Primary biliary cholangitis (PBC) is a rare autoimmune and chronic cholestatic liver disease affecting the small intrahepatic bile ducts, mainly the interlobular bile ducts [1]. In patients with PBC, the 10-year cumulative incidence of clinically significant portal hypertension (CSPH) is estimated to be approximately 40 % [2]. Moreover, among patients with chronic liver disease, the presence of CSPH is associated with an increased risk of hepatic decompensation and mortality [3,4]. The long-term prognosis may remain poor for PBC patients with CSPH despite a biochemical response to ursodeoxycholic acid treatment [5,6].
Unlike other chronic liver diseases, such as viral hepatitis, portal hypertension (pH) occurs in patients with PBC in the early stage without cirrhosis. Approximately 34 % of patients with pre-cirrhotic PBC had high-risk pH in Warnes et al.’s study [7]. An expert panel recommends the risk stratification method for PBC management, including age of onset, male sex, liver biochemistry, response to ursodeoxycholic acid therapy, and fibrosis stage [8]; however, no formal risk evaluation exists for pH. Some patients had pH complications as their first PBC manifestations, including ascites and bleeding from gastroesophageal varices [9,10]. Current guidelines do not recommend routine screening for pH in patients primarily diagnosed with PBC [11,12]; its incidence of pH in this population has been underestimated, increasing the risk of pH-related deaths.
A liver biopsy is not necessarily required for the diagnosis of PBC [1]. However, histopathological changes have essential predictive values for long-term prognosis of PBC [13]. Only a few studies reported the histopathologic features of PBC with comorbid pH, especially in the early histologic stage. Previous studies were limited to small samples or case reports [9,14,15]. A study of mere 12 patients demonstrated that 83.3 % of patients had severe peri‑portal inflammation and 25 % had perisinusoidal fibrosis and granulomatous inflammation in the early stage, and developed esophageal varices [14]. pH in patients with early-stage PBC may be presinusoidal and can progress towards sinusoidal pH in the advanced stage [16]; however, more evidence is required to support this claim. Understanding the pathologic features of PBC complicated with pH may contributes to risk assessment and prognostic prediction, leading to personalized treatment strategies. pH, a common complication of PBC, affects patient survival and prognosis. However, CSPH incidence has been underestimated due to insufficient understanding of CSPH in patients with pre-cirrhotic PBC. Therefore, this study aimed to investigate CSPH incidence and its clinicopathological features in patients with PBC, especially in the early-histologic stage. Furthermore, we investigated the value of baseline clinicopathological characteristic in predicting the prognosis of patients with PBC.
2Patients and Methods2.1Study populationA total of 280 patients with PBC were screened, all of whom were hospitalized in the Hepatology Department of the First Affiliated Hospital of Fujian Medical University between January 2013 and April 2022. Patients were enrolled if they met two of the following three inclusion criteria: 1) biochemical evidence of cholestasis determined by elevated alkaline phosphatase (ALP); 2) presence of antimitochondrial antibodies (AMA) or other PBC-specific autoantibodies if AMA was negative, including anti-Sp100 (Sp100) or anti-glycoprotein 210 antibodies (gp210); and 3) histologic evidence of non-suppurative destructive cholangitis and interlobular bile duct destruction [12]. The exclusion criteria included viral hepatitis, drug-induced liver injury, primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), overlap syndrome (AIH-PBC/PSC-PBC), metabolic-dysfunction-associated steatotic liver disease, parasitic infections, thrombosis, hereditary liver diseases, hematological diseases, vascular malformations, liver cancer, unknown diagnoses, and incomplete data.
CSPH was diagnosed if one or more of the following signs were presented according to the Baveno VI criteria [17]: hepatic ascites; portosystemic collateral circulation formation (including esophageal, gastric, and splenic varices); splenomegaly [the largest cross-sectional diameter of spleen is above 11 cm as determined by ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI)].
Demographic and clinical data were obtained from electronic medical records, and clinical outcomes were obtained from outpatient and inpatient records or telephonic follow-ups. The initial visit time for PBC diagnosis in our hospital was the starting point of follow-up, and the endpoints were defined as death, liver transplantation, or reaching the follow-up cut-off time (December 2022). During follow-up, all patients received standardized treatment with ursodeoxycholic acid (13–15 mg/kg/day).
2.2Baseline data collectionBaseline clinical characteristics were collected, including age, sex, previous medical history, clinical symptoms (fatigue, pruritus, and anorexia), laboratory parameters [white blood cell (WBC), platelets (PLT), hemoglobin (Hb), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, gamma-glutamyl transpeptidase (GGT), albumin (ALB), total cholesterol (TC), triglyceride (TG), prothrombin time (PT), AMA, gp210, Sp100, antinuclear antibodies (ANA), immunoglobulin M (IgM), and immunoglobulin G (IgG)], electronic gastroscopy (evaluation of esophagogastric varices), abdominal imaging (ultrasound, CT, and MRI were used to diagnose pH signs, including ascites, splenomegaly, or portosystemic collateral circulation formation), histological examination, and treatment status.
2.3Histological analysesUltrasound-guided percutaneous liver biopsies were performed in 104 patients with PBC. Liver tissue specimens (1–2 cm long) containing at least 11 complete portal areas were obtained, fixed in 4 % neutral formaldehyde solution, and embedded in paraffin. Serial tissue sections were stained with hematoxylin and eosin (HE), reticulin fiber, Masson's trichrome, periodic acid-Schiff with and without diastase, Perls’ stain, rhodamine, aldehyde fuchsin, and cytokeratins 7 and 19. Each specimen was independently examined by two experienced histopathologists blinded to the baseline information of CSPH and outcomes during follow-up, and a consensus was reached when there was a staging discrepancy. The modified Scheuer staging system was used to divide PBC into four stages [18]. In this study, PBC in Scheuer stages I–II was defined as early-histologic-stage PBC, while that in Scheuer stages III–IV was defined as advanced-histologic-stage PBC. Histopathological slides of 68 patients with early-histologic-stage PBC were reanalyzed for common histopathologic features of noncirrhotic pH mentioned in previous studies [7,19-21], including portal vein stenosis, portal vein dilatation, herniated portal vein, hypervascularized portal tract, periportal abnormal vessels, sinusoidal dilatation, disorganized hepatocyte cords, perisinusoidal fibrosis, and NRH.
2.4Statistical methodsContinuous data are presented as means ± standard deviation (SD) or median (interquartile range [IQR]), while categorical data are expressed as number (percentage). The t- or Mann–Whitney U test was used to analyze continuous variables, and the chi-square or Fisher's exact test was used for analysis of categorical data. A multivariate Cox proportional hazards model was used to identify the long-term survival prognostic factors. Forward stepwise procedures were applied for the final model selection. Additionally, nomograms were developed based on an optimized Cox regression model to facilitate point-of-care risk assessment and estimate the predicted survival probability. Kaplan–Meier (KM) plots were used to calculate liver transplant-free survival rates. Moreover, the inverse probability of treatment weighting (IPTW)-adjusted KM analysis and Cox regression model were performed to verify the robustness of the results. Relative risks were calculated using a Cox regression model in univariate and multivariate analyses. Statistical analyses were performed using SPSS version 25 (IBM Corp., Chicago, IL, USA) and R software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). P<0.05 was considered statistically significant.
2.5Ethical statementsThe study was conducted in accordance with the principles of the Declaration of Helsinki and Istanbul. The study conformed with the ethical standards for clinical studies and was approved by the Branch for Medical Research and Clinical Technology Application, Ethics Committee of the First Affiliated Hospital of Fujian Medical University on 13 January 2022 ([2015]084–2). Written informed consent for participation was obtained from all patients.
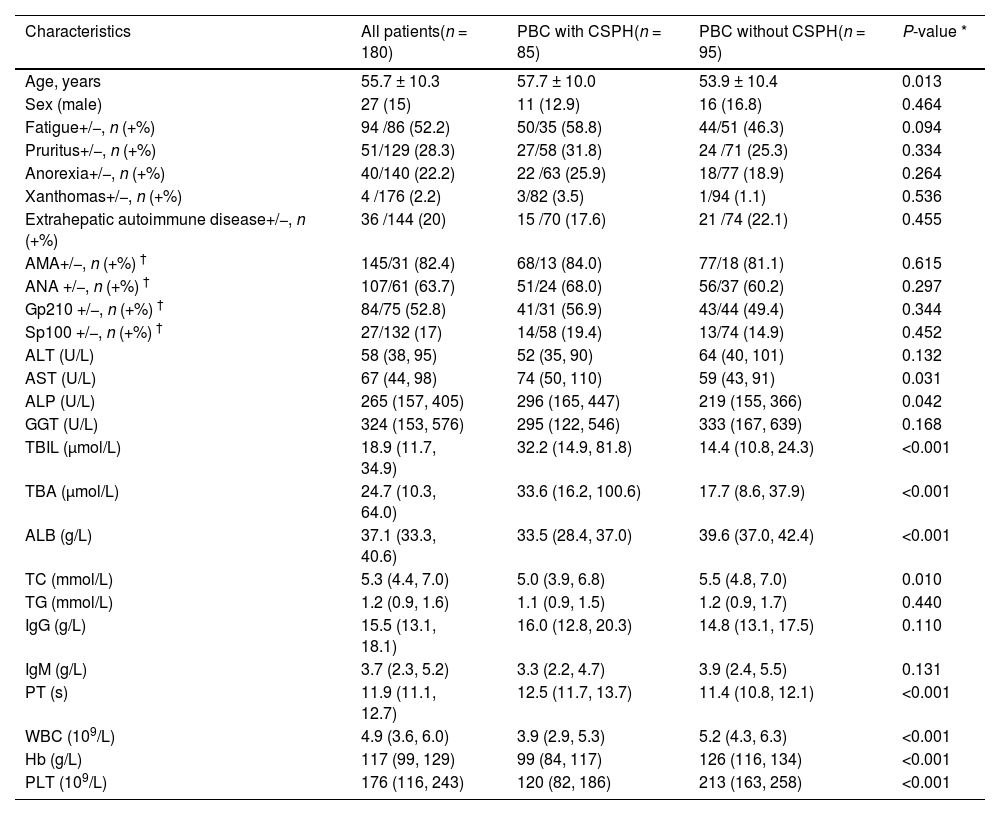
3Results3.1Clinical features of PBC patients with CSPHAfter excluding patients with AIH, PSC, viral hepatitis, or liver cancer, 180 patients with PBC were included (Fig. 1). The mean age was 55.7 ± 10.3 years, with a male-to-female ratio of 1:5.7 (Table 1). Among patients with PBC, 47.2 % (85/180) presented with CSPH at diagnosis, including 56 patients with esophagogastric varices, 48 with splenomegaly, 34 with moderate to severe ascites, and 11 with esophagogastric variceal bleeding (Supplementary Fig. 1D, Supplementary Fig. 1F). 104 patients underwent liver biopsy at baseline, 68 patients had early-histologic-stage of PBC, and CSPH was present in 20.6 % of these patients. During a median follow-up time of 5.1 (IQR, 2.1–7.2) years, there were three liver transplants, 32 deaths, and 14 new CSPH cases. Of the 85 patients with CSPH at baseline, 72 had hepatic decompensation, with a cumulative incidence of 84.7 % during follow-up. Stepwise forward binary logistic regression analysis revealed that lower Hb levels, lower PLT counts, and higher TBIL levels at baseline were associated with a higher risk of CSPH in patients with PBC (odds ratio [OR] 0.940, 95 % confidence interval [CI] 0.914–0.967; OR 0.989, 95 % CI 0.985–0.994; OR 1.035, 95 % CI 1.014–1.056, respectively).
Comparison of the clinical features with and without CSPH in PBC patients.
| Characteristics | All patients(n = 180) | PBC with CSPH(n = 85) | PBC without CSPH(n = 95) | P-value * |
|---|---|---|---|---|
| Age, years | 55.7 ± 10.3 | 57.7 ± 10.0 | 53.9 ± 10.4 | 0.013 |
| Sex (male) | 27 (15) | 11 (12.9) | 16 (16.8) | 0.464 |
| Fatigue+/−, n (+%) | 94 /86 (52.2) | 50/35 (58.8) | 44/51 (46.3) | 0.094 |
| Pruritus+/−, n (+%) | 51/129 (28.3) | 27/58 (31.8) | 24 /71 (25.3) | 0.334 |
| Anorexia+/−, n (+%) | 40/140 (22.2) | 22 /63 (25.9) | 18/77 (18.9) | 0.264 |
| Xanthomas+/−, n (+%) | 4 /176 (2.2) | 3/82 (3.5) | 1/94 (1.1) | 0.536 |
| Extrahepatic autoimmune disease+/−, n (+%) | 36 /144 (20) | 15 /70 (17.6) | 21 /74 (22.1) | 0.455 |
| AMA+/−, n (+%) † | 145/31 (82.4) | 68/13 (84.0) | 77/18 (81.1) | 0.615 |
| ANA +/−, n (+%) † | 107/61 (63.7) | 51/24 (68.0) | 56/37 (60.2) | 0.297 |
| Gp210 +/−, n (+%) † | 84/75 (52.8) | 41/31 (56.9) | 43/44 (49.4) | 0.344 |
| Sp100 +/−, n (+%) † | 27/132 (17) | 14/58 (19.4) | 13/74 (14.9) | 0.452 |
| ALT (U/L) | 58 (38, 95) | 52 (35, 90) | 64 (40, 101) | 0.132 |
| AST (U/L) | 67 (44, 98) | 74 (50, 110) | 59 (43, 91) | 0.031 |
| ALP (U/L) | 265 (157, 405) | 296 (165, 447) | 219 (155, 366) | 0.042 |
| GGT (U/L) | 324 (153, 576) | 295 (122, 546) | 333 (167, 639) | 0.168 |
| TBIL (μmol/L) | 18.9 (11.7, 34.9) | 32.2 (14.9, 81.8) | 14.4 (10.8, 24.3) | <0.001 |
| TBA (μmol/L) | 24.7 (10.3, 64.0) | 33.6 (16.2, 100.6) | 17.7 (8.6, 37.9) | <0.001 |
| ALB (g/L) | 37.1 (33.3, 40.6) | 33.5 (28.4, 37.0) | 39.6 (37.0, 42.4) | <0.001 |
| TC (mmol/L) | 5.3 (4.4, 7.0) | 5.0 (3.9, 6.8) | 5.5 (4.8, 7.0) | 0.010 |
| TG (mmol/L) | 1.2 (0.9, 1.6) | 1.1 (0.9, 1.5) | 1.2 (0.9, 1.7) | 0.440 |
| IgG (g/L) | 15.5 (13.1, 18.1) | 16.0 (12.8, 20.3) | 14.8 (13.1, 17.5) | 0.110 |
| IgM (g/L) | 3.7 (2.3, 5.2) | 3.3 (2.2, 4.7) | 3.9 (2.4, 5.5) | 0.131 |
| PT (s) | 11.9 (11.1, 12.7) | 12.5 (11.7, 13.7) | 11.4 (10.8, 12.1) | <0.001 |
| WBC (109/L) | 4.9 (3.6, 6.0) | 3.9 (2.9, 5.3) | 5.2 (4.3, 6.3) | <0.001 |
| Hb (g/L) | 117 (99, 129) | 99 (84, 117) | 126 (116, 134) | <0.001 |
| PLT (109/L) | 176 (116, 243) | 120 (82, 186) | 213 (163, 258) | <0.001 |
Data are presented as n (%), median (interquartile range), or mean ± standard deviation unless otherwise indicated.
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; AST, aspartate aminotransferase; CSPH, clinically significant portal hypertension; GGT, gamma-glutamyl transpeptidase; Gp210, anti-glycoprotein 210 antibody; Hb, hemoglobin; IgM, immunoglobulin M; IgG, immunoglobulin G; PBC, Primary biliary cholangitis; PLT, platelets; PT, prothrombin time; TBIL, total bilirubin; TBA, total bile acid; TC, total cholesterol; TG, triglyceride; WBC, white blood cell; Sp100, anti-Sp100 antibody.
In the whole cohort, the overall 1-, 3-, and 5-year liver transplant-free survival rates of patients with PBC were 91.3 %, 83.8 %, and 79.1 %, respectively. Kaplan–Meier analysis revealed that the 1-, 3-, and 5-year liver transplant-free survival rates for PBC patients with CSPH were 82.4 %, 68.4 %, and 58.6 %, respectively. The liver transplant-free survival rates were significantly lower in PBC patients with CSPH compared with those without CSPH (98.9 %, 96.2 %, and 94.6 %, respectively; supplementary Fig. 2A), regardless of with or without liver biopsy (P < 0.001, = 0.019, respectively; supplementary Fig. 2C–D). CSPH was a risk factor for liver transplantation-free survival in patients with PBC (hazard ratio [HR] 6.78, 95 % CI, 2.94–15.63; P < 0.001; supplementary Fig. 2A).
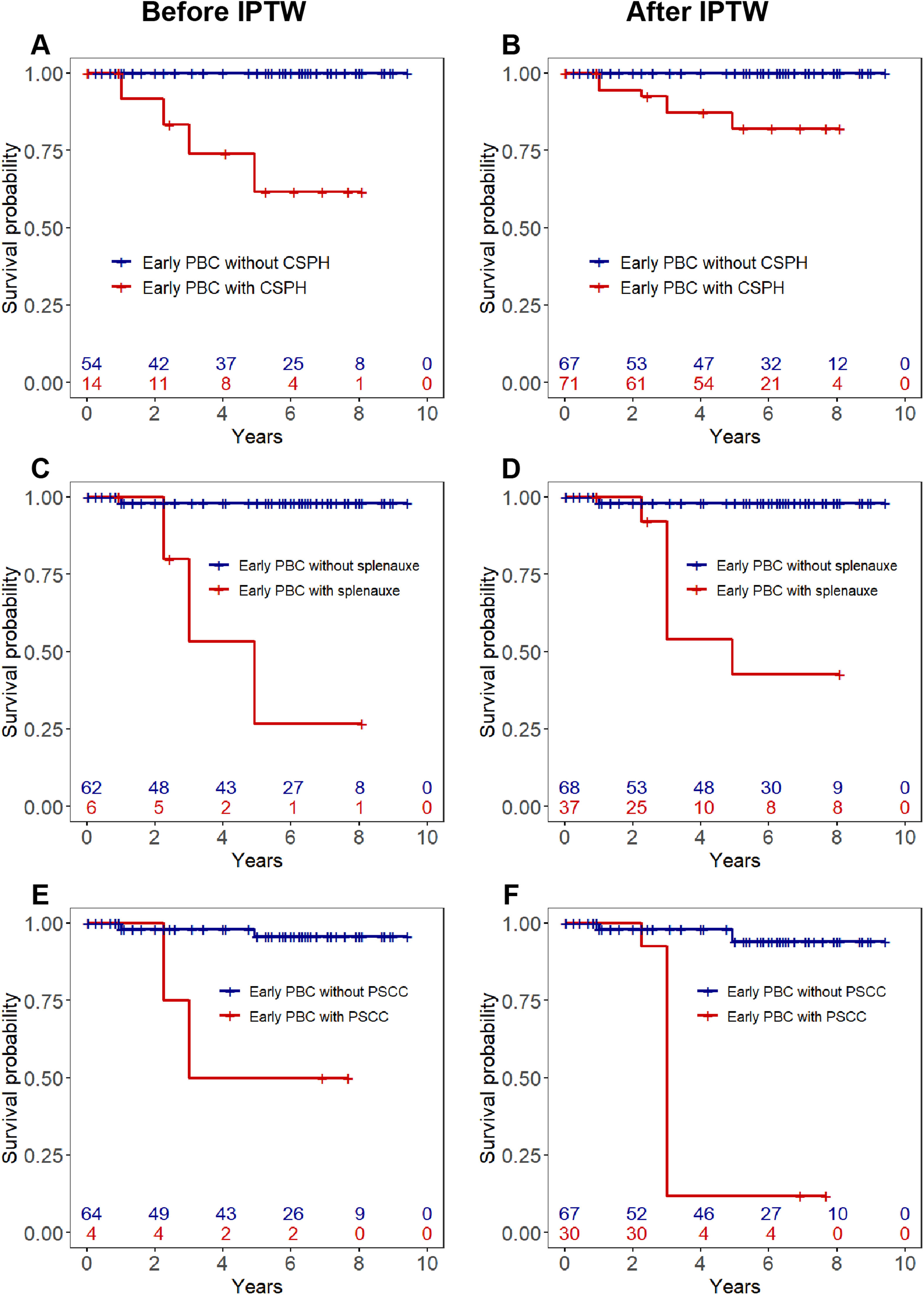
The liver transplant-free survival rates in early-histologic-stage PBC patients without CSPH were significantly higher than those with CSPH (Fig. 2A-B). In patients with early-histologic stage, splenomegaly was the most powerful adverse prognostic factor (IPTW-adjusted HR 28.78, 95 % CI 2.66–311.6, P = 0.006), followed by portosystemic collateral circulation (Fig. 2C-F).
KM survival analysis of liver transplant-free survival in early PBC patients.
Abbreviations: CSPH, clinically significant portal hypertension; Early PBC, early-histologic-stage PBC (PBC in Scheuer stages Ⅰ–Ⅱ); IPTW, inverse-probability of treatment-weighting analysis; PBC, primary biliary cholangitis. (A) Liver transplant-free survival of patients with early PBC with and without CSPH before IPTW. (B) Liver transplant-free survival of patients with early PBC with and without CSPH after IPTW. (C) Comparison of liver transplant-free survival between early PBC patients with and without splenomegaly before IPTW. (D) Comparison of liver transplant-free survival between early PBC patients with and without splenomegaly at baseline after IPTW. (E) Comparison of liver transplant-free survival between early PBC patients with and without PSCC at baseline before IPTW. (F) Comparison of liver transplant-free survival between early PBC patients with and without PSCC at baseline after IPTW.
Note: IPTW-adjusted (variables adjusted by age, sex, perisinusoidal fibrosis, and nodular regenerative hyperplasia)
Histopathological characteristic comparisons were conducted between patients with CSPH and without CSPH within the PBC group who underwent liver biopsy (Supplementary Table 1). These comparisons revealed markedly more advanced Scheuer histological and fibrosis stages in the CSPH group than in the non-CSPH group (P < 0.001). The rates of hepatic lobular structure disorder, fibrous septa formation in the hepatic lobules, cholestasis, and ductular reaction were significantly higher in patients with CSPH than in those without CSPH (P < 0.05).
Liver tissue sections were obtained from 68 patients with early histologic stage, including 28 patients with sinusoidal dilatation, 27 with portal vein stenosis, 26 with perisinusoidal fibrosis, 20 with fibrous septa formation in the hepatic lobules, 15 with disorganized hepatocyte cords, and 12 with NRH (Fig. 3A-F). Patients with PBC in the early histologic stage who had CSPH showed significantly higher incidences of perisinusoidal fibrosis and NRH than those without CSPH (P = 0.004 and P = 0.017, respectively) (Table 2).
Histopathology images of patients with early PBC.
Abbreviations: Early PBC, early-histologic-stage PBC (PBC in Scheuer stages Ⅰ–Ⅱ); (A) Perisinusoidal fibrosis (Masson stain, 100×). (B) Nodular regenerative hyperplasia, shown by arrow (reticulin fiber stain, 100×). (C) Dilated hepatic sinusoids (HE stain, 100×); (D) Portal vein stenosis, shown by arrow (HE stain, 100×). (E) Disorganized hepatocyte cords with fibrous septa formation in the hepatic lobules (reticular fiber stain, 100×). (F) Hepatocyte cholestasis, shown by arrow (HE stain, 200×).
Comparison of the histopathological features of patients with early PBC with and without CSPH (n = 68).
| Histopathological features | Early PBC with CSPH(n = 14) | Early PBC without CSPH(n = 54) | P-value |
|---|---|---|---|
| Chronic hepatitis grades 0 ≤ G ≤ 2 / 2 | 13 (92.9) / 1 (7.1) | 48 (88.9) / 6 (11.1) | 1.000 |
| Fibrosis stages0 ≤ S ≤ 2 / 2<S ≤ 4 | 13 (92.9) / 1 (7.1) | 51 (94.4) / 3 (5.6) | 1.000 |
| Herniated portal vein | 1 (7.1) | 8 (14.8) | 0.755 |
| Portal vein stenosis | 8 (57.1) | 19 (32.5) | 0.135 |
| Portal vein dilatation | 0 (0.0) | 1 (1.9) | 1.000 |
| Hypervascularised portal tract | 4 (28.6) | 17 (31.5) | 1.000 |
| Periportal abnormal vessels | 0 (0.0) | 7 (13.0) | 0.353 |
| Sinusoidal dilatation | 7 (50.0) | 21 (38.9) | 0.452 |
| Disorganized hepatocyte cords | 6 (42.9) | 9 (16.7) | 0.081 |
| Perisinusoidal fibrosis | 10 (71.4) | 16 (29.6) | 0.004 |
| NRH | 6 (42.9) | 6 (11.1) | 0.017 |
| Interface hepatitis | 5 (35.7) | 14 (25.9) | 0.694 |
| Fibrous septa formation in the hepatic lobules | 6 (42.9) | 14 (25.9) | 0.363 |
| Massive inflammatory cell infiltration in the portal area | 7 (50.0) | 23 (42.6) | 0.619 |
| Hepatic lobular structure disorder | 1 (7.1) | 3 (5.6) | 1.000 |
| Bile duct paucity | 10 (71.4) | 34 (63.0) | 0.782 |
| Ductular reaction | 3 (21.4) | 6 (11.1) | 0.567 |
| Cholestasis | 1 (7.1) | 4 (7.4) | 1.000 |
| Bridging necrosis | 1 (7.1) | 4 (7.4) | 1.000 |
| Granulomatous inflammation | 4 (28.6) | 17 (31.5) | 1.000 |
Data are presented as n (%).
Abbreviations: CSPH, clinically significant portal hypertension; NRH, nodular regenerative hyperplasia; PBC, Primary biliary cholangitis.
Early PBC: early-histologic-stage PBC (PBC in Scheuer stages Ⅰ–Ⅱ).
A total of 155 patients completed the final follow-up. 32 patients were dead and 3 patients received liver transplantations during the follow-up period (22.6 %, 35/155). Multivariate Cox regression analysis revealed that increased levels of TBIL (HR 1.023, 95 % CI 1.013–1.033, P < 0.001), lower ALB levels (HR 0.919, 95 % CI 0.849–0.995, P = 0.038), and decreased PLT levels (HR 0.992, 95 % CI 0.987–0.998, P = 0.010) were independent adverse predictors of liver transplant-free survival in patients with PBC (Supplementary Table 2 and supplementary Fig. 3).
3.5The predictive value of baseline histopathological characteristics for long-term outcomes in patients with PBCIn this study, 104 patients with PBC underwent baseline liver histological examination, 1 had a liver transplant, 12 died, and 12 were lost during follow-up. The univariate Cox analysis results revealed that the Scheuer histological staging, fibrous septa formation in the hepatic lobules, and cholestasis could predict the long-term prognosis of patients with PBC (all P < 0.05, Fig. 4). Subsequently, these variables were included in the multivariate Cox regression analysis, which demonstrated that fibrous septa formation in the hepatic lobules (HR 4.85, 95 % CI 1.51–15.52, P = 0.008) and cholestasis (HR 7.70, 95 % CI 2.56–23.18, P < 0.001) were independent adverse predictors of liver transplant-free survival in patients with PBC (Supplementary Fig. 4).
4DiscussionpH generally occurs after the progression of chronic liver disease to cirrhosis; however, some patients with PBC may present with pH signs in the early disease stage [9]. Furthermore, CSPH features, such as splenomegaly or portosystemic collaterals, may occur earlier than varices detected endoscopically. Overall, 180 patients with PBC were enrolled to our center and were followed up for a median of 5.1 years. We found that 47.2 % of patients with PBC developed CSPH at baseline, and its incidence in patients with early-histologic-stage PBC was 20.6 %. CSPH at diagnosis is a risk factor for predicting liver transplant-free survival in patients with PBC, especially among those at early histologic stage. As hepatic venous pressure gradient (HVPG) measurements are an invasive examination with high economic costs, their widespread utilization in clinical practice is challenging. The Baveno VII consensus states that the gold standard for the diagnosis of pH in patients with viral and alcoholic cirrhosis is HVPG; however, in patients with PBC, pH cannot be accurately assessed using HVPG because of the presinusoidal factor [3]. Our study confirmed that pre-cirrhotic pH is more common in PBC patients than previously recognized. The incidence of pH could be significantly underestimated if evaluation of pH was only performed in patients with cirrhosis. Baveno VII consensus recommends that liver stiffness ≤15 kPa as measured by transient elastography in combination with platelet count ≥150×109/L can rule out CSPH in patients with compensated advanced chronic liver disease [3]. However, the usage of this non-invasive method for the evaluation of pH in PBC cohort requires further study.
The 3- and 5-year liver transplantation-free survival rates of patients with PBC with baseline CSPH were 68.4 % and 58.6 %, respectively. Both the 3- and 5-year liver transplantation-free survival rates were significantly lower in patients with CSPH than those without CSPH. These findings indicated a poorer prognosis for patients with CSPH. An Austrian retrospective cohort study involving 333 PBC cases suggested that the risk of liver-related mortality in PBC patients with CSPH was 7.2 times that in those without CSPH. Meanwhile, splenomegaly and portosystemic collateral formation increased the risk of 5-year hepatic decompensation [22]. Similar findings were observed in our study and were particularly prominent in patients with early-histologic-stage PBC. The current guidelines recommend that only patients in whom the disease progresses to cirrhosis should undergo periodic gastroscopy and ultrasound imaging examinations to assess for pH manifestations [3]. This recommendation may underestimate the incidence of pH in patients with early-histologic-stage PBC, which is an essential factor in worse outcomes.
Currently, the histopathological characteristics of patients with PBC and comorbid pH are not well-defined. We evaluated the histopathological features of patients with early-histologic-stage PBC and found that perisinusoidal fibrosis and NRH were associated with CSPH. This is of great value for the early identification of pH in the pre-cirrhosis stage in patients with PBC. Perisinusoidal fibrosis could block portal blood flow, resulting in a sinusoidal pH [16]. NRH is another histopathological feature associated with pH. The development of pH may be related to the phenotypic shift in centrizonal atrophic hepatocytes and sinusoidal endothelial cells due to NRH [23]. Currently, it is believed that intrahepatic vessel compression or increased blood flow resistance due to NRH causes a presinusoidal or sinusoidal pH [24-26]. Additionally, portal vein stenosis, disorganized hepatocyte cords, and fibrous septa formation in the hepatic lobules were observed in this study and were considered as pH-related histomorphological signs. These histopathological signs are not characteristic features of pH in patients with PBC; however, they can provide a reference for diagnosing noncirrhotic pH.
Liver biopsies are useful for risk stratification of PBC, including staging and prediction of prognosis [27]. Our results suggest that fibrous septa formation in the hepatic lobules and baseline cholestasis are independent risk factors for the liver transplant-free survival in patients with PBC. Tsuneyama et al. [28]. suggested that fibrous septa formation in the hepatic lobules was caused by an increase and activation of perisinusoidal cells in the liver parenchyma, promoting the occurrence and development of liver parenchymal fibrosis. Nakanuma et al. [24] proposed the a PBC staging system where the degree of lichen red-positive particle deposition reflected cholestasis and was closely related to cirrhosis development. Recent studies have revealed that some histopathological features of liver including collagen area, fibrous septum thickness, microvascular density, and lymphatic duct density or their areas, can not only reflect the stage of liver fibrosis but also have a strong correlation with portal vein pressure [25,26,29]. These studies provide new information for diagnosing pH and assessing its severity.
In our study cohort, cumulative survival without liver transplantation was significantly higher among patients with early-histologic-stage PBC than those with advanced-histologic-stage PBC during follow-up (Supplementary Fig. 1A). It suggests that the histological stage is a crucial prognostic factor. Meanwhile, this study concluded that the baseline CSPH occurrence, along with TBIL, ALB, and PLT levels, had predictive value for the survival of patients with PBC. Previous studies have demonstrated that pH developed earlier than the elevation of TBIL levels in patients with PBC. Furthermore, some patients develop pH in the early stage, whereas elevated TBIL level is a typical manifestation of obvious cholestasis in the advanced disease stage [7]. A recent study showed that the Mayo risk score, calculated based on clinical indicators, such as TBIL and ALB, is also a good predictor of prognosis in patients with PBC [30]. The predictive value of serological indicator for PBC prognosis was further demonstrated in this study.
This retrospective study had some limitations. HVPG measurements were not performed; hence, pH was assessed by abdominal imaging such as ultrasound, CT or MRI, and gastroscopy imaging, which may have underestimated pH incidence in patients with PBC. The response to ursodeoxycholic acid was not investigated due to insufficient data. Finally, elastographic measurements were not used to exclude CSPH. The diagnostic value of elastographic measurements for CSPH in PBC remains to be ascertained.
5ConclusionsIn conclusion, PBC patients with baseline CSPH had a worse long-term outcome. Furthermore, the incidence of CSPH is high in patients with PBC in the early histologic stage. Perisinusoidal fibrosis and NRH are common histopathological features of early-histologic-stage PBC complicated by CSPH. Therefore, liver biopsy is of great value for risk-stratifying patients with PBC. Further exploration of the non-invasive methods and novel biomarkers to identify early pH without adverse outcomes is required.
FundingThis work was supported by the Clinical Research Centre for Liver and Intestinal Diseases of Fujian Province (grant number 2021Y2006) and the Fujian Province Science and Technology Plan Project of China (grant number 2023Y0015).
Data availability statementThe data that support the findings of this study are available from the corresponding authors upon reasonable request.
The authors thank Prof. Wang for his help in obtaining histopathologic information and for everyone who helped collect clinical data.