Studies have suggested that the presence of sarcopenia in patients with cirrhosis could be a predisposing risk factor for hepatic encephalopathy. This systematic review and meta-analysis were conducted to summarize all available evidence on this relationship. A systematic review was carried out in Medline and EMBASE database through December 2018 to identify studies that recruited patients with cirrhosis from any causes and collected data on the presence of minimal or overt hepatic encephalopathy as well as sarcopenia. All study designs (case–control, cohort and cross-sectional studies) were eligible for the meta-analysis. Odds ratio (OR) and 95% confidence interval (CI) were extracted from the included studies and were pooled together using random-effect, generic inverse variance method of DerSimonian and Laird. Five cross-sectional studies with a total of 1,713 patients met our eligibility criteria and were included into the meta-analysis. We found a significantly higher risk of both mild and overt hepatic encephalopathy among cirrhotic patients with sarcopenia when compared with cirrhotic patients without sarcopenia with the pooled OR of 3.34 (95% CI: 1.68–6.67; I2=37%) and 2.05 (95% CI: 1.28–3.29; I2=61%), respectively. This systematic review and meta-analysis demonstrated a significant association between sarcopenia and hepatic encephalopathy among patients with cirrhosis.
Hepatic encephalopathy is one of the most important complications of chronic liver disease that is found in approximately 40% of cirrhotic patients [1]. It is caused by hyperammonemia which is a consequence of liver dysfunction and porto-systemic shunt [2]. The reported inpatient mortality rate of hepatic encephalopathy is as high as 15% [3]. Known precipitating factors for exacerbation of hepatic encephalopathy include infection, gastrointestinal bleeding, diuretic overdose, electrolyte imbalance and constipation [4,5].
Sarcopenia is a syndrome of decreased muscle mass, strength and function [6]. It is commonly seen in patients with chronic diseases, including liver cirrhosis [7–10]. Interestingly, the presence of sarcopenia may serve as a predisposing factor for hepatic encephalopathy among patients with cirrhosis as muscle is involved in ammonia disposal process by converting ammonia into glutamine, which is subsequently excreted by the kidneys [11–15]. Therefore, muscle wasting can contribute to impairment of ammonia detoxification, leading to hyperammonemia and hepatic encephalopathy [16]. In fact, significant association between sarcopenia and risk of hepatic encephalopathy has been observed by several clinical studies although the true magnitude of the risk remains unclear because the reported relative risk varied considerably across the studies, possibly due to difference in patient population and methods used to identify sarcopenia and hepatic encephalopathy [11–15]. This systematic review and meta-analysis were conducted with the aims to shed more light into this possible relationship by identifying all relevant studies and summarizing their results together.
2Methods2.1Information sources and search strategyA systematic literature review of MEDLINE and EMBASE database was carried out by reviewing all indexed articles through December 2018 to identify all original studies that investigated the association between sarcopenia and hepatic encephalopathy. The systematic literature review was independently conducted by three investigators (K.W., P.P., and P.U.) using the search strategy that included the terms for “sarcopenia”, “frailty”, “muscle atrophy”, “hepatic encephalopathy” as described in online supplementary data 1. No language limitation was applied. A manual search for additional potentially applicable studies using references of selected included articles was also performed. This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement which is provided as online supplementary data 2.
2.2Selection criteriaStudies that were eligible for this meta-analysis needed to recruit patients with cirrhosis from any causes and collected data on the presence of minimal or overt hepatic encephalopathy as well as sarcopenia. All study designs, including case–control, cross-sectional and cohort studies, were eligible for this meta-analysis. The association between hepatic encephalopathy and sarcopenia needed to be investigated and odds ratios (ORs) and 95% confidence intervals (CIs) or sufficient raw data to calculate those ratios were provided. Inclusion was not limited by study size. When more than one study utilizing the same database/cohort was available, the study with the most comprehensive data/analyses was included.
Retrieved articles were independently reviewed for their eligibility by the same three investigators (K.W., P.P., and P.U.). Disagreement was resolved by conference with all investigators. The modified Newcastle-Ottawa scale as described by Herzog et al. was used for quality assessment of the included studies [17]. The scale evaluated quality of cross-sectional study based on seven factors, including representativeness of sample, sample size, non-respondent rate, ascertain of the exposure, comparability of the two groups, assessment of the outcome and statistical test.
2.3Data abstractionA structured information collection form was used to retrieve the following data from each study: title of the study, name of the first author, study design, year of publication, year of the study, country where the study was conducted, number of subjects, demographics of subjects, methods used to diagnose sarcopenia and hepatic encephalopathy (both overt and minimal hepatic encephalopathy), adjusted effect estimates with 95% CI and covariates that were adjusted in the multivariable analysis.
To ensure the accuracy, this data extraction process was independently performed by two investigators (K.W. and P.P.) and was reviewed by the senior investigator (P.U.).
2.4Statistical analysisData analysis was performed using the Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates from each study were consolidated by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study for the pooled analysis based on its variance (higher weight is given to study with lower variance) [18]. Random-effect model, rather than fixed-effect model, was used as the assumption of fixed-effect model that all studies, regardless of study design and background population, should give rise to the same result, is not true in almost all circumstances. Cochran's Q test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0–25% represents insignificant heterogeneity, 26–50% represents low heterogeneity, 51–75% represents moderate heterogeneity and more than 75% represents high heterogeneity [19]. If sufficient number of studies is identified, evaluation for publication bias will be performed using visualization of funnel plot.
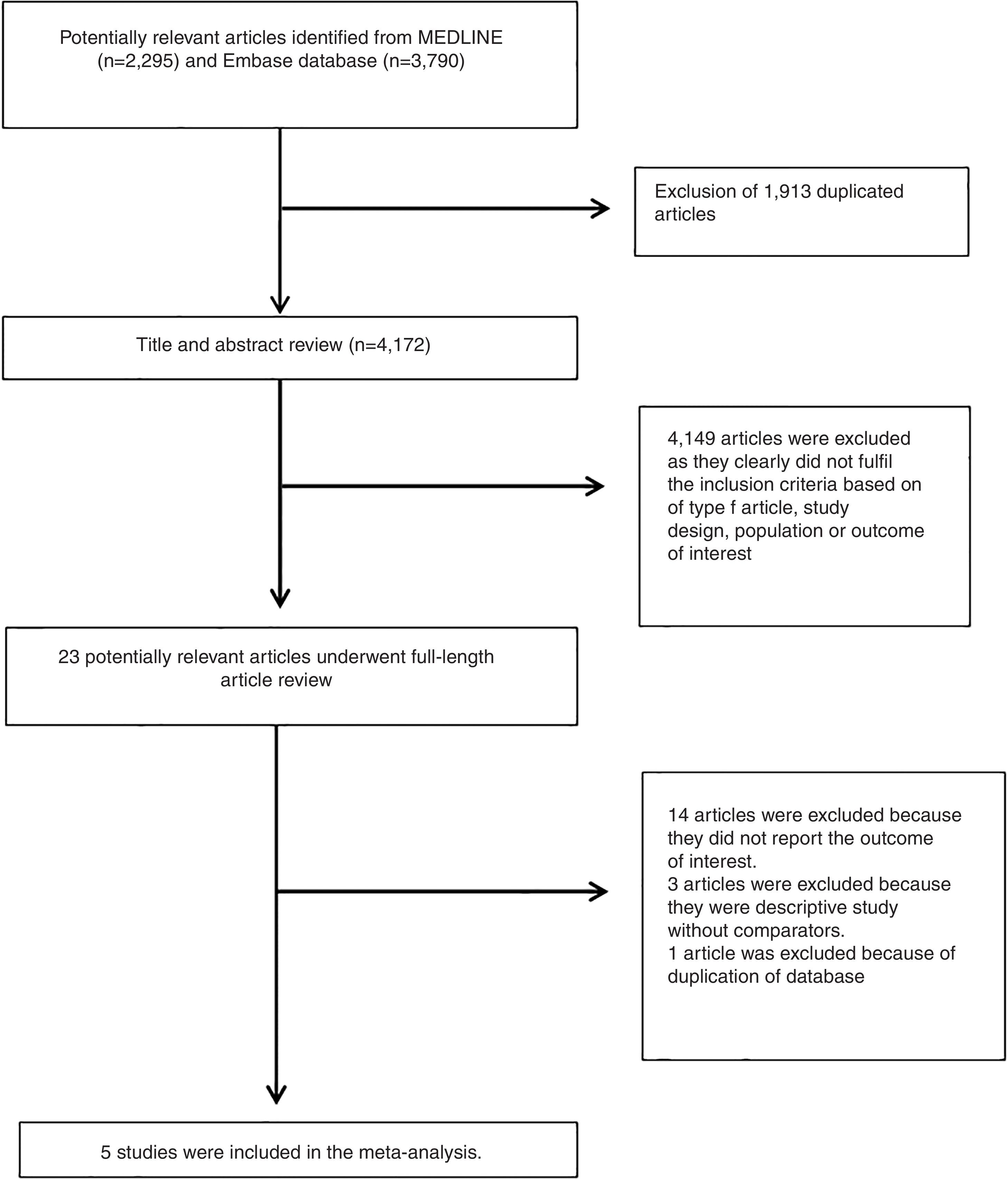
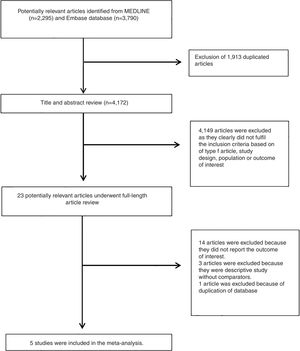
3ResultsA total of 6,085 potentially eligible articles were identified using our search strategy (2,295 articles from Medline and 3,790 articles from EMBASE). After exclusion of 1,913 duplicated articles, 4,172 articles underwent title and abstract review. Four-thousand and one hundred and forty-nine articles were excluded at this stage since they were case reports, case series, correspondences, review articles, in vitro studies, animal studies or interventional studies, leaving 23 articles for full-text review. Fourteen of them were excluded after the full-length review as they did not report the outcome of interest while three articles were excluded since they were descriptive studies without comparative analysis. A total of six cross-sectional studies [11–15] met the eligibility criteria. However, two studies [11,19] utilized the same database and only the study with more comprehensive data [19] was included into the meta-analysis to avoid double-counting of the same patients. Finally, five cross-sectional studies with 1,713 participants were included into the final analysis [11–15]. The literature review process is shown in Fig. 1. The characteristics and quality appraisal of the included studies are presented in Table 1. It should be noted that the inter-rater agreement for the quality assessment using the modified Newcastle-Ottawa scale was high with the kappa statistics of 0.72.
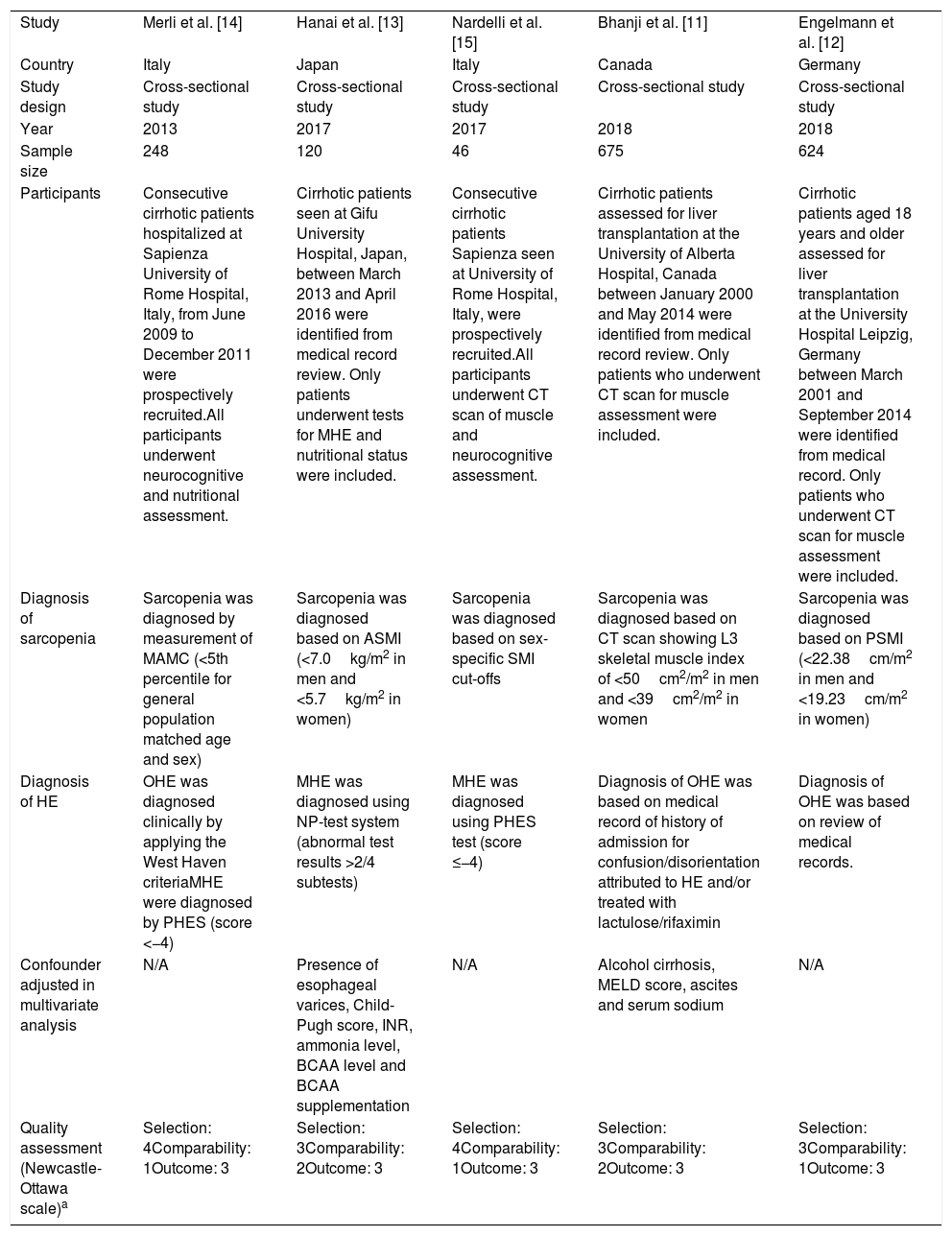
Main characteristics of the studies included in this meta-analysis.
| Study | Merli et al. [14] | Hanai et al. [13] | Nardelli et al. [15] | Bhanji et al. [11] | Engelmann et al. [12] |
| Country | Italy | Japan | Italy | Canada | Germany |
| Study design | Cross-sectional study | Cross-sectional study | Cross-sectional study | Cross-sectional study | Cross-sectional study |
| Year | 2013 | 2017 | 2017 | 2018 | 2018 |
| Sample size | 248 | 120 | 46 | 675 | 624 |
| Participants | Consecutive cirrhotic patients hospitalized at Sapienza University of Rome Hospital, Italy, from June 2009 to December 2011 were prospectively recruited.All participants underwent neurocognitive and nutritional assessment. | Cirrhotic patients seen at Gifu University Hospital, Japan, between March 2013 and April 2016 were identified from medical record review. Only patients underwent tests for MHE and nutritional status were included. | Consecutive cirrhotic patients Sapienza seen at University of Rome Hospital, Italy, were prospectively recruited.All participants underwent CT scan of muscle and neurocognitive assessment. | Cirrhotic patients assessed for liver transplantation at the University of Alberta Hospital, Canada between January 2000 and May 2014 were identified from medical record review. Only patients who underwent CT scan for muscle assessment were included. | Cirrhotic patients aged 18 years and older assessed for liver transplantation at the University Hospital Leipzig, Germany between March 2001 and September 2014 were identified from medical record. Only patients who underwent CT scan for muscle assessment were included. |
| Diagnosis of sarcopenia | Sarcopenia was diagnosed by measurement of MAMC (<5th percentile for general population matched age and sex) | Sarcopenia was diagnosed based on ASMI (<7.0kg/m2 in men and <5.7kg/m2 in women) | Sarcopenia was diagnosed based on sex-specific SMI cut-offs | Sarcopenia was diagnosed based on CT scan showing L3 skeletal muscle index of <50cm2/m2 in men and <39cm2/m2 in women | Sarcopenia was diagnosed based on PSMI (<22.38cm/m2 in men and <19.23cm/m2 in women) |
| Diagnosis of HE | OHE was diagnosed clinically by applying the West Haven criteriaMHE were diagnosed by PHES (score <−4) | MHE was diagnosed using NP-test system (abnormal test results >2/4 subtests) | MHE was diagnosed using PHES test (score ≤−4) | Diagnosis of OHE was based on medical record of history of admission for confusion/disorientation attributed to HE and/or treated with lactulose/rifaximin | Diagnosis of OHE was based on review of medical records. |
| Confounder adjusted in multivariate analysis | N/A | Presence of esophageal varices, Child-Pugh score, INR, ammonia level, BCAA level and BCAA supplementation | N/A | Alcohol cirrhosis, MELD score, ascites and serum sodium | N/A |
| Quality assessment (Newcastle-Ottawa scale)a | Selection: 4Comparability: 1Outcome: 3 | Selection: 3Comparability: 2Outcome: 3 | Selection: 4Comparability: 1Outcome: 3 | Selection: 3Comparability: 2Outcome: 3 | Selection: 3Comparability: 1Outcome: 3 |
Abbreviations: MHE: minimal hepatic encephalopathy; OHE: overt hepatic encephalopathy; HE: hepatic encephalopathy; MAMC: mid-arm-muscle-circumference; HGS: handgrip strength; PHES: Psychometric Hepatic Encephalopathy Score; CT: computed tomography; MELD: Model for End-Stage Liver Disease; ASMI: appendicular skeletal muscle mass index; NP-test: neuropsychiatric test; INR: international normalized ratio; BCAA: branched chain amino acids; SMI: skeletal muscle index; PSMI: paraspinal muscle index.
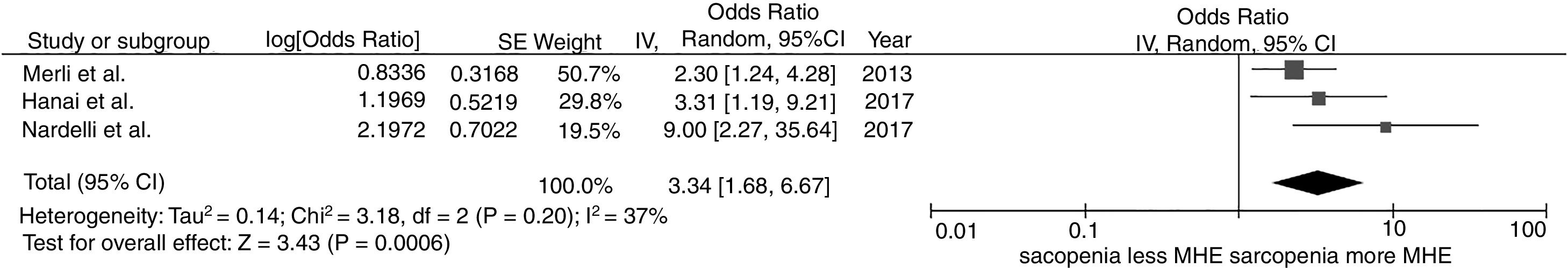
We found a significantly increased risk of minimal hepatic encephalopathy among patients with sarcopenia with the pooled OR of 3.34 (95% CI, 1.68–6.67), as demonstrated in Fig. 2. The between-study heterogeneity was low with an I2 of 37%.
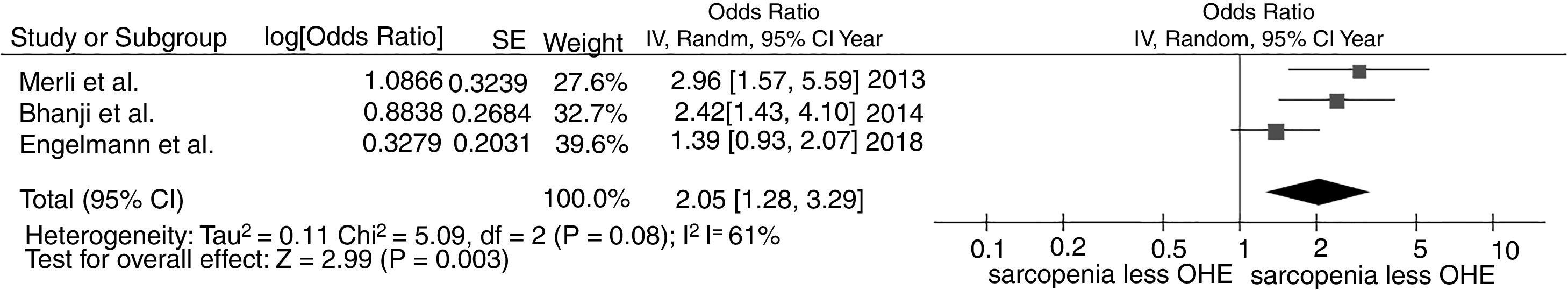
3.2Association between sarcopenia and overt hepatic encephalopathyWe found a significantly increased risk of overt hepatic encephalopathy among patients with sarcopenia with the pooled OR of 2.05 (95% CI, 1.28–3.29), as demonstrated in Fig. 3. The between-study heterogeneity was moderate with an I2 of 61%.
3.3Evaluation for publication biasEvaluation for publication bias using funnel plot could not be performed due to the limited number of the included studies.
4DiscussionSarcopenia is common among patients with cirrhosis because of several contributing factors. The major factor is malnutrition as a result of physiological and anatomical changes associated with end stage liver disease such as ascites causing early satiety, elevated inflammatory cytokines affecting appetite, abnormal gut motility and poor nutrient absorption due to portal hypertension [20–22]. Patients with cirrhosis are also in hypermetabolic state and have a decreased capacity of glucoeneogenesis, which would lead to muscle breakdown to mobilize energy and muscle amino acids [23]. In addition, some specific etiologies of chronic liver disease are associated with the increased risk of sarcopenia. For instance; alcoholic cirrhosis, especially when associated with active alcoholism and poverty, can lead to poor diet and macro- and micronutrients deficiencies [21]; chronic viral hepatitis can lead to a state of chronic inflammation, resulting in decreased appetite [21,23]; elevated insulin resistance in NAFLD could impair the uptake of glucose and subsequently trigger gluconeogenesis via muscle breakdown [20].
The current systematic review and meta-analysis found that the presence of sarcopenia in patients with cirrhosis is associated with an approximately two-fold and three-fold increased risk of overt and minimal hepatic encephalopathy, respectively. This observation suggests that the presence of sarcopenia may adversely affect the outcome of cirrhosis. The most plausible explanation for this increased risk lies in the metabolic function of skeletal muscle as it can convert ammonia into glutamine, which can then be excreted by the kidneys [24]. This is an important alternative pathway for ammonia homeostasis when liver function is impaired. In sarcopenic patients, because of the lack of muscle mass, this compensatory mechanism is decreased or absent and, thus, increase the risk of hyperammonemia and hepatic encephalopathy [25].
Nonetheless, due to the cross-sectional nature of the primary studies, it is also possible that the observed association may have a reverse cause-and-effect direction. It is possible that hyperammonemia, the main underlying etiology of hepatic encephalopathy, could predispose cirrhotic patients to sarcopenia by promoting muscle autophagy and reducing muscle protein synthesis [26–28]. In fact, a study in patients with cirrhosis has demonstrated that once hyperammonemia was reversed, a significant recovery in the rate of protein synthesis was observed [29].
Although the quality of the included studies was high as reflected by the high modified Newcastle-Ottawa scores and the literature review process was thorough, we acknowledge that this study has some limitations and the results should be interpreted with caution. First, we could not assess for the presence of publication bias due to the limited number of the included studies. Therefore, publication bias in favor of studies with positive association may have been presented. Second, as discussed earlier, all of the included studies were cross-sectional in nature, leaving the uncertainty in the direction of the cause-and-effect. Last, between-study heterogeneity was not low in the meta-analysis of overt hepatic encephalopathy which may jeopardize the validity of the pooled result. The difference in patient population (some studies included all cirrhotic patients whereas some studies included only cirrhotic patients evaluated for liver transplantation) and methods used to identify sarcopenia and hepatic encephalopathy were probably responsible for this variation in effect size. Unfortunately, further analysis, such as subgroup analysis and meta-regression, to explore this between-study heterogeneity could not be performed due to the limited number of included studies.
In summary, this study demonstrated a significant association between hepatic encephalopathy and sarcopenia among patients with cirrhosis. Further investigations are still required to determine whether this association is causal and the role of management of sarcopenia among patients with cirrhosis in clinical practice.AbbreviationsOR odds ratio confidence interval Preferred Reporting Items for Systematic Reviews and Meta-Analysis non-alcoholic fatty liver disease
All authors had access to the data and a role in writing the manuscript.
Wijarnpreecha K, acquisition of data, analysis and interpretation of data, drafting the articles, final approval; Werlang M, acquisition of data, drafting the articles, final approval; Panjawatanan P, acquisition data, interpretation the data, final approval; Kroner PT, interpretation the data, revising the article, final approval; Cheungpasitporn W, acquisition of data, interpretation of data, final approval; Lukens FJ, Pungpapong S, interpretation the data, revising the article, final approval; Ungprasert P, conception and design of the study, critical revision, final approval.
FundingNone declared.
Conflict of interestWe do not have any financial or non-financial potential conflicts of interest.