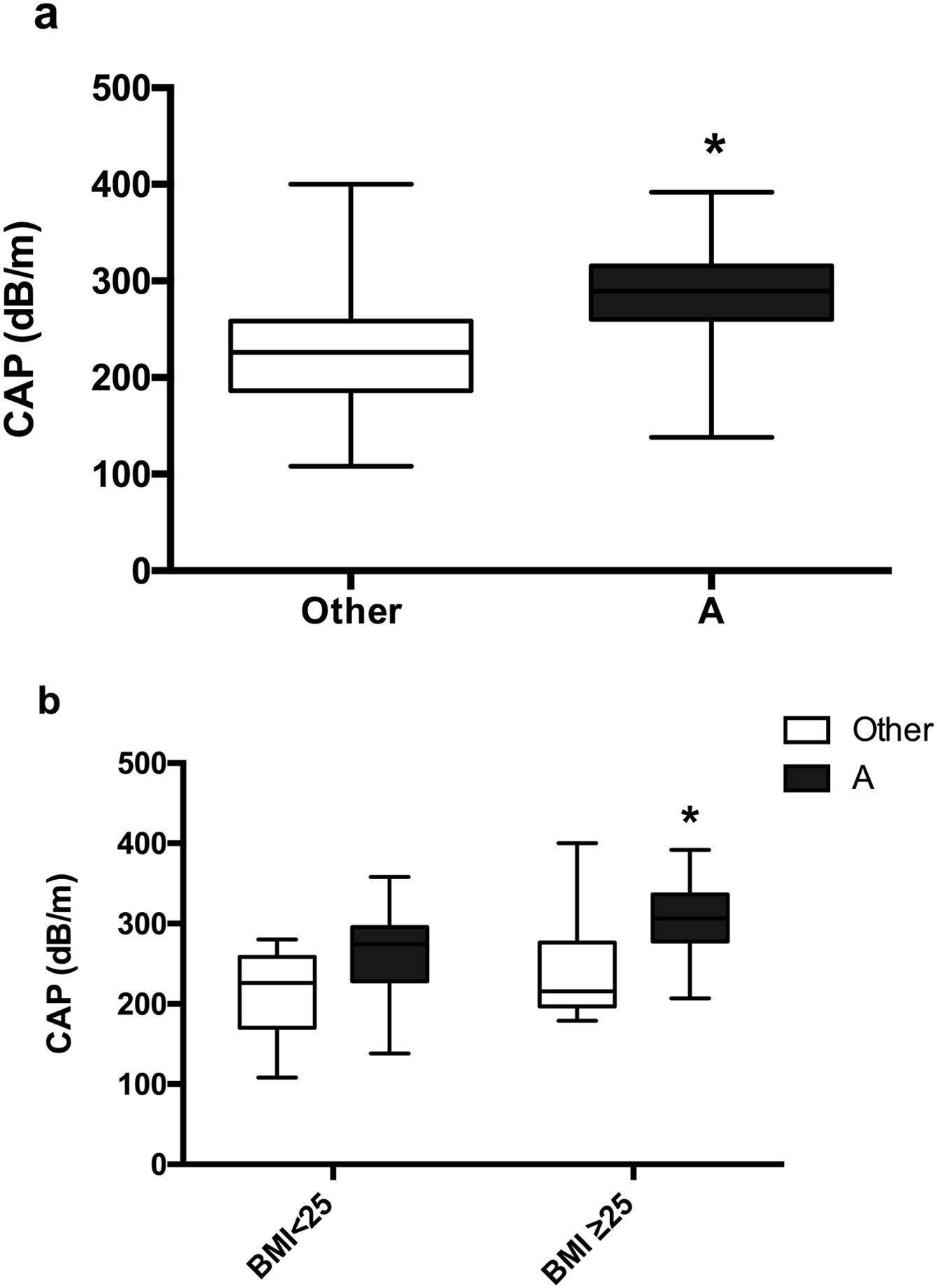
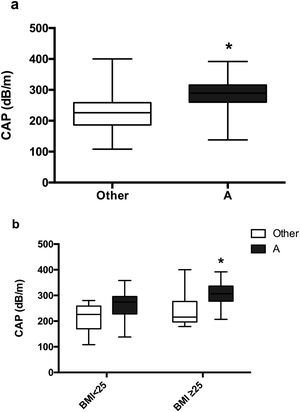
Women with phenotype A have more frequency of NAFLD. We observed the prevalence of NAFLD at 84.3% in patients with phenotype A while in the presentation of PCOS with another phenotype; the prevalence was 41.1%. The NAFLD prevalence estimates among the patients were 94.4% for PA-BMI≥25, 37.5% for another phenotype PCOS-BMI≥25, and 71.4% for PA-BMI<25, 44.4% for other phenotypes PCOS-BMI<25.
Moreover, a significantly higher CAP was observed in the classic phenotype A than in other PCOS phenotypes, 287.16dB/m vs. 225.41dB/m (p=0.0009) (Fig. 5a). Non-differences were found between women with phenotype A and PCOS-BMI<25, 260.9±15.23dB/m, compared to other phenotype and BMI<25, 211±18.53, However, patients with phenotype A and PCOS-BMI≥25 (307.6±10.51dB/m) had a significant difference (p=0.0086) in contrast to other phenotypes and PCOS-BMI<25 (242.6±25.65dB/m) (Fig. 5b).
Mean CAP in patients with PCOS around different phenotypes. (a) Classic phenotype A (black) vs. others (white). Data represent the mean±standard deviation. * Refers compared to the PCOS presentation with another phenotype p=0.0009. (b) Phenotype presentation according to BMI. Data represent the mean±standard deviation. * Refers compared to the presence of another PCOS phenotype and BMI≥25 group, p=0.0086. Body mass index (BMI), controlled attenuation parameter (CAP).
Approximately 25% of the world's population has NAFLD [10,11], which represents multiple expenses to the public health system [11]. NAFLD patients generally present some other conditions, including metabolic syndrome, atherosclerosis, coronary vascular disease, or extrahepatic tumors that confer lower survival compared to the general population [24,25]. Recent evidence shows that PCOS increases NAFLD prevalence in childbearing age women [1]. In the present study, it was observed that NAFLD prevalence is higher in patients with PCOS than in control patients (69.3% vs. 34.6%), following a previous study published by Gutierrez-Grobe et al., in which ultrasonography diagnosed a 62% NAFLD prevalence in Mexican with PCOS [26]; similarly Karoli et al. reported a 67% prevalence of hepatic steatosis in women with PCOS diagnosed by ultrasound compared to a 25% prevalence in control women [27].
Between 61 and 76% of women with PCOS are overweight or obese [28], which exacerbates the hormonal and clinical characteristics of PCOS [29]. Also, the affinity of obesity with NAFLD is well recognized [30] and is related to the disease progression and a more severe phenotype [31]. For this reason in clinical practice, the evaluation of BMI should improve metabolic risk stratification [32]; however, the association between PCOS and obesity is neither universal nor necessary to integrate the diagnosis [33]. Most studies that seek to determine NAFLD prevalence in patients with PCOS are performed in women with obesity; however, some studies that include lean patients report a higher NAFLD prevalence when presenting PCOS (6%) than in patients without this condition (2.8%) [34]. Nevertheless, more recent studies show that NAFLD prevalence in thin people was 10.2% (95% confidence interval: 7.6–13.6%) and nonobese patients was 15.7% (95% confidence interval: 12.5–19.6%) [35].
It would seem that the prevalence is relatively low in lean patients; nevertheless, according to our results, patients with PCOS have a significantly higher NAFLD risk (OR=4.26, 95% CI 1.83–9.93), and this occurs not only in patients with BMI≥25 but also in women with BMI<25, observing a prevalence of 60.9% in PCOS-BMI<25, while only 4.3% of patients in the control-BMI group<25 presented it. Indeed, this study clearly shows how lean patients with PCOS present the typical hepatic steatosis behavior of an overweight or obese patient. In this way, PCOS importance is supported as a predisposing factor for NAFLD development regardless of BMI; therefore, it could be considered essential to study the metabolic profile of the patient with PCOS, even in lean patients.
On the other hand, Zhang et al. reported that NAFLD prevalence was higher in patients with PCOS-BMI≥25 than in patients with PCOS without obesity, with a prevalence of 64% and 16% respectively [36], which occurred similarly in our patients, where NAFLD prevalence in patients with PCOS-BMI≥25 was 76.9%. 50% of patients with PCOS-BMI≥25 had severe steatosis, while in patients with PCOS-BMI<25 it was only present in 17.3% and only in 3.8% of patients without PCOS-BMI≥25. This suggests that the limitation of high-risk factors through bodyweight control is essential to prevent the occurrence and decrease the severity of NAFLD [36]. The role of abdominal obesity in the NAFLD and PCOS pathogenesis is supported by the hepatic steatosis reduction after weight loss [37]. Therefore, we suggest that more strict and individualized follow-up be performed in all patients with PCOS, but mainly in those with high associated BMI.
In parallel, PCOS patients with overweight or obesity showed a slightly higher LSM than controls (4.6 versus 3.9kPa, p=0.004); this information suggests that NAFLD progression can be accelerated in patients with PCOS and associated BMI higher than 25. Since the apoptotic processes initiated by androgens actively contribute to NAFLD evolution, women with concomitant PCOS and NAFLD could be more likely to develop fibrosis; however, liver fibrosis is a complex inflammatory and fibrogenic process that results from chronic liver injury [38]. In our study, the average age of patients with PCOS was 26 years, which is possibly a very short time to develop fibrosis; still, they would probably present NASH and eventually develop fibrosis.
Sarkar and colleagues observed that the risk of presenting NAFLD in patients with PCOS was maintained even in patients without obesity or IR, elucidating the possible role of HA in the hepatic steatosis development [39], since differences were observed between the CAP mean in patients with PCOS and clinical HA and those who did not present it 277.83dB/m vs. 191.57dB/m respectively (p<0.001). Similarly, a cross-sectional study in the Chinese population, which included 400 women with PCOS and 100 controls matched by BMI, reported that the NAFLD prevalence increased in the PCOS subgroup with HA (72% vs. 33%, p<0.001), which It reinforces the theory that HA plays a vital role in the NAFLD development in women with PCOS [1]. It is currently recognized that patients with PCOS and HA have a significantly higher risk of presenting NAFLD, compared to the control group without HA (OR=3.31; 95% CI 2.58–4.24) [40].
Moreover, Vassilatou et al. found that women with PCOS-NAFLD had higher levels of androgens and decreased sex hormone-binding globulin than women with NAFLD without PCOS, which is consistent with other studies reporting that androgen excess is one of the parameters that make patients with PCOS more susceptible to NAFLD development [41]. Also, in a physiological state, the androgen secretion induced by luteinizing hormone increases the presence of insulin. IR leads to a state of compensatory hyperinsulinemia, which in turn stimulates theca cells to secrete testosterone and androstenedione that are sensitive to luteinizing hormone. The ovaries have abundant insulin receptors, and signaling deregulation could increase androgens production in theca cells, being the primary source of excessive androgen biosynthesis in women with PCOS [1,40].
The molecular mechanisms through which PCOS and NAFLD might be linked are IR that may generate a dysregulation in the sex hormone-binding globulin expression and synthesis, which initiate a vicious cycle since the bioavailability of androgens would be higher. Consequently, the HA of the patient with PCOS will be perpetuated and aggravated; in this way, HA and IR can contribute to the severity of PCOS clinical and metabolic presentation, as well as NAFLD development and progression [9]. Therefore, we might think that the severity of the IR and the HA maintain a bidirectional relationship, where each one perpetuates and aggravates the other condition.
We are one of the earliest researchers in establish the association between the severity of NAFLD with HA and in doing so in Mexican women. Consequently, medical specialists could pay particular attention to patients who present the complete phenotype and perform more accurate screening, as well as discard women who do not have HA from a possible NAFLD risk. Another advantage of the study is that Fibroscan was used as a diagnostic tool for NAFLD, because it has greater validation compared to ultrasound, in addition it has greater sensitivity since it detects lower levels of lipids in the liver and is a more economical method compared to magnetic resonance.
Our study presents some limitations that include lack of liver biopsies, the gold standard for NAFLD diagnosis, the invasive quality of this method, and sampling error and requirements for highly trained physicians and pathologists introduced another disadvantage. This limitation was diminished as much as possible using TE by only one experience operator guided by the standard TE protocol for NAFLD diagnosis by the FibroScan® 502 Touch with regular machine inspections and validation. Laboratory assessment and sample size could improve the impact of our study.
5ConclusionIn conclusion, the prevalence of NAFLD in Mexican women with PCOS is 69.39% PCOS, which is an independent risk factor for NAFLD development. Based on our results, we suggest that NAFLD screening needs to be considered in all PCOS patients independently of BMI, except in patients with PCOS without HA and BMI<25.AbbreviationsPCOS polycystic ovary syndrome non-alcoholic fatty liver disease body mass index hyperandrogenism polycystic ovarian morphology controlled attenuation parameter liver stiffness measurement kilopascals decibels per meter insulin resistance
The authors have no conflicts of interest.
We appreciate the financing of this study to Medica Sur Clinical Foundation.
We confirm that this work is original and has not been published nor is it currently under consideration for publication elsewhere, in whole or in part, and we have not had any competing financial interests or commercial relationships that might pose a conflict of interest.