Different patterns of liver injury have been reported in association with the SARS-CoV-2 vaccines. The aim of this study was to describe a nationwide cohort of patients with SARS CoV-2 vaccine-induced liver injury, focusing on treatment and the evolution after further booster administration.
Patients and Methodsmulticentre, retrospective-prospective study, including subjects who developed abnormal liver tests within 90 days after administration of SARS-CoV-2 vaccination.
Results47 cases were collected: 17 after prime dose and 30 after booster. Age was 57 years, 30 (63.8 %) were female, and 7 (14.9 %) had a history of prior autoimmune hepatitis (AIH). Most cases were non-severe, though 9 (19.1 %) developed acute liver injury or failure (ALF). Liver injury tended to be more severe in those presenting after a booster (p=0.084). Pattern of liver injury was hepatocellular (80.9 %), mixed (12.8 %) and 3 (6.4 %) cholestatic. Liver biopsy was performed on 33 patients; 29 showed findings of AIH. Forty-one (87.2 %) patients received immunosuppressants, mostly corticosteroids (35/41). One required liver transplantation and another died due to ALF. Immunosuppression was discontinued in 6/41 patients without later rebound. Twenty-five subjects received at least one booster and 7 (28.0 %) relapsed from the liver injury, but all were non-severe. Recurrence was less frequent among patients on immunosuppressants at booster administration (28.6 % vs. 88.9 %, p=0.007).
ConclusionsSARS CoV-2 vaccine-induced liver injury is heterogeneous but mostly immune-mediated. Relapse of liver injury after re-exposure to vaccine is frequent (28.0 %) but mild. Immunosuppression at booster administration is associated with a lower risk of liver injury.
The current worldwide strategy against the Coronavirus disease 2019 (COVID-19) pandemic is based on prevention of severe infection by vaccination [1]. However, generalized vaccination against severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) has led to the description of multiple associated adverse events, ranging from self-limited pseudo-viral symptoms to severe acute myocarditis [2].
Overall, the main side effects linked to vaccines are related to the inflammatory response they trigger, either local or systemic. Nevertheless, autoimmune phenomena due to cross-reaction between auto-antigens from the host and antigenic components of the inoculum have been described in almost all vaccines [3]. With regards to the SARS CoV-2 ones, autoimmune and autoinflammatory adverse events have been widely documented, e.g., thrombotic thrombocytopenia purpura (TTP) and Guillain-Barré syndrome [4]. The underlying mechanism for these reactions is not fully understood, though the activation of directed antibodies by the SARS CoV-2 vaccines seems to play a determinant role, as in the case of the TTP [5].
Liver involvement has also been documented as a consequence of SARS-CoV-2 vaccination. A first report of a woman who developed an autoimmune hepatitis (AIH) after a prime dose of a messenger (m)RNA-SARS CoV-2 vaccine turned up in the description of a few more cases of AIH [6], both among subjects with and without underlying liver disease [7–12]. Nevertheless, later reports have shown that the spectrum of liver injury associated with both the mRNA and viral-vector vaccines is not limited to vaccine-induced AIH [13]. Hepatocellular hepatitis due to AIH appears as the most common presentation, but also immune-mediated hepatitis or drug-induced liver injury[12]. Moreover, mixed or cholestatic hepatitis has also been described in association with an immune-mediated mechanism triggered by the spike protein of the mRNA vaccines [14]. The heterogeneous presentation of vaccine-induced liver injury, as well as the need for future boosters for protection against the new SARS CoV-2 strains, hinders management decisions for these patients.
The aim of our study was to carry out a nationwide description of the different patterns of liver injury after SARS CoV-2 vaccination in Spain, in both subjects with and without previous autoimmune hepatitis, as well as the outcome in terms of severity, need for immunosuppression and rebound after re-exposition to the vaccine.
2Materials and methods2.1Study design and patientsThis was a multicentre, retrospective-prospective study involving 15 academic hospitals participating in the Spanish Registry for Autoimmune and Cholestatic Diseases (ColHai registry). The study included subjects aged over 18 years, with or without previous autoimmune hepatitis, who have received at least one dose of any of the approved SARS CoV-2 vaccines in Spain and, afterwards, presented with increased transaminases levels within the 90 days after first (prime) or successive doses (boosters) of immunization. In Spain, there was no monitoring protocol after vaccination for the general population. Consequently, most of the patients in the study were diagnosed with liver injury mainly due to the development of symptoms and, more rarely, incidentally.
Among patients with a prior diagnosis of autoimmune hepatitis, only those without changes in corticosteroids or immunosuppressant dosages within the previous three months to vaccination were included.
The available vaccines in Spain at the time of the study included those m-RNA-based (Pfizer/BioNTech and Moderna), and viral vector (Oxford-AstraZeneca and Johnson & Johnson). Five of the cases included in the study have been recently published [15].
2.2MethodsDemographic and clinical variables were recorded at the time of the liver injury and included gender, age, cardiovascular history, previous diagnosis of liver disease or other autoimmune disorders. In subjects with previous diagnosis of autoimmune hepatitis, date of diagnosis and treatment records were collected. Data on the consumption of drugs or herbal products were registered, as well as whether there was a previous diagnosis of COVID-19, its date and severity. Data on SARS-CoV-2 vaccine consisted of type, number of doses (prime or booster), date of administration, presence of adverse effects and time to liver injury.
Laboratory parameters consisted of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), alkaline phosphatase (AP), total and direct bilirubin, total proteins, international normalized ratio (INR), gammaglobulin, and immunoglobulin (IgG, IgM, IgA) levels.
In all cases included, a systematic work-up for ruling out acute viral hepatitis was performed: anti-HAV, HBsAg, anti-HBc IgM, anti-HCV, anti-HEV IgM, anti-CMV and anti-EBV IgM plus/minus and nucleic acid amplification testing.
Autoantibodies evaluation included antinuclear (ANA), anti-smooth muscle (SMA), anti-mitochondrial (AMA), anti-liver-kidney microsomal (LKM) and anti-neutrophil cytoplasmic antibodies (ANCA). ANAs titter ≥ 1/80 were considered as significant. Histological findings were recorded if available. The simplified criteria for the diagnosis of AIH were applied to patients without previous history of AIH [16]. Causal relationship between vaccination and liver injury was assessed by the Roussel Uclaf Causality Assessment Method (RUCAM) [17]. Liver injury severity was established according to the EASL criteria as acute liver injury-ALI (liver dysfunction defined as INR>1.5) or acute liver failure (ALF) for those with both coagulopathy and encephalopathy [18].
Prospective data included the need and type of immunosuppressant therapy, biochemical follow-up (normalization), clinical and analytical evolution after booster vaccines and after withdrawal of immunosuppression.
2.3Statistical analysisQuantitative variables were expressed as median and interquartile range (IQR) or range and analysed with the Mann-Whitney U or the Student t test, as appropriate. Categorical variables were expressed as frequency and percentage and compared using the Chi-squared or Fisher's exact test when frequencies were less than 5 %. Data of patients were contrasted according to previous history of AIH (AIH vs. de novo AIH), prime vs. booster dose and type of SARS CoV-2 vaccination (mRNA vs. viral-vector). Results were considered statistically significant when the p-value was <0.05. All statistical analyses were performed using IBM SPSS, version 26.0 (SPSS Inc, Armonk, NY, USA).
2.4TheoryAlthough, a recently published systematic review has indicated that vaccination against COVID-19 is associated with a higher rate of vaccine-associated liver injury than immunization against other viruses, such as influenza [19], the need for periodical boosters for general population arises concern about the incidence and prognosis of patients with a history of prior SARS CoV-2 vaccine-associated liver injury (SVALI).
2.5Ethical statementsThe study was approved by the Spanish Agency of Medicines and Medical Devices (AEMPS) and the Gregorio Marañón Hospital Ethics Committee (VAC-COVID19_COLHai, report 19/2021) and was conducted in compliance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements. Informed consent forms were provided to participating subjects, and all data were anonymized.
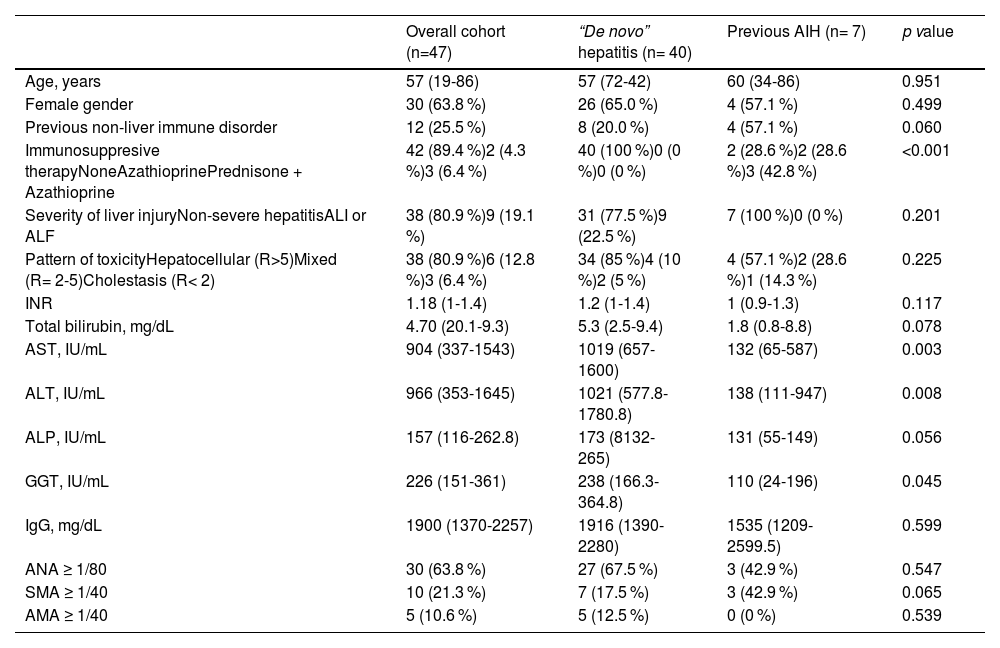
3Results3.1Characteristics of patients at the time of the SARS CoV-2 vaccine-associated liver injuryFrom February/2021 to February/2022, 47 cases of SVALI were recorded at 15 Spanish academic hospitals. Main characteristics of patients are summarized in Table 1. Briefly, 30 (63.8 %) were female, median age was 57 years and 12 (25.5 %) had a prior non-liver autoimmune disorder. Furthermore, 7 (14.9 %) patients had a previous diagnosis of autoimmune hepatitis (AIH) and, therefore, the remaining 40 (85.1 %) cases were considered as probably “de novo” hepatitis. Four (8.5 %) patients had COVID-19 infection prior to vaccination. All these COVID-19 infections were mild and without development of respiratory distress or need for hospital admission.
Characteristics of patients at the time of the SVALI and comparison according to the prior history of AIH.
| Overall cohort (n=47) | “De novo” hepatitis (n= 40) | Previous AIH (n= 7) | p value | |
|---|---|---|---|---|
| Age, years | 57 (19-86) | 57 (72-42) | 60 (34-86) | 0.951 |
| Female gender | 30 (63.8 %) | 26 (65.0 %) | 4 (57.1 %) | 0.499 |
| Previous non-liver immune disorder | 12 (25.5 %) | 8 (20.0 %) | 4 (57.1 %) | 0.060 |
| Immunosuppresive therapyNoneAzathioprinePrednisone + Azathioprine | 42 (89.4 %)2 (4.3 %)3 (6.4 %) | 40 (100 %)0 (0 %)0 (0 %) | 2 (28.6 %)2 (28.6 %)3 (42.8 %) | <0.001 |
| Severity of liver injuryNon-severe hepatitisALI or ALF | 38 (80.9 %)9 (19.1 %) | 31 (77.5 %)9 (22.5 %) | 7 (100 %)0 (0 %) | 0.201 |
| Pattern of toxicityHepatocellular (R>5)Mixed (R= 2-5)Cholestasis (R< 2) | 38 (80.9 %)6 (12.8 %)3 (6.4 %) | 34 (85 %)4 (10 %)2 (5 %) | 4 (57.1 %)2 (28.6 %)1 (14.3 %) | 0.225 |
| INR | 1.18 (1-1.4) | 1.2 (1-1.4) | 1 (0.9-1.3) | 0.117 |
| Total bilirubin, mg/dL | 4.70 (20.1-9.3) | 5.3 (2.5-9.4) | 1.8 (0.8-8.8) | 0.078 |
| AST, IU/mL | 904 (337-1543) | 1019 (657-1600) | 132 (65-587) | 0.003 |
| ALT, IU/mL | 966 (353-1645) | 1021 (577.8-1780.8) | 138 (111-947) | 0.008 |
| ALP, IU/mL | 157 (116-262.8) | 173 (8132-265) | 131 (55-149) | 0.056 |
| GGT, IU/mL | 226 (151-361) | 238 (166.3-364.8) | 110 (24-196) | 0.045 |
| IgG, mg/dL | 1900 (1370-2257) | 1916 (1390-2280) | 1535 (1209-2599.5) | 0.599 |
| ANA ≥ 1/80 | 30 (63.8 %) | 27 (67.5 %) | 3 (42.9 %) | 0.547 |
| SMA ≥ 1/40 | 10 (21.3 %) | 7 (17.5 %) | 3 (42.9 %) | 0.065 |
| AMA ≥ 1/40 | 5 (10.6 %) | 5 (12.5 %) | 0 (0 %) | 0.539 |
Factors were expressed as n (%) and median (interquartile range).
AIH, autoimmune hepatitis; ALI, acute liver injury; ALF, acute liver failure; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibodies; ANA, antinuclear antibodies; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; Ig, immunoglobulin; INR, international normalized ratio; SMA, anti-smooth muscle antibodies; SVALI, SARS CoV-2 vaccine-associated liver injury.
Overall, 17 (36.2 %) cases of SVALI were reported after the prime dose of vaccine against SARS CoV-2. Of the remaining cases, 22 (46.8 %) occurred after the first booster dose and 8 (17.0 %) after the second booster. Most cases were associated with mRNA vaccines (76.6 %). Among the 17 patients developing SVALI after the first dose, 9 (52.9 %) had received a mRNA vaccine (6 Pfizer, 3 Moderna) and 8 (47.1 %) a viral-vector (6 Oxford-AstraZeneca, 3 Johnson & Johnson). However, the majority of subjects with liver injury after a booster had been exposed to a mRNA vaccine (booster 90 % vs. prime 52.9 %, p=0.006), mainly Pfizer (18/30).
Median time from the last vaccine dose to diagnosis of liver injury was 22 (IQR 11-41) days. Twelve patients (25 %) reported adverse events (AEs) related to the vaccine dose. Most frequently reported AEs were asthenia, dyspepsia and febricula. There were no significant differences in the rate and severity of the AEs between the type of vaccine.
At the time of the liver injury, 38 (80.9 %) patients reported any symptoms. Most frequently described symptoms were jaundice (42.1 %), asthenia (34.2 %), abdominal pain (28.9 %) and nausea (13.1 %). The remaining 9 (19.1 %) patients were asymptomatic and, thus, the diagnosis was considered incidental.
Thirty-eight (80.9 %) patients presented a non-severe acute hepatitis, whereas 6 (12.8 %) presented with ALI, and 3 (6.4 %) with ALF. Liver injury tended to be more severe in those presenting after a booster dose, with a higher percentage of patients with ALI or ALF (26.7 % vs. 5.9 %, p=0.084). However, no differences in severity were observed regarding the type of vaccine despite the fact that the 3 cases of ALF were associated with an mRNA vaccine.
The most frequent pattern of liver injury was hepatocellular (38, 80.9 %), followed by mixed (6, 12.8 %) and cholestatic in only 3 (6.4 %) cases. Type of liver injury was similar regardless of the dose (prime vs. booster) or type of SARS CoV-2 vaccine.
Analytical data at the time of the SVALI is summarized in Table 1. Briefly, median AST and ALT values were roughly 1000 IU/mL, total bilirubin 4.7 mg/dL and IgG 1900 mg/dL, with 30 (63.8 %) presenting with IgG above upper limit of normality.
Thirty-two patients (68.1 %) had any positive autoantibody, with ANAs as the most commonly observed (63.8 %). It should be highlighted that only 1 out of the five subjects with positive AMA presented a cholestatic pattern.
A liver biopsy was performed in 33 patients, and all but 4 (87.9 %) showed histological findings compatible with AIH. Three (9.1 %) cases had features of drug-induced liver injury, with relevant eosinophilic infiltrate, with one of them informed as probable immune-allergenic origin. The remaining case was reported as nonspecific. Considering only “de novo” AIH patients with available liver biopsy, simplified AIH Score showed at least probable AIH (≥6 points) in 23 out of 33 patients (69.7 %).
The RUCAM score suggested an at least possible association between liver injury and SARS CoV-2 vaccination in 38 (80.9 %) cases and probable in 20 (42.6 %).
3.2Liver injury in patients with underlying autoimmune hepatitisSeven patients had a history of AIH, and five (71.4 %) of them were still undergoing immunosuppression at the time of the liver injury. As summarized in Table 1, patients with prior AIH more commonly have history of autoimmune disorders (p=0.06), whereas symptoms at the diagnosis of liver injury were more frequently reported by those with de novo hepatitis (p=0.018).
No differences were observed in terms of severity, although no cases of severe hepatitis were observed among those with prior AIH, in comparison with 22.5 % among patients with de novo hepatitis. Transaminases at SVALI were higher among subjects with de novo hepatitis (AST, p=0.003; ALT, p=0.008), whereas the presence of SMA tended to be more frequent among those with prior AIH (p=0.065). In no case of SVALI with underlying AIH was a liver biopsy carried out.
3.3Treatment of liver injury and outcome after withdrawal of immunosuppressionForty-one patients (87.2 %) received specific treatment based on immunosuppressants, including the 7 cases with history of underlying AIH. One of the 3 cases of SVALI-induced ALF required liver transplantation with favourable outcome; another died as a consequence of the ALF, and the remaining presented a good outcome after corticosteroid pulses.
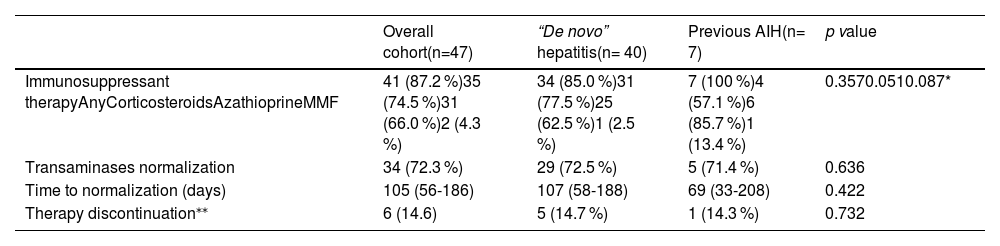
Thirty-four (72.3 %) subjects had completely normalized transaminase values within a median time of 109 (IQR 56-195) days from the date of the SVALI. Although the number of subjects who did not receive any immunosuppression was limited (n=6), no differences were observed in the rate of transaminase normalization (78.4 % vs. 66.7 %, p=0.429) or the time to this achievement (159 vs. 105, p=0.521) when contrasted with subjects who did receive immunosuppression.
Regarding patients with underlying AIH, there were no differences either in the rate or the time to transaminase normalization when compared to subjects with de novo hepatitis, as summarized in Table 2.
Outcome of the overall cohort and according to the prior history of AIH.
| Overall cohort(n=47) | “De novo” hepatitis(n= 40) | Previous AIH(n= 7) | p value | |
|---|---|---|---|---|
| Immunosuppressant therapyAnyCorticosteroidsAzathioprineMMF | 41 (87.2 %)35 (74.5 %)31 (66.0 %)2 (4.3 %) | 34 (85.0 %)31 (77.5 %)25 (62.5 %)1 (2.5 %) | 7 (100 %)4 (57.1 %)6 (85.7 %)1 (13.4 %) | 0.3570.0510.087* |
| Transaminases normalization | 34 (72.3 %) | 29 (72.5 %) | 5 (71.4 %) | 0.636 |
| Time to normalization (days) | 105 (56-186) | 107 (58-188) | 69 (33-208) | 0.422 |
| Therapy discontinuation⁎⁎ | 6 (14.6) | 5 (14.7 %) | 1 (14.3 %) | 0.732 |
Factors were expressed as n (%) and median (interquartile range).
AIH, autoimmune hepatitis; MMF, mycophenolate mofetil; SVALI, SARS CoV-2 vaccine-associated liver injury.
During follow-up, immunosuppression was maintained in the majority of patients (35 out of 41 initially treated), including 6 out of 7 with prior AIH and 29 out of 34 with probably de novo hepatitis. No later rebound was observed in the six patients who discontinued immunosuppression, despite the fact that 3 of them presented findings compatible with AIH at liver biopsy.
3.4Outcome after re-exposition to SARS CoV-2 vaccinationTwenty-five subjects received at least a booster of any SARS CoV-2 vaccine after recovering from the episode of SVALI. Of these, 17 received one booster and 8 two booster doses.
Vaccination rechallenge was more frequent among those who had developed the SVALI after the prime immunization (70.6 % vs. 43.3 %, p=0.067) or those who presented a non-severe hepatitis (63.2 % vs. 11.1 %, p=0.006). In fact, only one out of the nine subjects with prior ALI or ALF-induced SVALI received a booster. Moreover, only 2 (28.6 %) subjects with prior AIH were rechallenged in comparison with 57.5 % subjects with de novo hepatitis.
Of the 25 subjects who were re-exposed to vaccination, 22 had received immunosuppressant therapy at the time of the SVALI and 18 were still on therapy at the time of revaccination.
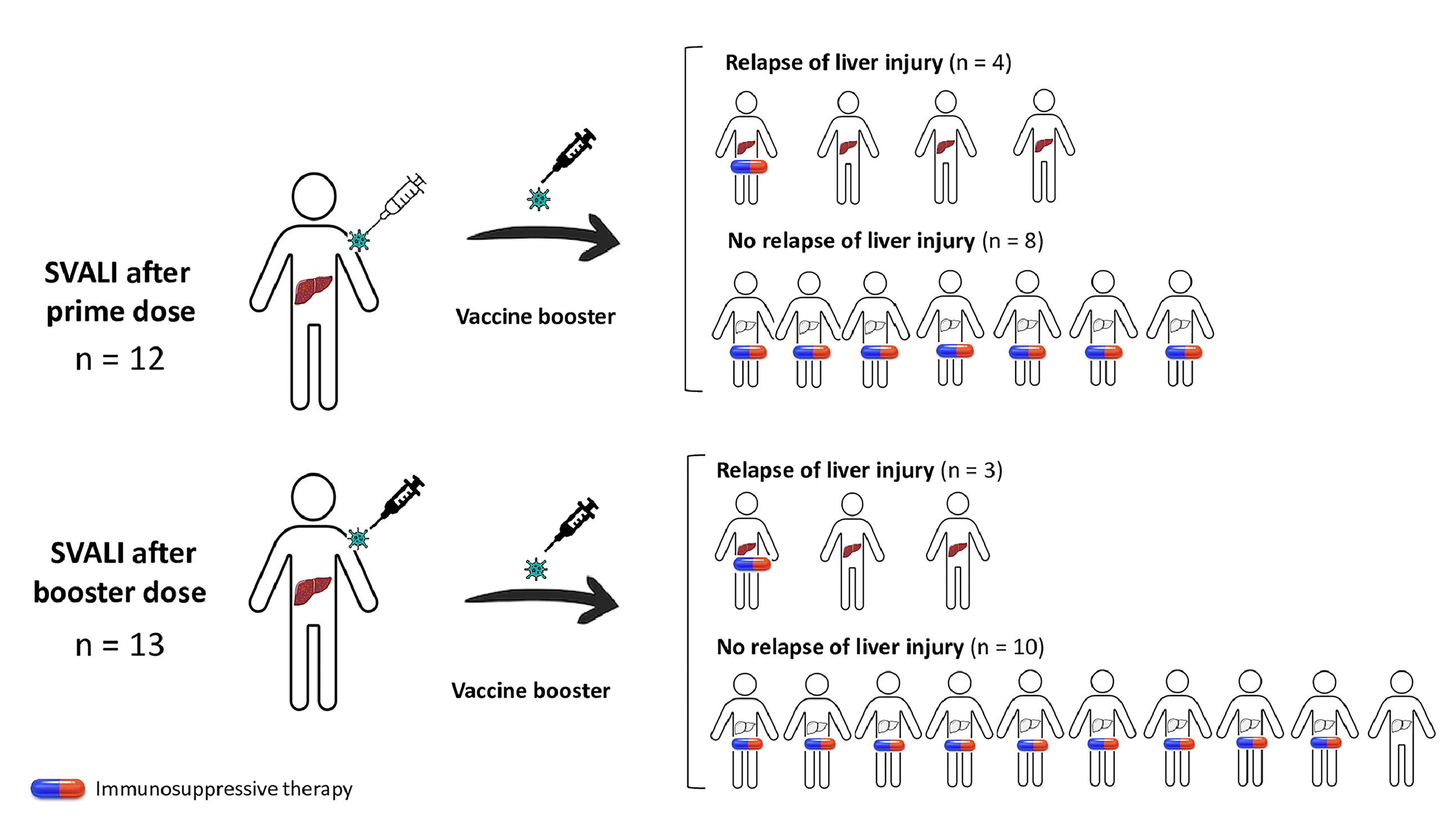
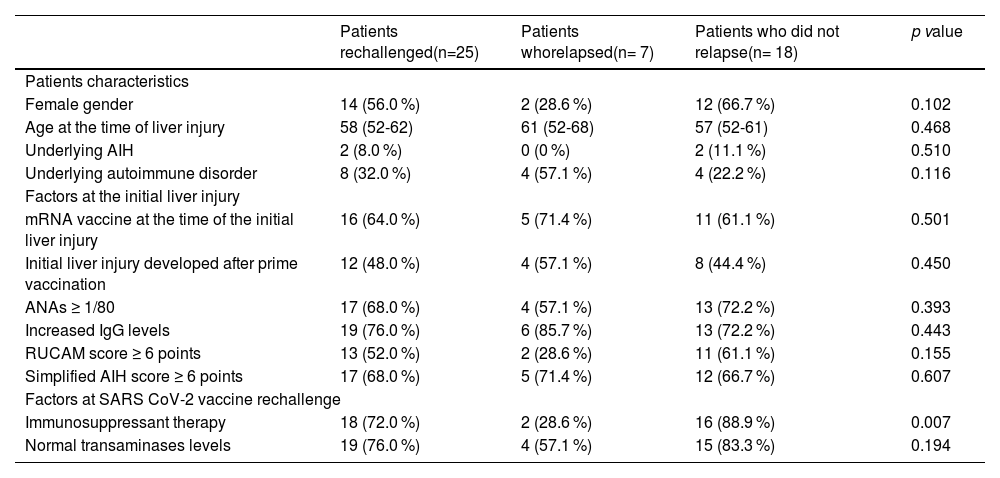
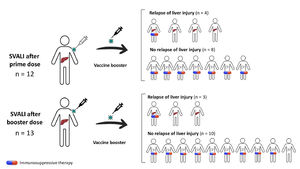
On the whole, 7 (28.0 %) relapsed from the liver injury when re-exposed to a new dose of vaccine, and all cases were mild. Factors associated with the risk of relapse of SVALI are summarized in Table 3. Males and individuals with prior autoimmune disorders were more prone to develop a flare of the liver injury (p=0.102 and p=0.116, respectively). However, the rate of relapse was higher among those who were not undergoing immunosuppressants at the time of the booster administration (p=0.007), as shown in Fig. 1.
Factors associated with relapse of SVALI among patients retreated with a SARS CoV-2 vaccine.
| Patients rechallenged(n=25) | Patients whorelapsed(n= 7) | Patients who did not relapse(n= 18) | p value | |
|---|---|---|---|---|
| Patients characteristics | ||||
| Female gender | 14 (56.0 %) | 2 (28.6 %) | 12 (66.7 %) | 0.102 |
| Age at the time of liver injury | 58 (52-62) | 61 (52-68) | 57 (52-61) | 0.468 |
| Underlying AIH | 2 (8.0 %) | 0 (0 %) | 2 (11.1 %) | 0.510 |
| Underlying autoimmune disorder | 8 (32.0 %) | 4 (57.1 %) | 4 (22.2 %) | 0.116 |
| Factors at the initial liver injury | ||||
| mRNA vaccine at the time of the initial liver injury | 16 (64.0 %) | 5 (71.4 %) | 11 (61.1 %) | 0.501 |
| Initial liver injury developed after prime vaccination | 12 (48.0 %) | 4 (57.1 %) | 8 (44.4 %) | 0.450 |
| ANAs ≥ 1/80 | 17 (68.0 %) | 4 (57.1 %) | 13 (72.2 %) | 0.393 |
| Increased IgG levels | 19 (76.0 %) | 6 (85.7 %) | 13 (72.2 %) | 0.443 |
| RUCAM score ≥ 6 points | 13 (52.0 %) | 2 (28.6 %) | 11 (61.1 %) | 0.155 |
| Simplified AIH score ≥ 6 points | 17 (68.0 %) | 5 (71.4 %) | 12 (66.7 %) | 0.607 |
| Factors at SARS CoV-2 vaccine rechallenge | ||||
| Immunosuppressant therapy | 18 (72.0 %) | 2 (28.6 %) | 16 (88.9 %) | 0.007 |
| Normal transaminases levels | 19 (76.0 %) | 4 (57.1 %) | 15 (83.3 %) | 0.194 |
Factors were expressed as n (%).
AIH, autoimmune hepatitis; ANA, antinuclear antibodies; IgG, immunoglobulin G; (m)RNA, messenger RNA; RUCAM, Roussel Uclaf causality assessment method; SARS CoV-2, severe acute respiratory syndrome coronavirus-2.
Rate of relapse of SARS CoV-2 vaccine-induced liver injury after rechallenge and impact of concomitant immunosuppression. This diagram shows patients with initial SVALI after prime (n=12) or booster dose (n=13), and the occurrence of relapse according to the presence or absence of concomitant immunosuppressant therapy. Overall, the risk of relapse was statistically lower in those undergoing immunosuppression at the time of vaccine rechallenge (28.6 % vs. 88.9 %, p=0.007). SVALI, SARS CoV-2 vaccine-induced liver injury.
This nationwide multicenter study describes the characteristics of 47 patients presented with liver injury after administration of a SARS CoV-2 vaccine and its outcome, including the evolution after consecutive booster doses.
In our cohort, SVALI occurred most frequently after the administration of mRNA vaccines, mainly Pfizer, with data consistent with those reported in a recent international study [13]. Hitherto, Official Spanish government data show that almost 95,000,000 doses of coronavirus vaccine have been administered in Spain, suggesting that the incidence of liver injury following COVID-19 immunization is a rare event [20]. However, the fact that SVALI among patients with prior AIH was asymptomatic and, as well, some of the de novo hepatitis, implies that the number of cases may be greater than reported.
We did not observe differences in terms of severity according to the type of vaccine, though the 3 cases of ALF were associated with the prior administration of an mRNA vaccine. To note, one of these three patients required liver transplantation, with this case as one of the few reported linked to SARS CoV-2 vaccination up to now [21,22]. However, it should be highlighted that liver injury developed after boosters tended to be more severe than those reported after prime doses.
Analytical and histological patterns in our cohort are in line with those observed in two previous cohorts published so far [12,13], with hepatocellular patterns and findings compatible with AIH at the liver biopsy as the most commonly reported.
Yet the rate of patients from our cohort who were treated with corticosteroids or other immunosuppressant drugs was higher than previously stated [13]. This may be partially explained by the greater number of cases with transaminases 10-fold normality and also the fact that the majority of patients with liver biopsy presented with findings of AIH. In our cohort, up to 87 % of subjects received corticosteroids and 82.5 % received concomitant azathioprine or mycophenolate. Moreover, in 35 out of 41 initially treated patients, immunosuppression was maintained after resolution of the SVALI. The percentage of therapy discontinuation is lower than that reported for an international cohort [13], a fact that may be once again, caused by the large number of cases in our cohort with previous AIH or with AIH findings at the biopsy at diagnosis of SVALI.
The most interesting data from our study is the potential protective role of immunosuppression at the time of exposure to the SARS CoV-2 vaccine. In general, the role of immunosuppression in association with the COVID-19 pandemic has been variable, with a protective role against severe infection and inflammatory-cytokine storm linked to acute infection, but also being deleterious by lowering the response to vaccination. Recently, therapy with ursodeoxycholic acid has been associated with milder SARS-CoV-2 infection among patients with liver cirrhosis [23], but literature is scarce regarding the prevention of potential toxicity induced by COVID-19 vaccination. Our study reports, for the first time, a possible benefit of preventing relapse of liver injury in case of vaccination rechallenge.
Our study has some limitations. Although it encompasses most hospitals in Spain, some cases of SVALI may be missed since, in our country, there is no systematic research on side effects linked to vaccination. Some of the results concerning the type of vaccines might be associated with their availability throughout the -Cov-2 pandemic, such as the higher exposure to mRNA immunization at booster doses. Also, in this line, the fact that the majority of subjects who presented with ALI or ALF had received mRNA vaccines may be more related to the booster dose than to the type of immunization itself. The decision to administer further doses of vaccination after the SVALI episode was at the discretion of patients and the treating physician, and therefore, rechallenge was very limited in some subgroups of patients, such as prior SVALI-induced ALI or ALF or patients with underlying AIH.
Nevertheless, our study brings to light interesting data, highlighting, on the one hand, the heterogeneous characteristics of liver injury induced by SARS CoV-2vaccination, and on the other hand, the tendency to a greater severity in those developing after booster doses, a fact that raises the question about the need for immunosuppression prior to new boosters for patients with history of AIH or prior vaccine-induced liver injury, especially in light of the clearly lower risk of relapse in those undergoing immunosuppressants.
5ConclusionsIn summary, herein we report the Spanish nationwide cohort of SARS CoV-2 vaccine-induced liver injury, confirming the heterogeneous characteristics of these patients and showing novel and relevant data on the higher risk of relapse of liver injury in patients who are not undergoing immunosuppression at the time of the booster exposure.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
English writing support was provided by Fidelma Greaves.