Cohort studies reported controversial results regarding the long-term prognosis of patients with lean non-alcoholic fatty liver disease (NAFLD) compared to non-lean NAFLD patients. This updated meta-analysis aimed to estimate the magnitude of the association between lean body mass index and all-cause mortality risk in NAFLD patients.
Materials and MethodsWe systematically searched the EMBASE and MEDLINE databases from inception to March 2023 to identify observational studies that reported hazard ratio (HR) for all-cause mortality of patients with lean NAFLD versus those with non-lean, overweight, or obese NAFLD. Multivariable-adjusted hazard ratios (HRs) for all-cause mortality were pooled using a random effects model.
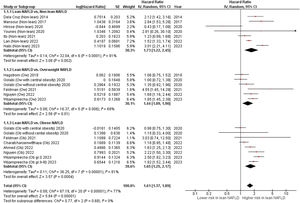
ResultsFourteen studies with 94,181 NAFLD patients (11.3 % with lean NAFLD) and 7,443 fatal events over a median follow-up of 8.4 years (IQR, 6.6–17.4 years) were included. Patients with lean NAFLD had a higher risk of all-cause mortality than those with non-lean NAFLD (random-effects HR 1.61, 95 % CI 1.37–1.89; I2=77 %). The magnitude of this risk remained unchanged even after stratified analysis by measures of NAFLD diagnosis, study country, cohort setting, length of follow-up, adjustment with fibrosis stage/cirrhosis, and the Newcastle-Ottawa Scale. The risk was independent of age, sex, and cardiometabolic risk factors. Sensitivity analyses did not alter these findings. The funnel plot and Egger's test revealed no significant publication bias.
ConclusionsThis meta-analysis revealed that lean NAFLD is associated with an approximately 1.6-fold increased mortality risk. Further studies are needed to unravel the existing but complex link between lean NAFLD and an increased risk of death.
Non-alcoholic fatty liver disease (NAFLD) is a potentially severe liver disease that affects about one-quarter of the adult population worldwide [1,2]. NAFLD can progress from simple steatosis to non-alcoholic steatohepatitis, cirrhosis, liver decompensation, and hepatocellular carcinoma (HCC) [3]. Patients with NAFLD have been shown to have a higher risk of death from any cause than those without the disease [4,5]. Although obesity is a major contributing factor for NAFLD, approximately 20 % of NAFLD patients are lean [6]. NAFLD with normal weight (body mass index [BMI] <23 kg/m2 in Asians and <25 kg/m2 in people of other ethnicities), considered to be lean NAFLD, is thought to be more benign than NAFLD with higher BMI. Since individuals with lean NAFLD usually present with more favorable metabolic and histologic profiles, it is commonly believed that this lean group will follow a relatively benign clinical course than their overweight or obese counterparts [7,8]. However, an international cohort study by Dela Cruz et al. first reported increased mortality for patients with lean NAFLD compared with those with higher BMI [9]. Later, several studies on the mortality risk of lean NAFLD have discovered controversial results [10–17].
The impact of adiposity measures on the long-term prognosis of patients with NAFLD is currently a topic of intense scientific debate. In 2022, the first meta-analysis investigating the association between lean NAFLD and mortality risk was published. It included ten observational studies and concluded that the risk of all-cause mortality in lean NAFLD patients is comparable with that in patients with non-lean NAFLD [18]. However, these findings were criticized because baseline factors, especially cardiovascular disease (CVD) risk factors that might influence survival, are not evenly distributed between the lean and non-lean NAFLD. Thus, the pooled analysis only combined unadjusted risk estimates from studies, potentially leading to biased inferences about the exposure-outcome association. Furthermore, over the recent years, several large cohort studies in adults from the United States and Asia have examined the association between lean NAFLD and the risk of all-cause mortality [19–23]. Therefore, this systematic review and updated meta-analysis was conducted to comprehensively assess the association between a lean body mass and the long-term risk of all-cause mortality among patients with NAFLD by performing a pooled analysis of covariate-adjusted hazard ratios (HRs).
2Materials and Methods2.1Search methods for identification of studiesTwo researchers independently performed a systematic review of the literature in the EMBASE and MEDLINE databases from inception to March 1st, 2023, to identify all longitudinal cohort studies that examined all-cause mortality of patients with lean NAFLD compared to populations with overweight or obese NAFLD. Supplementary Data 1 contains the search strategy outlining the search terms for “non-alcoholic fatty liver disease,” “lean,” “mortality,” and “survival”. To maximize the comprehensiveness of the systematic review, we searched and reviewed the references of included papers. However, the authors of potentially relevant articles were not contacted. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) was used for this study protocol (Supplementary data 2). The study is registered on Open Science Framework, number osf.io/a9qfn.
2.2Study eligibility criteriaStudies were included in the meta-analysis if they met the following criteria: 1) longitudinal cohort studies comparing mortality between NAFLD patients with lean body mass and those with either non-lean, overweight, or obese body mass; 2) studies reporting adjusted HRs with 95 % confidence intervals (95 % CIs) values for the outcome of interest; and 3) studies diagnosing NAFLD with liver biopsy, imaging techniques, or surrogate indices. Both conference abstracts and full-text articles were eligible. Articles published in non-English languages were excluded. Lean or non-lean body mass was defined as BMI <23 or ≥23 kg/m2 for Asians and <25 or ≥25 kg/m2 for non-Asians, respectively. Overweight body mass was defined as BMI between 23 and 24.9 kg/m2 for Asians and BMI between 25 and 29.9 kg/m2 for non-Asians. Obese body mass was defined as BMI ≥25 kg/m2 for Asians and ≥30 kg/m2 for non-Asians. The first round of screening of titles and abstracts and the second round of full-text review were performed independently by two investigators (WW and NC) to ensure that the included studies met all eligible criteria. Any disagreements were resolved by consensus and, if necessary, a third author (PC).
2.3Data extraction and quality assessmentThe following details were extracted using a standardized record form: the author, the year of publication, the country where the study was conducted, the study design, the number of participants, the identification and ascertainment of NAFLD participants, baseline characteristics of participants, including metabolic disorders of all patients, lean and non-lean patients, the average or median duration of follow-up, and covariates included in multivariable models. The primary outcome of interest was the comparative risk of all-cause mortality among individuals with lean NAFLD compared to non-lean NAFLD patients. A secondary outcome was to estimate the cause-specific mortality risks for patients with lean NAFLD. Covariate adjusted HR and 95 % CI for all-cause mortality in patients with lean-NAFLD versus those with non-lean NAFLD were recorded. Two researchers (WW and NC) utilized the Newcastle-Ottawa Scale (NOS) to evaluate the quality of the relevant cohort studies [24], and the senior investigator (PC) settled any disagreements.
2.4Statistical analysis and data synthesisHRs were pooled via the inverse variance method using the DerSimonian and Laird random effects model to compare mortality between individuals with lean NAFLD and non-lean NAFLD patients [25]. The participants in the reference group were categorized into three groups based on whether they had non-lean, overweight, or obese NAFLD. Statistical heterogeneity was tested using the I2 test. An I2 ≥50 % or p <0.1 was suggestive of considerable heterogeneity among the studies [26].
To investigate potential sources of heterogeneity among the eligible studies and test the robustness of the observed associations, we stratified analyses according to methods for NAFLD diagnosis, study country, study setting (hospital-based vs. population-based studies), length of follow-up (<10 years vs. ≥10 years), NOS category (<8 stars vs. ≥8 stars), adjustment with fibrosis stage/cirrhosis, and a full adjustment for traditional CVD risk factors (defined as studies adjusting at least for age, sex, smoking history, type 2 diabetes mellitus, hypertension, and hyperlipidemia). We performed univariable meta-regression analyses to determine the impact of specific moderator variables (i.e., age, sex, smoking status, and percentage of type 2 diabetes mellitus, hypertension, and hyperlipidemia) on the effect size for adjusted HRs of all-cause mortality. We further conducted sensitivity analyses to test for the possible excessive influence of each study on overall results by excluding one study at a time. Finally, the possibility of publication bias was evaluated using the funnel plot and Egger's test if at least 10 studies were available. Review Manager 5.4 software from the Cochrane Collaboration (London, United Kingdom) was used for data analysis.
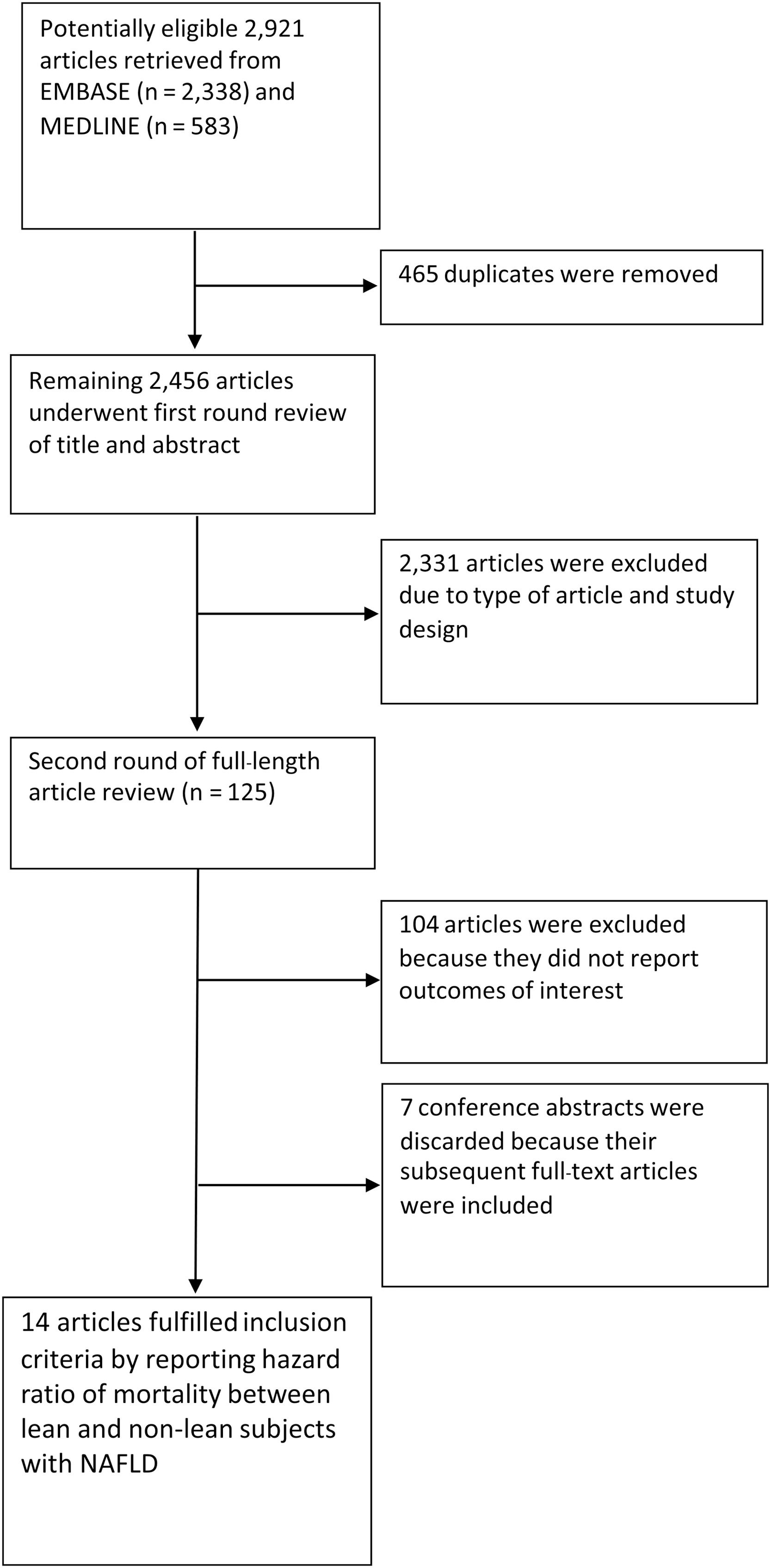
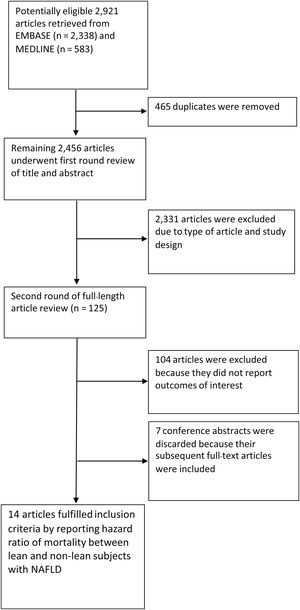
3Results3.1Characteristics of the included studiesA total of 2921 articles, including 2338 from EMBASE and 583 from MEDLINE, were retrieved. After 465 duplicated articles were found and eliminated, the remaining 2456 articles underwent the first-round review of titles and abstracts. A total of 2331 articles were removed because they didn't fulfill the eligibility criteria based on the study design and the outcome of interest. After the second round of reviews of 125 publications, 14 relevant observational studies with a total of 94,181 patients with NAFLD (11.3 % with lean NAFLD) were included in this meta-analysis. Eleven full-text articles [10-15,17,19-22] and three conference abstracts [9,16,23] explored the impact of lean body mass on all-cause mortality in the NAFLD population. One of the full-text articles, a study by Ito et al. [15], was a meta-analysis of individual patients from two cohorts in Japan [27,28]. These original cohort studies did not report the effect of lean body mass on our prerequisite outcomes. However, a study by Ito et al. re-analyzed original raw data and reported an HR of lean NAFLD on all-cause mortality [15]. Then, we included this study as a cohort study. Two studies examined the association between lean NAFLD and the risk of cause-specific mortality [12,20]. Fig. 1 provides an overview of the literature review and study selection process.
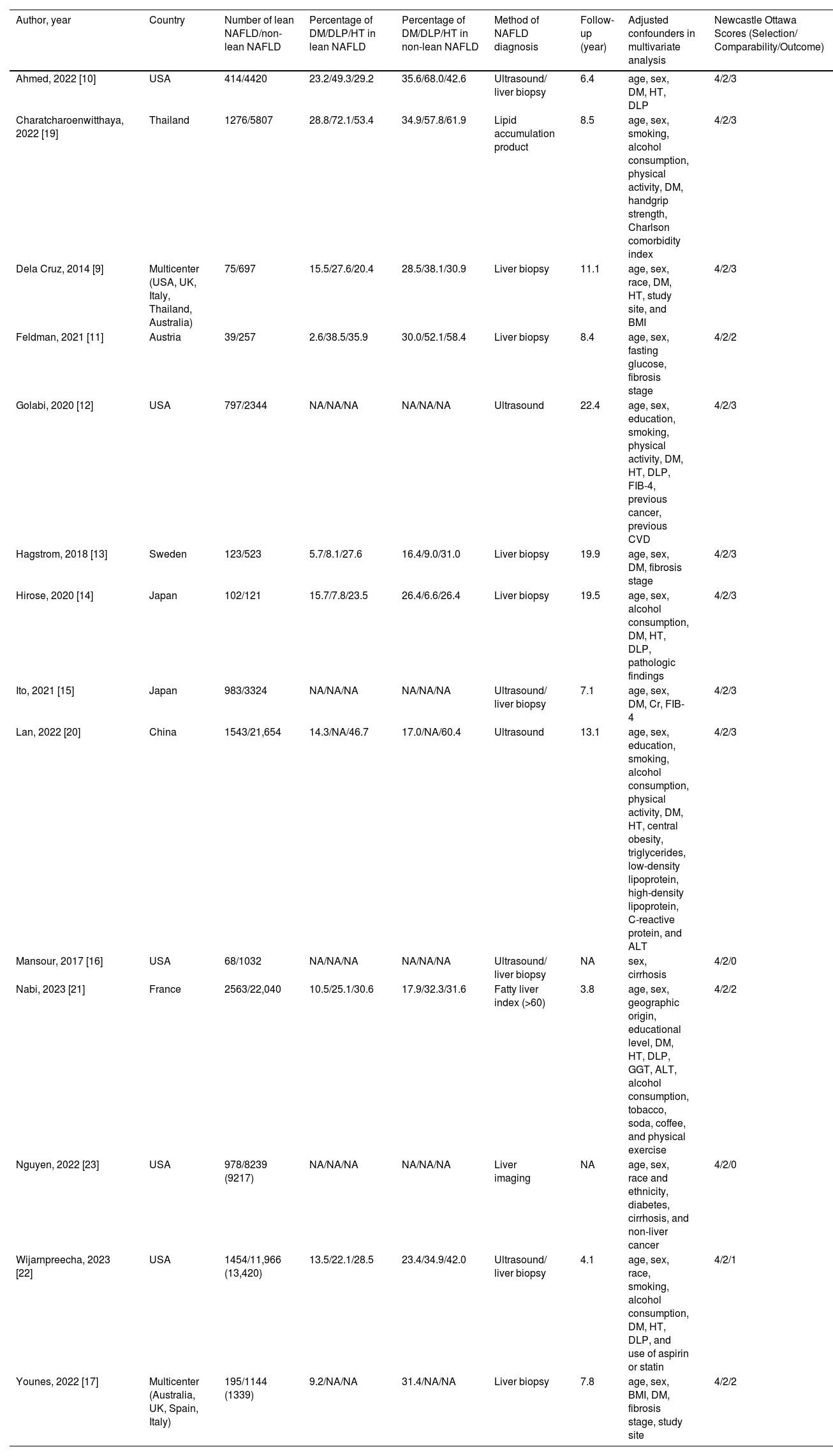
Table 1 summarizes the study design, characteristics of participants, and the NOS of the included studies. The majority of these studies enrolled participants from general populations, national health examination surveys, or outpatient cohorts, in which NAFLD was diagnosed by liver biopsy, imaging techniques (mostly ultrasonography), or surrogate indices of fatty liver. Five studies were carried out in the United States; four studies were carried out in Asia (China, Japan, and Thailand); three studies were carried out in Europe (Austria, France, and Sweden); and two studies were international research collaborations. Eight studies received a score of nine stars on the NOS (studies at relatively low risk of bias), four studies received seven or eight stars (studies at medium risk of bias), and two studies received six stars (studies at high risk of bias). Patients with lean NAFLD in most included studies were more likely to have a lower prevalence of components of the metabolic syndrome, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, than non-lean counterparts.
Characteristics of cohort studies assessing overall mortality between lean and non-lean NAFLD populations included in the review.
| Author, year | Country | Number of lean NAFLD/non-lean NAFLD | Percentage of DM/DLP/HT in lean NAFLD | Percentage of DM/DLP/HT in non-lean NAFLD | Method of NAFLD diagnosis | Follow-up (year) | Adjusted confounders in multivariate analysis | Newcastle Ottawa Scores (Selection/ Comparability/Outcome) |
|---|---|---|---|---|---|---|---|---|
| Ahmed, 2022 [10] | USA | 414/4420 | 23.2/49.3/29.2 | 35.6/68.0/42.6 | Ultrasound/ liver biopsy | 6.4 | age, sex, DM, HT, DLP | 4/2/3 |
| Charatcharoenwitthaya, 2022 [19] | Thailand | 1276/5807 | 28.8/72.1/53.4 | 34.9/57.8/61.9 | Lipid accumulation product | 8.5 | age, sex, smoking, alcohol consumption, physical activity, DM, handgrip strength, Charlson comorbidity index | 4/2/3 |
| Dela Cruz, 2014 [9] | Multicenter (USA, UK, Italy, Thailand, Australia) | 75/697 | 15.5/27.6/20.4 | 28.5/38.1/30.9 | Liver biopsy | 11.1 | age, sex, race, DM, HT, study site, and BMI | 4/2/3 |
| Feldman, 2021 [11] | Austria | 39/257 | 2.6/38.5/35.9 | 30.0/52.1/58.4 | Liver biopsy | 8.4 | age, sex, fasting glucose, fibrosis stage | 4/2/2 |
| Golabi, 2020 [12] | USA | 797/2344 | NA/NA/NA | NA/NA/NA | Ultrasound | 22.4 | age, sex, education, smoking, physical activity, DM, HT, DLP, FIB-4, previous cancer, previous CVD | 4/2/3 |
| Hagstrom, 2018 [13] | Sweden | 123/523 | 5.7/8.1/27.6 | 16.4/9.0/31.0 | Liver biopsy | 19.9 | age, sex, DM, fibrosis stage | 4/2/3 |
| Hirose, 2020 [14] | Japan | 102/121 | 15.7/7.8/23.5 | 26.4/6.6/26.4 | Liver biopsy | 19.5 | age, sex, alcohol consumption, DM, HT, DLP, pathologic findings | 4/2/3 |
| Ito, 2021 [15] | Japan | 983/3324 | NA/NA/NA | NA/NA/NA | Ultrasound/ liver biopsy | 7.1 | age, sex, DM, Cr, FIB-4 | 4/2/3 |
| Lan, 2022 [20] | China | 1543/21,654 | 14.3/NA/46.7 | 17.0/NA/60.4 | Ultrasound | 13.1 | age, sex, education, smoking, alcohol consumption, physical activity, DM, HT, central obesity, triglycerides, low-density lipoprotein, high-density lipoprotein, C-reactive protein, and ALT | 4/2/3 |
| Mansour, 2017 [16] | USA | 68/1032 | NA/NA/NA | NA/NA/NA | Ultrasound/ liver biopsy | NA | sex, cirrhosis | 4/2/0 |
| Nabi, 2023 [21] | France | 2563/22,040 | 10.5/25.1/30.6 | 17.9/32.3/31.6 | Fatty liver index (>60) | 3.8 | age, sex, geographic origin, educational level, DM, HT, DLP, GGT, ALT, alcohol consumption, tobacco, soda, coffee, and physical exercise | 4/2/2 |
| Nguyen, 2022 [23] | USA | 978/8239 (9217) | NA/NA/NA | NA/NA/NA | Liver imaging | NA | age, sex, race and ethnicity, diabetes, cirrhosis, and non-liver cancer | 4/2/0 |
| Wijarnpreecha, 2023 [22] | USA | 1454/11,966 (13,420) | 13.5/22.1/28.5 | 23.4/34.9/42.0 | Ultrasound/ liver biopsy | 4.1 | age, sex, race, smoking, alcohol consumption, DM, HT, DLP, and use of aspirin or statin | 4/2/1 |
| Younes, 2022 [17] | Multicenter (Australia, UK, Spain, Italy) | 195/1144 (1339) | 9.2/NA/NA | 31.4/NA/NA | Liver biopsy | 7.8 | age, sex, BMI, DM, fibrosis stage, study site | 4/2/2 |
BMI, body mass index; Cr, creatinine; CVD, cardiovascular disease; DLP, dyslipidemia; DM, diabetes mellitus; FIB-4, fibrosis-4 index; FLI, fatty liver index; HT, hypertension; NA, Not available; NAFLD, non-alcoholic fatty liver; UK, the United Kingdom; USA, United States of America.
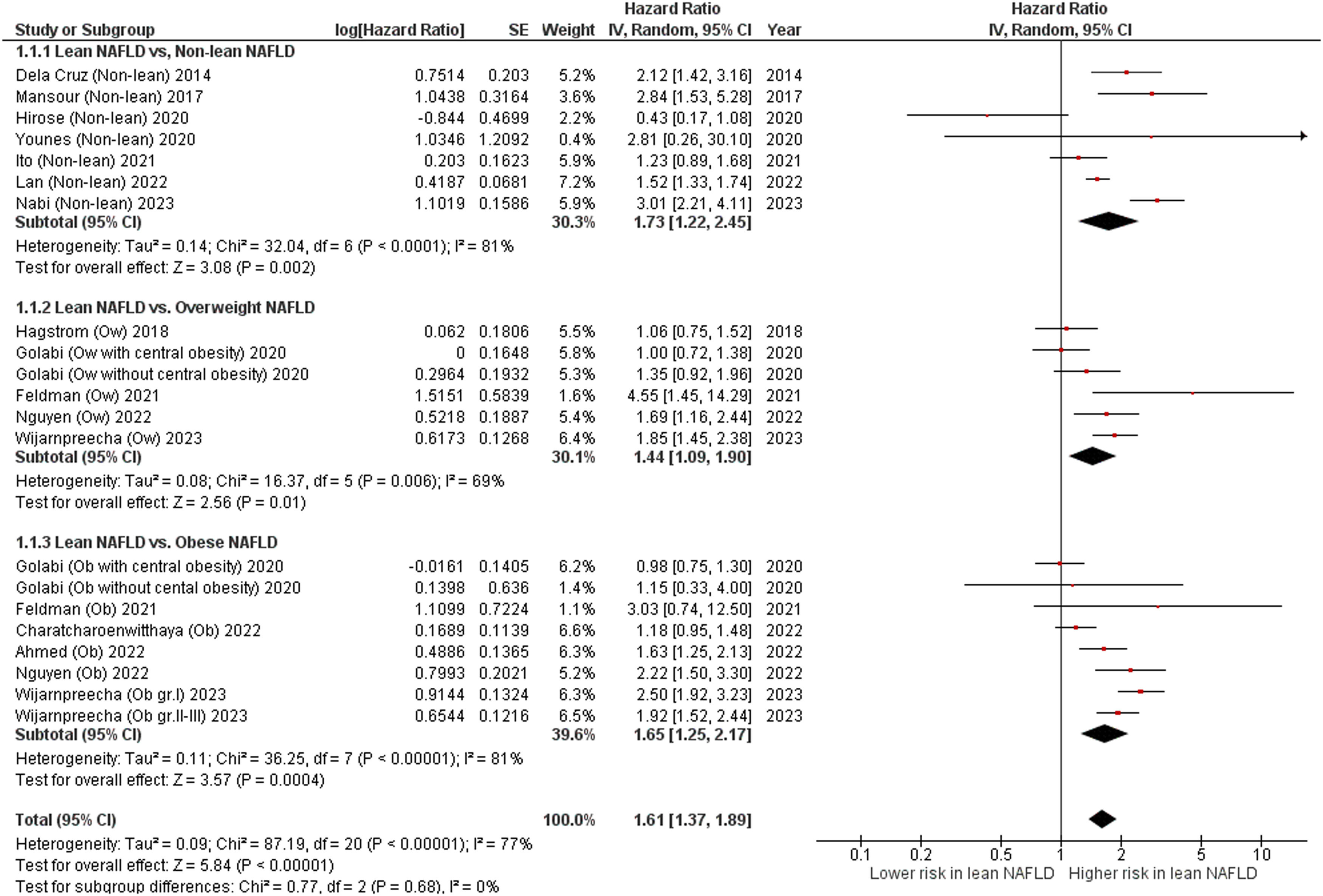
Overall, these studies had aggregate data on 94,181 individuals with NAFLD (10,610 patients with lean NAFLD and 83,571 patients with non-lean NAFLD) and 7443 incident cases of fatal events over a median of mean/median follow-up of 8.4 years (interquartile range, 6.6–17.4 years). Patients with lean NAFLD were significantly associated with an increased risk of all-cause mortality compared to non-lean NAFLD patients with a pooled HR of 1.73 (95 % CI, 1.22–2.45; I2 = 81 %) [9,14-17,20,21], overweight NAFLD patients with a pooled HR of 1.44 (95 % CI, 1.09–1.90; I2 = 69 %) [11-13,22,23], obese NAFLD patients with a pooled HR of 1.65 (95 % CI, 1.25–2.17]; I2 = 81 %) [10-12,19,22,23], and their overall combined non-lean counterparts with a pooled HR of 1.61 (95 % CI, 1.37–1.89; I2 = 77 %) [9-17,19-23], as displayed in Fig. 2. Pooled estimates for all-cause mortality remained consistent in direction when the analysis was limited to the 11 full-text articles (pooled random-effects HR 1.51, 95 % CI, 1.26–1.81; test for overall effect Z = 4.42, P<0.0001; I2 = 79 %) [10-15,17,19-22].
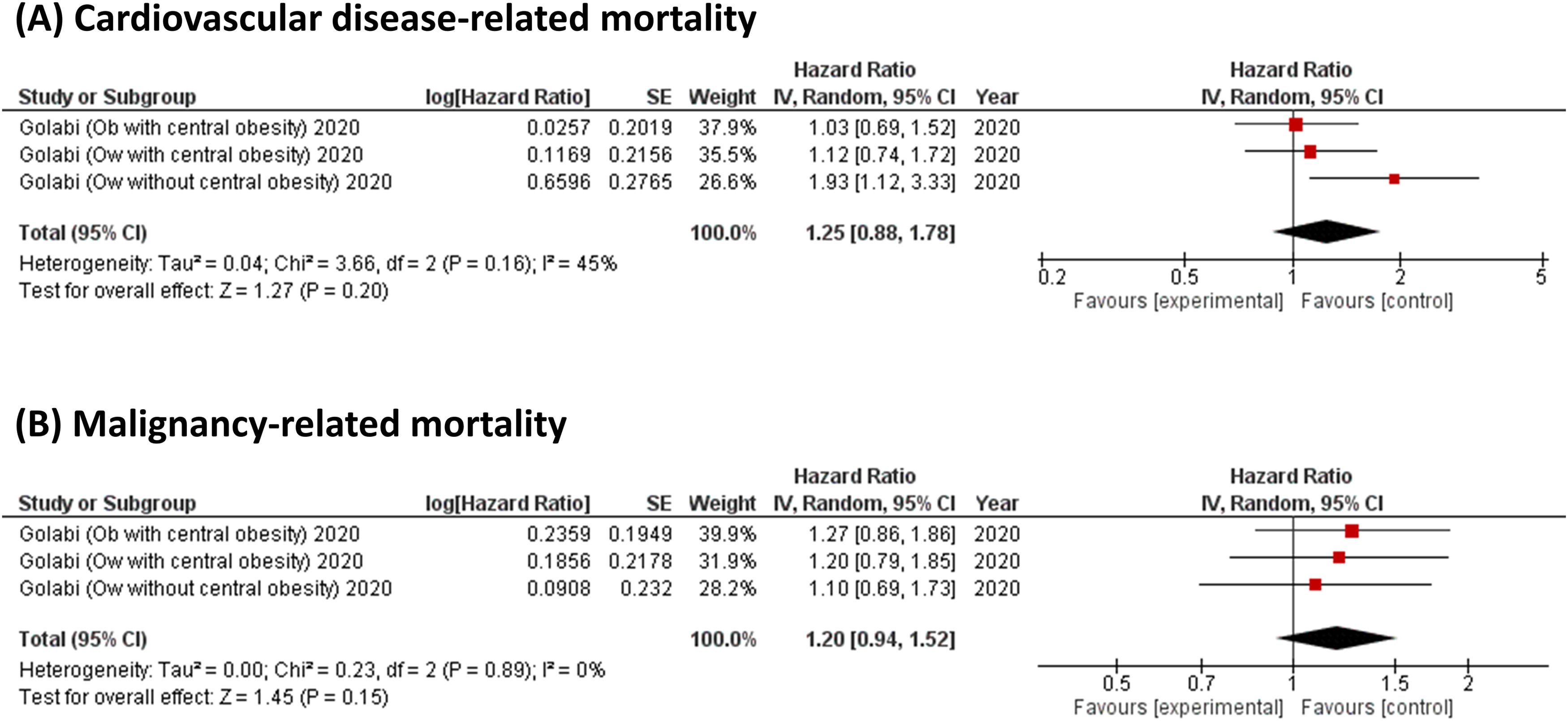
For cause-specific mortality, Golabi et al. showed that there was no significant difference in the risk of CVD-related mortality (a pooled HR 1.25, 95 % CI, 0.88–1.78; I2 = 45 %, Fig. 3A) and malignancy-related mortality (a pooled HR 1.20, 95 % CI, 0.94–1.52; I2 = 0 %, Fig. 3B) between patients with lean NAFLD and those with overweight or obese NAFLD [12]. On the other hand, Lan et al. found a significant association between patients with lean NAFLD and an increased risk of liver-related mortality, with an adjusted HR of 2.77 (95 % CI, 1.23–6.24), when compared to those with non-lean NAFLD [20].
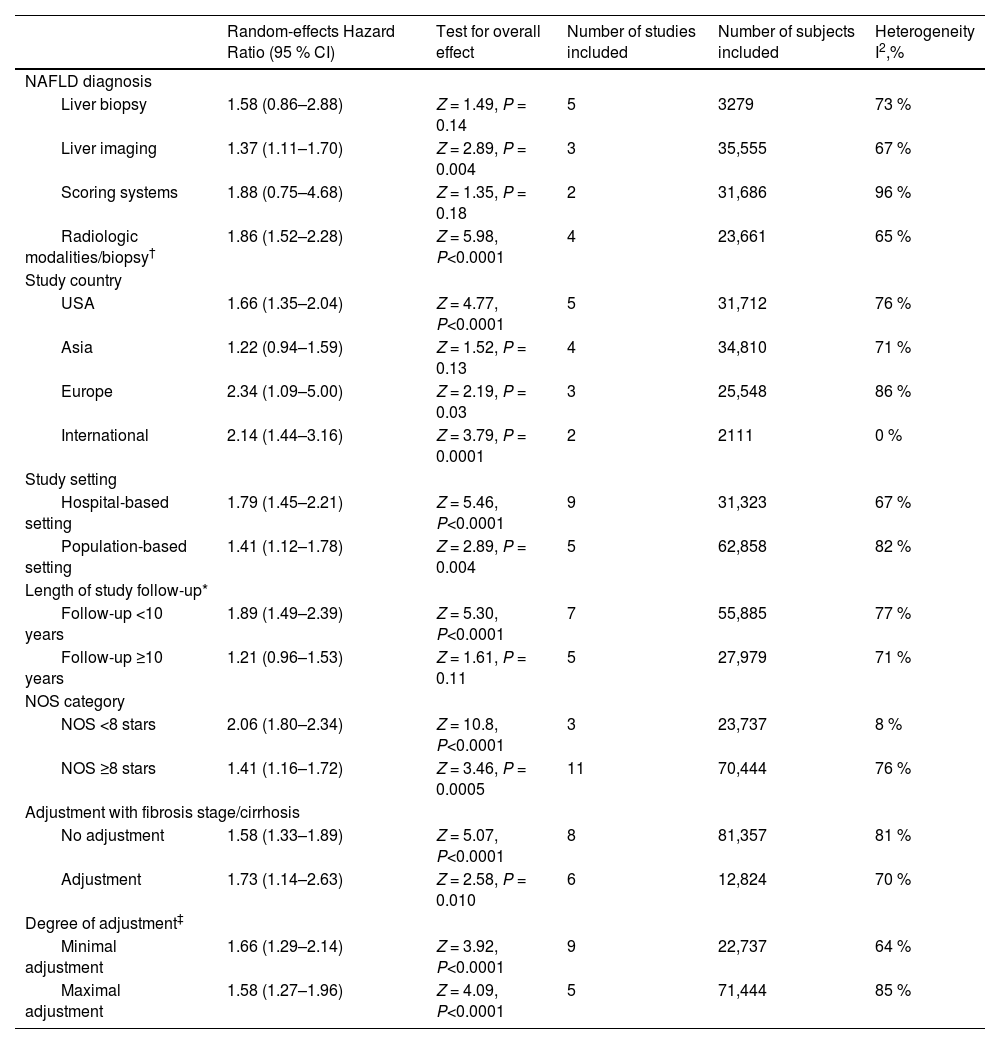
3.3Subgroup analyses, meta-regressions, and sensitivity analysesWe performed subgroup analyses to explore the possible sources of heterogeneity across the eligible studies (Table 2). The significant association between lean body mass and higher all-cause mortality risks in NAFLD subjects remained consistent across all the subgroups. In particular, the pooled random-effects HRs were comparable after stratification by the modality of NAFLD diagnosis, study country, types of cohort setting, length of follow-up, NOS quality scale, fibrosis stage/cirrhosis adjustment or degree of covariate adjustment.
Subgroup analyses for associations between lean NAFLD and risk of all-cause mortality.
| Random-effects Hazard Ratio (95 % CI) | Test for overall effect | Number of studies included | Number of subjects included | Heterogeneity I2,% | |
|---|---|---|---|---|---|
| NAFLD diagnosis | |||||
| Liver biopsy | 1.58 (0.86–2.88) | Z = 1.49, P = 0.14 | 5 | 3279 | 73 % |
| Liver imaging | 1.37 (1.11–1.70) | Z = 2.89, P = 0.004 | 3 | 35,555 | 67 % |
| Scoring systems | 1.88 (0.75–4.68) | Z = 1.35, P = 0.18 | 2 | 31,686 | 96 % |
| Radiologic modalities/biopsy† | 1.86 (1.52–2.28) | Z = 5.98, P<0.0001 | 4 | 23,661 | 65 % |
| Study country | |||||
| USA | 1.66 (1.35–2.04) | Z = 4.77, P<0.0001 | 5 | 31,712 | 76 % |
| Asia | 1.22 (0.94–1.59) | Z = 1.52, P = 0.13 | 4 | 34,810 | 71 % |
| Europe | 2.34 (1.09–5.00) | Z = 2.19, P = 0.03 | 3 | 25,548 | 86 % |
| International | 2.14 (1.44–3.16) | Z = 3.79, P = 0.0001 | 2 | 2111 | 0 % |
| Study setting | |||||
| Hospital-based setting | 1.79 (1.45–2.21) | Z = 5.46, P<0.0001 | 9 | 31,323 | 67 % |
| Population-based setting | 1.41 (1.12–1.78) | Z = 2.89, P = 0.004 | 5 | 62,858 | 82 % |
| Length of study follow-up* | |||||
| Follow-up <10 years | 1.89 (1.49–2.39) | Z = 5.30, P<0.0001 | 7 | 55,885 | 77 % |
| Follow-up ≥10 years | 1.21 (0.96–1.53) | Z = 1.61, P = 0.11 | 5 | 27,979 | 71 % |
| NOS category | |||||
| NOS <8 stars | 2.06 (1.80–2.34) | Z = 10.8, P<0.0001 | 3 | 23,737 | 8 % |
| NOS ≥8 stars | 1.41 (1.16–1.72) | Z = 3.46, P = 0.0005 | 11 | 70,444 | 76 % |
| Adjustment with fibrosis stage/cirrhosis | |||||
| No adjustment | 1.58 (1.33–1.89) | Z = 5.07, P<0.0001 | 8 | 81,357 | 81 % |
| Adjustment | 1.73 (1.14–2.63) | Z = 2.58, P = 0.010 | 6 | 12,824 | 70 % |
| Degree of adjustment‡ | |||||
| Minimal adjustment | 1.66 (1.29–2.14) | Z = 3.92, P<0.0001 | 9 | 22,737 | 64 % |
| Maximal adjustment | 1.58 (1.27–1.96) | Z = 4.09, P<0.0001 | 5 | 71,444 | 85 % |
NAFLD, non-alcoholic fatty liver disease; NOS, Newcastle-Ottawa Scale.
The diagnosis of NAFLD was established using either radiological modalities or liver biopsy in each of the individual studies.
Maximal covariate adjustment included studies that adjusted the results for fewer traditional cardiovascular disease risk factors: age, sex, smoking history, diabetes, hypertension, and dyslipidemia (or plasma lipid profile). Minimal covariate adjustment included all other eligible studies that have adjusted the results for a lower number of traditional cardiovascular disease risk factors than those listed above.
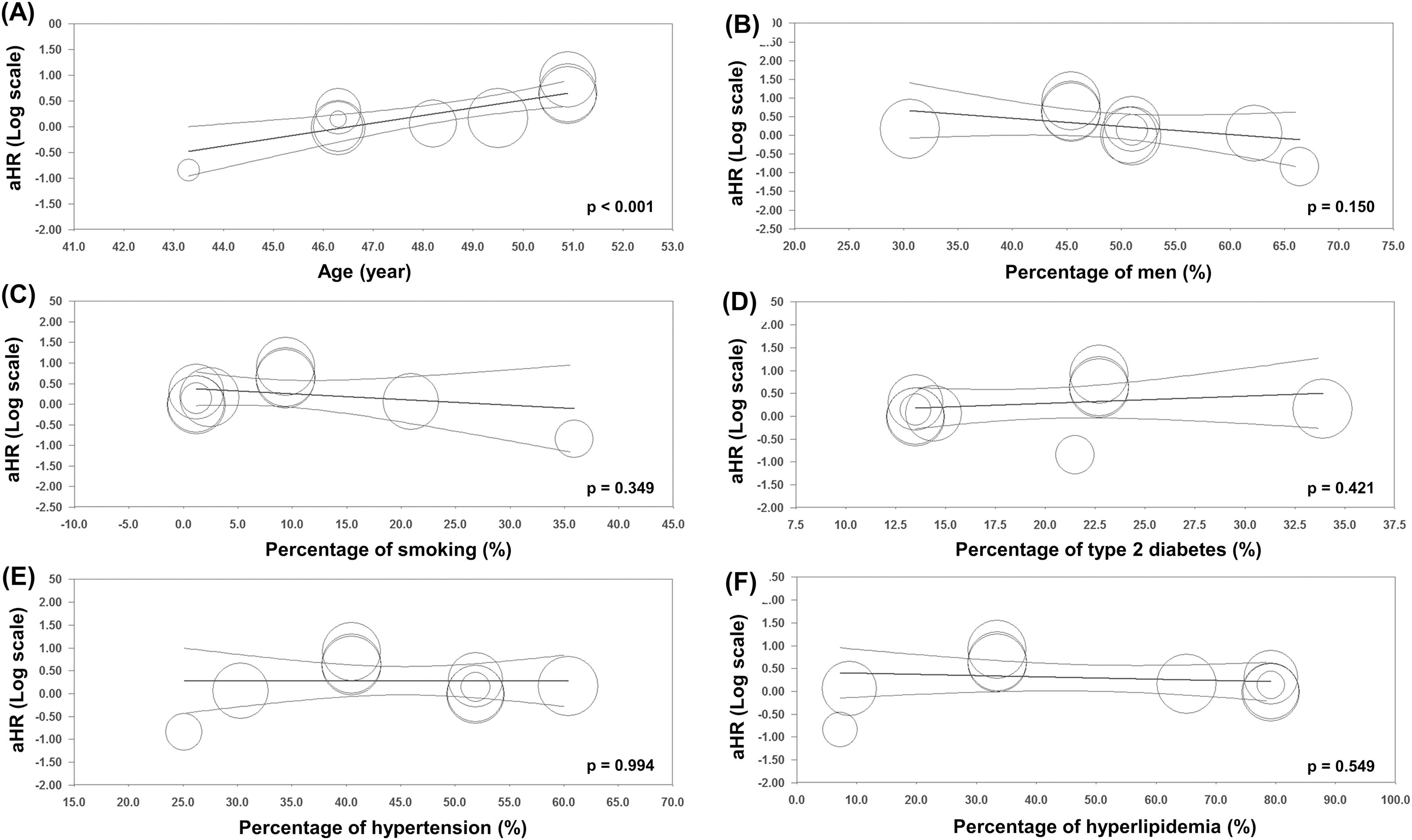
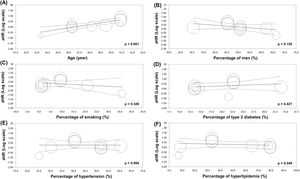
As shown in Fig. 4, the results of univariable meta-regression analyses to examine the effect of potential moderator variables showed a significant positive association between mean age and the risk of all-cause mortality (panel A). There were no significant effects of the proportion of male patients and smoking status, and the percentage of pre-existing type 2 diabetes mellitus, hypertension, and hyperlipidemia on the association between lean subjects and the risk of all-cause mortality in the population with NAFLD.
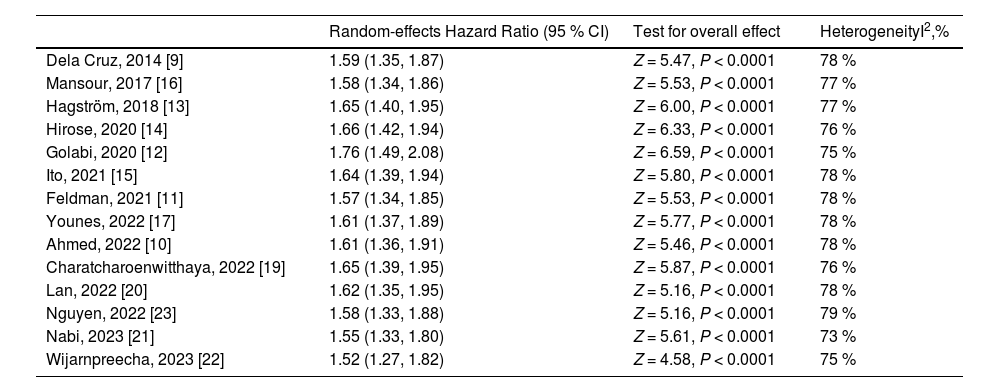
In sensitivity analyses, we found that the increased risk of all-cause mortality in lean NAFLD remained statistically significant when each study was removed from analysis at a time with the range of pooled HRs from 1.52 to 1.76, indicating no significant effect of any individual study on the overall risk of all-cause mortality (Table 3).
Sensitivity analyses for the association between lean NAFLD and risk of all-cause mortality.
| Random-effects Hazard Ratio (95 % CI) | Test for overall effect | HeterogeneityI2,% | |
|---|---|---|---|
| Dela Cruz, 2014 [9] | 1.59 (1.35, 1.87) | Z = 5.47, P < 0.0001 | 78 % |
| Mansour, 2017 [16] | 1.58 (1.34, 1.86) | Z = 5.53, P < 0.0001 | 77 % |
| Hagström, 2018 [13] | 1.65 (1.40, 1.95) | Z = 6.00, P < 0.0001 | 77 % |
| Hirose, 2020 [14] | 1.66 (1.42, 1.94) | Z = 6.33, P < 0.0001 | 76 % |
| Golabi, 2020 [12] | 1.76 (1.49, 2.08) | Z = 6.59, P < 0.0001 | 75 % |
| Ito, 2021 [15] | 1.64 (1.39, 1.94) | Z = 5.80, P < 0.0001 | 78 % |
| Feldman, 2021 [11] | 1.57 (1.34, 1.85) | Z = 5.53, P < 0.0001 | 78 % |
| Younes, 2022 [17] | 1.61 (1.37, 1.89) | Z = 5.77, P < 0.0001 | 78 % |
| Ahmed, 2022 [10] | 1.61 (1.36, 1.91) | Z = 5.46, P < 0.0001 | 78 % |
| Charatcharoenwitthaya, 2022 [19] | 1.65 (1.39, 1.95) | Z = 5.87, P < 0.0001 | 76 % |
| Lan, 2022 [20] | 1.62 (1.35, 1.95) | Z = 5.16, P < 0.0001 | 78 % |
| Nguyen, 2022 [23] | 1.58 (1.33, 1.88) | Z = 5.16, P < 0.0001 | 79 % |
| Nabi, 2023 [21] | 1.55 (1.33, 1.80) | Z = 5.61, P < 0.0001 | 73 % |
| Wijarnpreecha, 2023 [22] | 1.52 (1.27, 1.82) | Z = 4.58, P < 0.0001 | 75 % |
As shown in Supplementary Figure 1, visual inspection of funnel plot and Egger's test did not show evidence of publication bias in the overall analysis for the association between lean NAFLD and risk of all-cause mortality (P = 0.682).
4DiscussionThis updated meta-analysis of 14 observational studies reporting adjusted HRs (involving a total of 94,181 patients with NAFLD from different countries with 7443 incident cases of fatal events over a median follow-up of 8.4 years) revealed that lean NAFLD was associated with a ∼1.6-fold increased long-term risk of all-cause mortality (a pooled random-effects HR of 1.61, 95 % CI 1.37 to 1.89; test for overall effect Z = 5.84, P<0.0001; I2 = 77 %). The magnitude of this risk remained significant in those studies where analyses were adjusted for age, sex, smoking, and common cardiometabolic risk factors.
The findings of our meta-analysis with pooled estimates from adjusted HRs revealed significantly higher mortality in patients with lean NAFLD than those with non-lean NAFLD. These results differed from a previous meta-analysis of a pooled unadjusted relative risk (RR), which found a comparable risk of death between lean NAFLD and non-lean NAFLD (RR 1.09, 95 % CI 0.67–1.77; I2 = 97.7 %) [18]. The pooled HR is more relevant than the pooled RR when incorporating summary time-to-event data into meta-analysis. Because HR considers the number and timing of events and the time until the last follow-up for each patient who has not experienced an event, whereas RR only considers the number of events and disregards the timing [29]. The use of such dichotomous measures in a previous meta-analysis of time-to-event outcomes can lead some biases. When the total number of events reported for each cohort study with variable follow-up is used to calculate and combine RRs, this risk estimate is unreliable and difficult to interpret. Therefore, four cohort studies previously included in a meta-analysis by Ha et al. were excluded from our updated meta-analysis because they did not provide statistical information to estimate the HR [7,30-32]. Of note, our systematic review included five additional full-text articles [15,19-22] and three conference abstracts [9,16,23] of large cohort studies from China, Japan, Thailand, and the United States with an additional 83,699 NAFLD patients in quantitative analysis. In these studies, adjusted HRs were performed using the multivariate Cox models accounting for known risk factors for disease progression and the severity of liver fibrosis to determine the impact of lean body mass on mortality risk throughout follow-up. Therefore, our meta-analysis was more comprehensive regarding the number of eligible studies and the magnitude of a risk assessment.
The mechanisms underlying the increased mortality risk among patients with lean NAFLD remain unknown. Some evidence suggests that patients with lean NAFLD may face an elevated risk of clinically significant liver-related outcomes. A retrospective cohort study of patients with biopsy-proven NAFLD in Sweden revealed that patients with lean NAFLD are at a higher risk of developing cirrhosis, decompensated liver disease, or HCC, resulting in a higher incidence of liver-related death than their non-lean counterparts, despite having a lower prevalence of advanced fibrosis and steatohepatitis at baseline [13]. Consistent results were reported in a large community-based Chinese cohort study conducted by Lan et al., unveiling the significant association between lean NAFLD and an increased risk of liver-related mortality [20]. These observed differences in liver outcomes between lean and non-lean NAFLD patients could be attributed to differences in the genotype distribution of the patatin-like phospholipase domain-containing 3 (PNPLA3) gene among both groups. Lean NAFLD individuals are more likely to have the PNPLA3 GG genotype [33,34], which has been shown to modify the risk of NAFLD progression [35]. Furthermore, a nationwide population study in the United States reveals that the PNPLA3 rs738409 G variant is associated with increased liver-related mortality compared to the C allele [36]. Nonetheless, a retrospective cohort study from the University of Michigan Health System demonstrates that the PNPLA3 GG genotype is associated with a higher incidence of liver cirrhosis, but not all-cause mortality or non-liver outcomes, in both obese and non-obese individuals with NAFLD [22]. Further research is needed to better understand the underlying mechanisms and potential genetic factors contributing to the increased mortality risk in this patient population.
Many studies have highlighted favorable cardiometabolic profiles in individuals with lean NAFLD compared to those with non-lean NAFLD. A recent meta-analysis of 85 studies revealed that patients with lean NAFLD exhibited less metabolic dysfunction, including a lower prevalence of diabetes, lower fasting blood glucose levels, waist circumference, and blood pressure when compared to overweight or obese patients with NAFLD [37]. Our study aligns with previous reports, as we observed that lean NAFLD patients at the time of diagnosis had significantly lower rates of diabetes, dyslipidemia, and hypertension compared to their non-lean counterparts (Table 1) [9-11,13,14,17,19-22]. Since patients with lean NAFLD usually present with fewer metabolic comorbidities, it has been commonly believed that they would have a lower risk of developing CVD compared to those with higher BMI. However, a prospective cohort study of patients with NAFLD from the United States demonstrated that individuals without obesity are at a similar risk of developing metabolic disease and CVD during long-term follow-up as those with obesity [38]. This association is further supported by the longitudinal data of the general U.S. population reported by Golabi et al., showing comparable risk of CVD-related mortality between patients with lean NAFLD and non-lean NAFLD (Fig. 3A) [12]. Together, these findings suggest that although patients with lean NAFLD have a healthier cardiometabolic profile at baseline, the risk of developing CVD-related morbidity and mortality during follow-up is similar between patients with lean NAFLD and non-lean NAFLD. However, a causal relationship between lean NAFLD and cardiovascular outcomes needs to be confirmed through further prospective studies.
Our meta-analysis has several important strengths. We provide the most comprehensive and updated assessment of the prognostic relevance of lean body mass on the long-term risk of all-cause mortality among NAFLD patients. The data was incorporated from large cohort studies from Asia, Europe, and the United States. It is likely to accurately represent patients with NAFLD seen in clinical practice. Notably, the higher mortality risks associated with lean NAFLD were consistently observed across all subgroup analyses (such as references of non-lean, overweight, or obese subjects, modalities of NAFLD diagnosis, study country, cohort setting, length of follow-up, NOS quality scale, adjustment with fibrosis stage/cirrhosis, and degree of adjustment for potential confounders) and sensitivity analysis, supporting consistent effect of lean NAFLD and robustness of this evidence. Furthermore, since the results from three eligible studies were published as conference abstracts, the risk estimates for mortality risk may be inaccurate. However, when the pooled analyses were limited to 11 full-text articles, the direction of the association between lean NAFLD and mortality risk remained unchanged, suggesting a consistent effect of the disease. Finally, we believe that our comprehensive search did not miss any published reports, and visual inspection of the funnel plot and a formal statistical test revealed no evidence of any publication bias.
Our meta-analysis had some limitations. First, although we used a random-effects model, the results of this meta-analysis should be interpreted with caution, similar to a previously published meta-analysis [18], given the relatively high heterogeneity observed in a pooled analysis (I2 = 77 %, Fig. 2). We explored potential sources of statistical heterogeneity using stratified analyses, meta-regressions, and sensitivity analyses. This high heterogeneity could be attributed to differences in the demographic characteristics of study populations, the length of study follow-up, and methods used for NAFLD diagnosis. Our meta-regression analyses also showed that the association between lean NAFLD and all-cause mortality was modified by the mean age of the study population, suggesting an impact of the onset of lean NAFLD on adverse outcomes. Second, the majority of the eligible studies adjusted the outcome of interest for age, sex, type 2 diabetes, hypertension, hyperlipidemia, and other traditional CVD risk factors; however, the possibility of residual confounding by some unmeasured factors cannot be completely ruled out.
5ConclusionsThis comprehensive systematic review and updated meta-analysis provides evidence for a significant association between lean body mass and the long-term risk of all-cause mortality in the NAFLD population. Due to the lower prevalence of metabolic comorbidities in patients with lean NAFLD, the management of lean NAFLD may need to differ from conventional lifestyle modifications focused on weight loss and metabolic control. Further studies exploring potential explanations for these findings would be essential to guide the management of lean NAFLD and eventually impact patient outcomes.
Author contributionsWasit Wongtrakul and Natthinee Charatcharoenwitthaya: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Phunchai Charatcharoenwitthaya: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.