Liver fibrosis (LF) is a pathological repair response of the liver to chronic liver injury caused by various factors. It is manifested by increased extracellular matrix (ECM) production and reduced degradation, leading to diffuse excessive deposition and abnormal distribution in the liver [1]. LF involves complex interactions between multiple lineages of non-hematopoietic cells, with hepatic stellate cells (HSCs) being the most important [2]. They are the primary sources of ECM secretion and play a vital role in the occurrence and development [3]. Normally HSCs are located in the Disse gap, where they are at rest and store vitamin A. However, HSCs transform into myofibroblasts in response to injury, begin to express α-smooth muscle actin (α-SMA), migrate to tissue repair sites, and secrete large amounts of ECM [4].
The causes of LF include viral infection, drug toxicity, and fatty liver. Studies have shown that the severity of fibrosis is closely related to adverse liver outcomes [5]. Despite treatment with antiviral drugs, 7.2 % of patients with chronic HBV infection have advanced LF compared to 2.9 % of controls without hepatitis B infection [6]. HBV-related mortality remains a major problem due to the high prevalence of hepatitis B. Besides viral hepatitis, fatty liver disease, alcohol-induced LF, and cirrhosis should not be ignored. At present, the treatment for liver fibrosis mainly includes etiological treatment and anti-fibrosis treatment, but there are no specific anti-liver fibrosis drugs in clinical practice. Hence, it is critical to identify patients at high risk for significant fibrosis regardless of the causes and seek a new target.
Additionally, LF staging depends on liver biopsy, which is an invasive procedure with some risks for patients [7]. First of all, the patient's acceptance is low; there is a risk of bleeding, perforation, and other complications, and he needs to be hospitalized during the puncture examination. Secondly, it is easy to be limited by the technology and experience of operators and pathologic film readers [8]. A variety of non-invasive tools are already available to assess the stage of liver fibrosis and monitor its progression. Transient elastography (TE) is a safe and non-invasive method to estimate the degree of liver fibrosis by detecting the elastic value of the liver [9]. However, obesity, stenosis of the costal space, extrahepatic cholestasis, and ascites may increase the rate of operation failure and affect the interpretation of the fibrosis degree. In contrast, the use of serum biomarkers for evaluation has many advantages, such as easy sample collection, low risk to patients, and repeatability of the test. Direct serum markers [9], such as hyaluronic acid, type III procollagen peptide, type IV collagen, laminin, etc., can be easily and repeatable for follow-up. However, the results exhibit a relatively low correlation with the pathological stages of liver fibrosis, along with low accuracy and specificity. Additionally, there is still a dearth of a unified diagnostic threshold for clinical application. And a variety of serum indexes of another joint diagnosis of liver fibrosis score models such as Aspartate aminotransferase and Platelet ratio index (Aspartate aminotransferase to Platelet Ratio Index, APRI), The Fibrosis Index Based on 4 Factors (FIB-4) has not yet reached a consensus. A study of HBV-infected people in China showed that APRI and FIB-4 were not relatively effective in the diagnosis of liver fibrosis and cirrhosis, and the non-invasive model was more suitable for HBeAg-positive patients than HBeAg-negative patients [10]. Therefore, there is an urgent need to develop new alternative non-invasive biomarkers to identify patients with significant liver fibrosis.
Data mining has been applied in many fields, and bioinformatics analysis can help identify differentially expressed genes and molecular mechanisms. Liver tissue transcriptome analysis of fibrosis patients has been performed to fully understand the molecular abnormalities involved in their disease progression. However, the discovery of omics-based biomarkers has not been fully utilized. In this study, we sequenced 66 liver tissue samples from patients with HBV-LF, analyzed the differential genes in different fibrosis stages using bioinformatics, and combined it with human HSCs sequencing to find the factors related to LF development. Then, we focused on Latent transforming growth factor β binding protein 2 (LTBP2) and evaluated the viability of LTBP2 as a new biomarker using serum from 151 patients who underwent liver biopsy. The study overview is in Supplementary Fig. 1.
2Patients and Methods2.1Liver fibrosis patientsLiver biopsy samples from 66 naive HBV-infected patients were obtained. Among the recruited patients, patients were admitted to the Shanghai Public Health Clinical Center affiliated with Fudan University. All patients were diagnosed based on the criteria recommended by the Asian Pacific Association for the Study of the Liver (APASL) [11] and had not previously received antiviral therapy.
A total of 151 serum samples were collected from patients undergoing liver biopsy at the Shanghai Public Health Clinical Center affiliated with Fudan University. Clinical data and laboratory examination were obtained using standard data collection forms from electronic medical records.
All the samples were sent to the Pathology Department of Shanghai Public Health Clinical Center, affiliated with Fudan University, for histopathology diagnosis. The stages of fibrosis (Scheuer S) and inflammation (Scheuer G) were interpreted independently by two experienced pathologists [12,13]. According to the guidelines for diagnosing and treating liver fibrosis, S0-1 indicates non-significant fibrosis (NSF), while Scheuer ≥ S2 is defined as significant fibrosis (SF) [14].
2.2Enzyme-linked immunosorbent assayThe serum samples were kept in a − 80 °C freezer until measurements. Serum LTBP2 levels were examined with the ELISA (ELK Biotechnology, Wuhan, China) kit following the manufacturer's protocols.
2.3Traditional noninvasive fibrosis diagnostic modelAPRI [15] index: APRI ={(AST/ASTULN)/PLT(109/L)} × 100
FIB-4 [16] index: FIB-4 =age × AST(U/L)/PLT(109/L) × ALT(U/L)1/2
2.4Statistical analysisThe data were processed using SPSS 25.0 and GraphPad 7.0 software and presented as the mean ± standard deviation (mean ± SD). Unless otherwise stated, categorical variables are expressed as frequency and percentage (n%), and continuous variables are expressed as median (IQR). Comparison between groups was performed by t-test, Mann Whitney U test, χ2 test, or Fisher exact test. The correlation between the two parameters was assessed using Spearman's rank-order correlation analysis. The best cut-off value is found by the Youden index. The risk factors related to patients with significant fibrosis were analyzed using univariate and multivariate logistic regression models. Based on the results of the multivariate analysis, a nomogram model was drawn using the R rms package. An analysis of the area under the receiver operating characteristic curve (AUROC) was performed on the model. The calibration curves of the training cohort and the verification cohort were adjusted after 1000 self-weightlifting samples, and the consistency of prediction probability and observation probability was analyzed. By quantifying the net benefits under different threshold probabilities, decision curve analysis (DCA) was performed to determine the clinical applicability of the model. P-value < 0.05 indicated that the difference was statistically significant.
All other information on materials and methods is provided in the Supplementary Data.
2.5Ethical statementThis study was in accordance with the ethical standards of the institutional and national research committee and with the Declaration of Helsinki (as revised in 2013). The ethics committee of Shanghai Public Health Clinical Center, Fudan University, approved this study (2023-S081-02).
3Results3.1Clinical characteristics of HBV-related LF patientsOur study included 66 adult patients who were diagnosed with HBV-related liver fibrosis. There are 38 (57.6 %) males, and the average age of the whole cohort is 38.28 ± 9.8. Liver biopsy is the standard to diagnose fibrosis, and sequential histological staging of fibrosis (Scheuer score ‘S’) and grading of inflammation (Scheuer score ‘G’) can be used to assess the LF progression [17]. The numbers of patients by fibrosis stage (1/2/3/4) and inflammation grade (1/2/3) were 27/12/16/11 and 27/31/8, respectively. Virological markers, including HBV DNA, HBsAg, and HBeAg, were used to monitor viral replication and guide antiviral therapy. The four items of serum liver fibrosis also indicated the progression of fibrosis. The clinical features of each patient are listed in Supplementary Table 2.
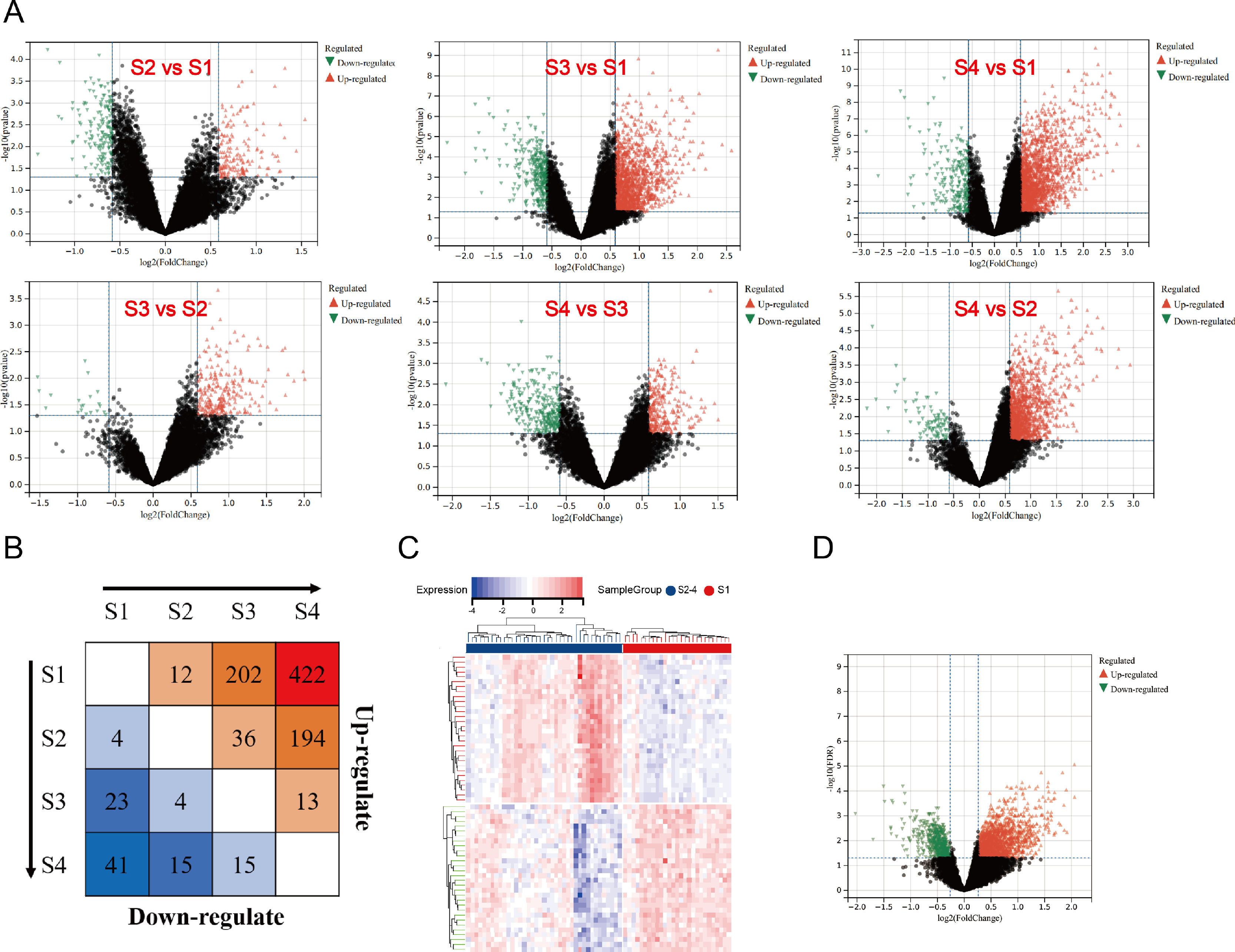
3.2Differential mRNA screening, GO, and pathway enrichment analysis of HBV-related LF patientsSince the liver histological Scheuer score is the most relevant fibrosis assessment, we analyzed DEGs at different stages. The volcano plots of DEGs between different groups are shown in Fig. 1A. S2 had 12 upregulated and four downregulated genes, S3 had 202 upregulated and 23 downregulated genes, and S4 had 422 upregulated and 41 downregulated genes, with stage S1 as the control (Fig. 1B). From our results, we observed that the number of upregulated DEGs among patients in stages S3 and S4 is more than in the early stage of disease progression. In addition, it can be seen that the number of downregulated DEGs associated with fibrosis also increases with the progression of the disease (Fig. 1B).
The results of DEGs in different histological stages. A. Volcano plots visualizing the DEGs were analyzed among each fibrosis stage. The X-axis shows the fold change, while the Y-axis shows the p-value. A vertical line indicates an increase or decrease of 1.2 times. The horizontal line indicates p value = 0.05. Red dots indicate up-regulated genes, black dots indicate no significant change genes, and green dots indicate down-regulated genes. B. The number of DEGs was shown by a heatmap. The number of up-regulated genes is shown in the upper half, and down-regulated genes are shown in the lower half. C. The heatmap of DEGs among the significant fibrosis group and non-significant fibrosis group. D. The volcano plot of DEGs among the significant fibrosis group and non-significant fibrosis group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We compared the SF and NSF groups to identify key genes involved in fibrosis development. There were 1833 upregulated and 608 downregulated genes in SF, with NSF as a control. The heatmap and volcano plot of DEGs are shown in Fig. 1C& D.
GO and KEGG pathway enrichment analysis was employed to investigate the functions of DEGs between the SF and NSF groups. The results showed that the DEGs were enriched in 17 KEGG pathways, 212 GO-BP pathways, 16 GO-CC pathways, and 14 GO-MF pathways. We summarized the top three of each way (Supplementary Fig. 2A). ECM-receptor interaction was the most enriched pathway for DEGs. DEGs were enriched for ECM structural constituents in the MF category. DEGs were primarily enriched in collagen-containing ECM in the CC category. The DEGs were mainly enriched in ECM organization in the BP category. These results suggested that biological processes associated with fibrosis are strongly activated. The gene expression enriched into each pathway of the top five was further demonstrated in detail (Supplementary Fig. 2B and Supplementary Table 3).
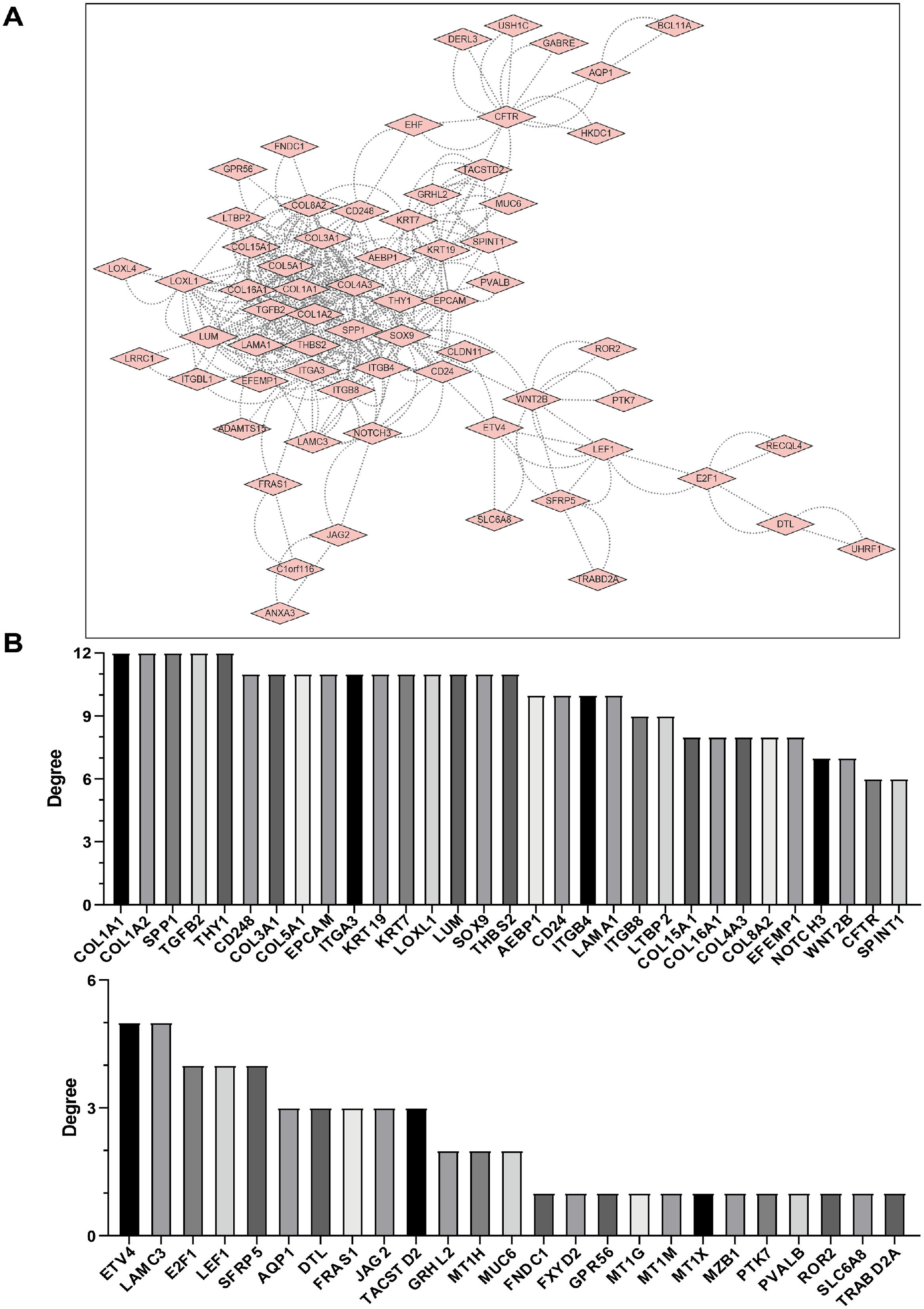
3.3PPI network construction and identification of hub genesA PPI network for these DEGs was constructed using the search tool to retrieve interacting genes (STRING) and identify which DEGs were most probably the key genes in the LF process. A total of 127 DEGs were filtered into the PPI network complex and visualized using the Cytoscape software (Fig. 2A).
PPI network analysis of DEGs with HBV-related fibrosis. A: Using the STRING online database, a total of 127 DEGs (116 up-regulated genes and 11 down-regulated genes) were screened into a DEG PPI network complex by cytoscape. Each node represents a gene, and the edges stand for the interactions between nodes (the disconnected nodes are not shown) B. Key genes were screened using CytoHubba in Cytoscape software. To ensure the reliability of the screened genes, 12 kinds of scoring (MCC, DMNC, MNC, Degree, EPC, BottleNeck, EcCentricity, Closeness, Radiality, Betweenness, Stress and ClusteringCoefficient) were used and the first 30 genes obtained from each scoring standard were selected, and then their intersection was taken to calculate the occurrence times of each gene. If a gene can appear in the list of more than 6 key genes, it is considered a key gene with high confidence.
We used the CytoHubba [18] plug- in Cytoscape [19] to identify the hub genes with 12 built-in centrality indices: Maximal Clique Centrality (MCC), Density of Maximum Neighborhood Component (DMNC), Maximum Neighborhood Component (MNC), Degree, Edge Percolated Component (EPC), Bottleneck, Eccentricity, Closeness, Radiability, Betweenness, Stress and Clustering Coefficient to calculate the hub scores of significant modules. The top 30 genes in each centrality index were considered highly credible hub candidates. We applied a strict filter criterion to 12 lists of potential hub genes ranked by different hub scores. Only genes within the intersection of ≥ 6 lists were considered high-confidence hub genes with potential biological significance [20] (Fig. 2B and Supplementary Table 4). The high-confidence hub genes as follows: COL1A1, COL1A2, SPP1, TGFB2, THY1, CD248, COL3A1, COL5A1, EPCAM, ITGA3, KRT19, KRT7, LOXL1, LUM, SOX9, THBS2, AEBP1, CD24, ITGB4, LAMA1, ITGB8, LTBP2, COL15A1, COL16A1, COL4A3, COL8A2, EFEMP1, NOTCH3, WNT2B, CFTR, SPINT1.
3.4Differential mRNA screening in the HSC-activated and validate the high-confidence hub genesHSC activation is essential for LF progression. We analyzed the HSC transcriptome GSE68001 [21] from the GEO database to ascertain whether the dysregulated expression patterns of the identified LF-specific genes were consistent across liver tissues and HSCs. HSCs were isolated from healthy liver and culture-activated as aHSC in GSE68001. Activated HSCs had 3221 upregulated and 2180 downregulated genes compared to qHSCs (Supplementary Fig. 3). Based on these results, we used bioinformatics tools to obtain the common DEGs among GSE68001 and high-confidence hub genes in HBV-related LF. The results showed that 16 genes were upregulated, while none were downregulated. The common genes as follows: AEBP1, COL1A1, COL3A1, COL1A2, COL5A1, LOXL1, KRT7, LTBP2, COL16A1, THBS2, NOTCH3, ITGA3, THY1, LUM, CD248, EFEMP1. Spearman correlation was constructed using expression values and histological scores (Supplementary Fig. 4). Genes highly associated with disease severity (R > 0.6) were AEBP1, COL1A1, COL3A1, COL1A2, COL5A1, LOXL1, KRT7, LTBP2, COL16A1, THBS2 and NOTCH3.
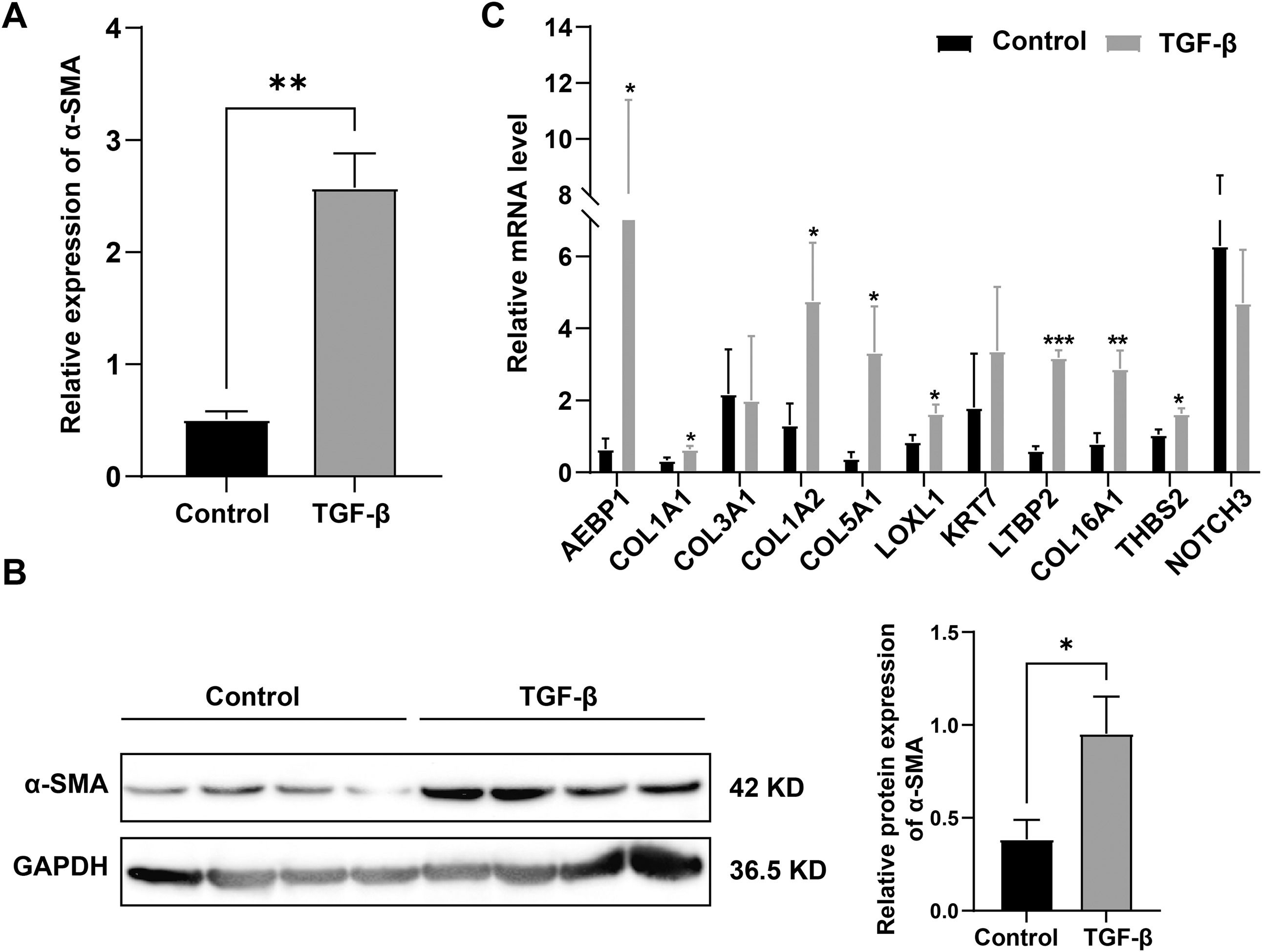
High-confidence hub gene expression was confirmed in LX-2 cells treated with recombinant human TGF-β, and LX-2 cells expressed more α-SMA, indicating the successful establishment of the cell model. (Fig. 3A and B). Fig. 3C displays a statistical analysis of 11 high-confidence hub genes relative expression to GAPDH; eight of them were upregulated significantly. Some gene expression trends may be inconsistent due to incomplete representation of activated HSCs or gene expression in other cells, such as hepatic parenchymal and Kupffer cells.
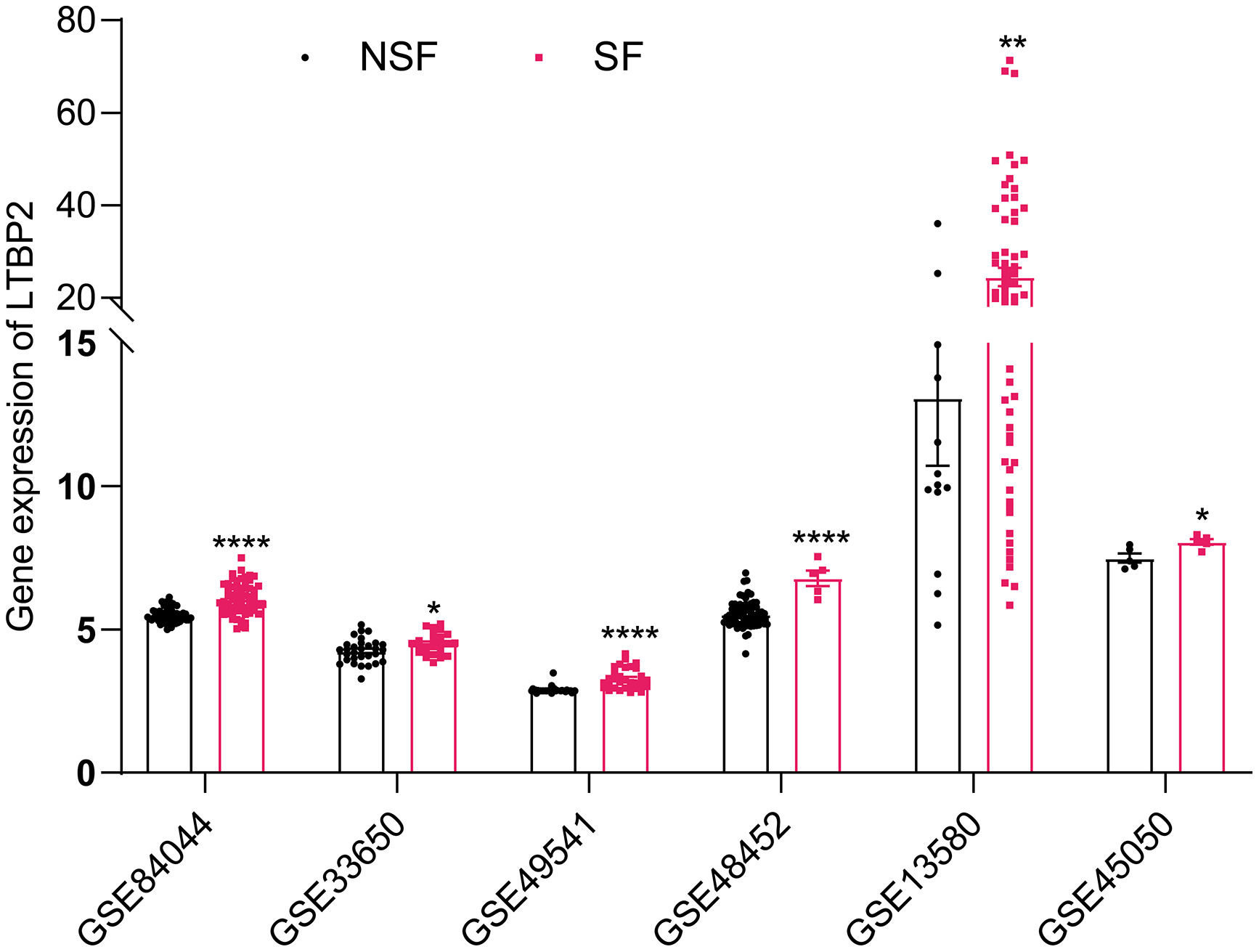
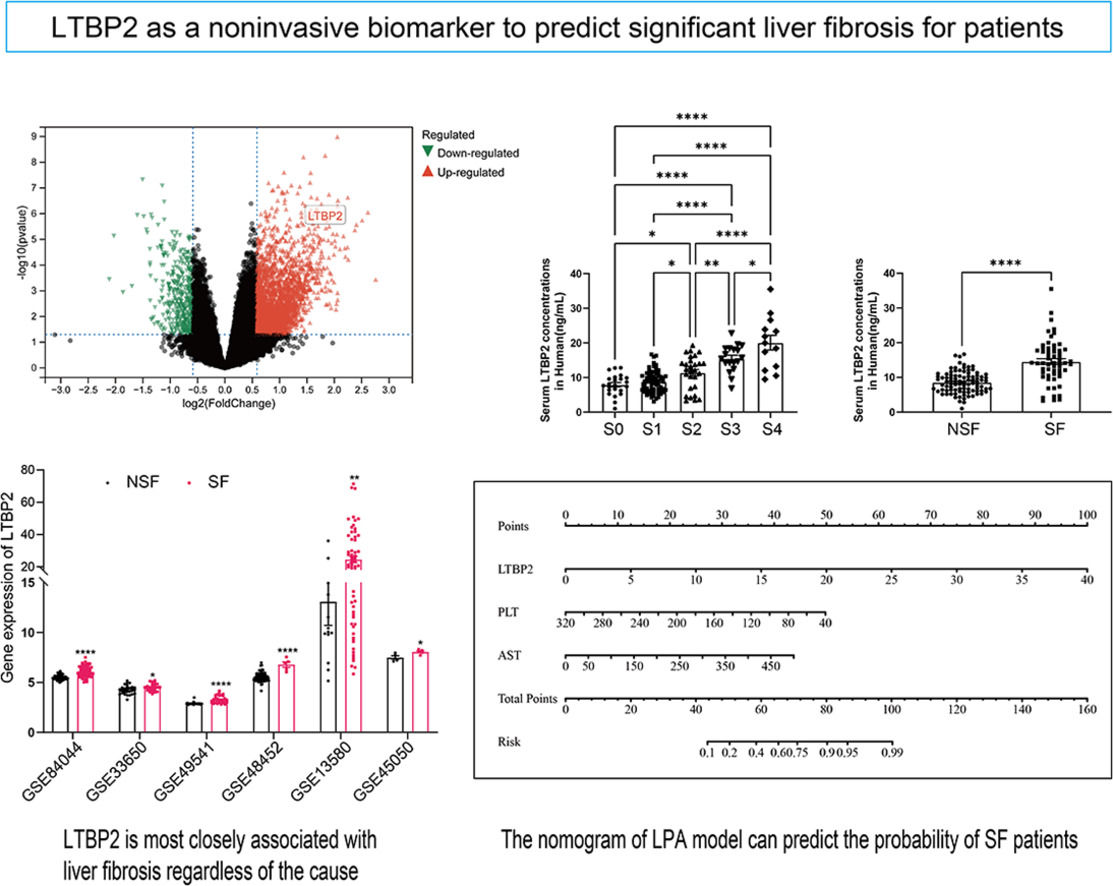
COL1A1, COL1A2, COL5A1 and COL16A1 are key structural components where collagen family members participate in ECM. LOXL1 [22], AEBP1 [23,24] and THBS2 [25,26] have been extensively studied in LF. LTBP2, which has never been studied in LF, aroused our interest. We searched additional databases of LF caused by HBV, HCV, NAFLD and alcoholism to demonstrate further whether LTBP2 is an etiologically dependent agent of LF. We found that LTBP2 expression increased, regardless of the causes of LF (Fig. 4).
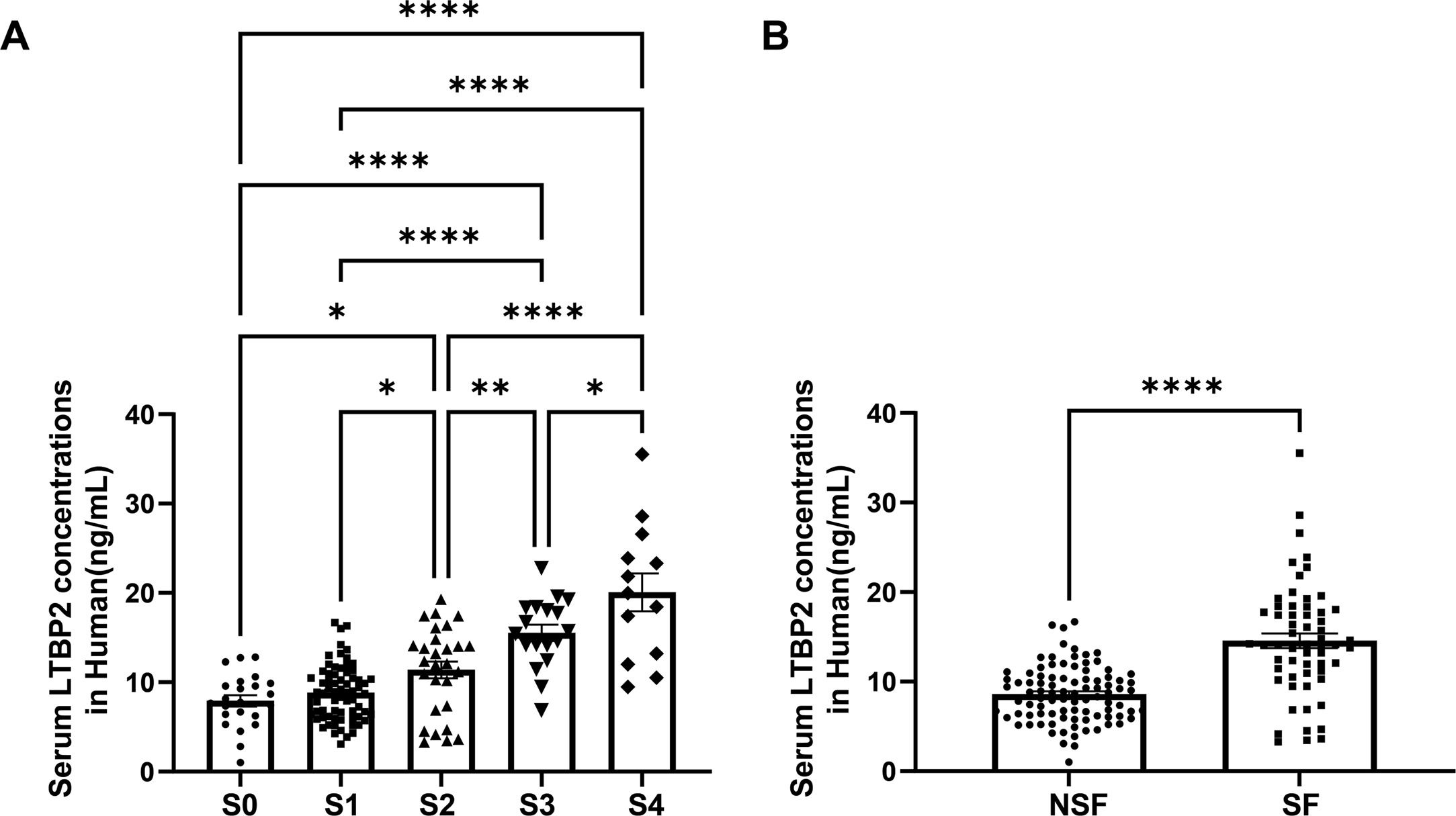
3.5Serum LTBP2 levels identified patients with significant LFIn our transcriptome data set, LTBP2 expression levels showed stepwise up-regulation parallel to the fibrosis stage (Supplementary Fig. 4H), and the expression levels of COL1A1 and ACTA2 were strongly correlated with LTBP2 (Supplementary Fig. 5). The AUROC of intrahepatic LTBP2 mRNA levels for significant fibrosis was significantly higher than that of the FIB-4 index and APRI index (Supplementary Fig. 6). LTBP2 is a multi-domain extracellular matrix protein soluble in blood. Next, we investigated the potential of serum LTBP2 as a noninvasive biomarker to distinguish patients with SF using 151 patients with biopsy. The numbers of patients by fibrosis stage (0/1/2/3/4) and inflammation grade (0/1/2/3) were 23/69/28/18/13 and 2/99/32/18 respectively. There was no difference in age between the two groups (92 patients with NSF and 59 patients with SF), with a higher proportion of males in the SF group (P < 0.05) (Supplementary Table 5). Serum LTBP2 levels showed a stepwise elevation along with the fibrosis stage, and patients in the SF group are much higher than those with NSF (14.16 ng/mL vs. 8.72 ng/mL, P< 0.0001) with a cut-off value of 11.38 ng/mL (Fig. 5 and Supplementary Table 5).
3.6Nomogram and validation of the modelWe wanted to construct a new clinical model to predict the probability of patients developing SF. The number of patients in the training and the validation datasets was close to 7:3, as previous studies described [27]. There are 106 patients in the training cohort and 45 patients in the validation cohort. In the training cohort, we detected several differences between the NSF and the SF patients in laboratory findings, including high LTBP2 level (8.32 ng/mL vs. 13.23 ng/mL, P < 0.0001), lower PLT level (189.00 (10×9/L) vs. 147.00 (10×9/L), P < 0.0001), higher RDW-CV level (12.40 vs. 12.80, P = 0.005), higher AST (27.00 U/L vs. 44.00 U/L, P = 0.0002), lower ALB (43.80 g/L vs. 41.00 g/L, P = 0.037) and PA level (231.30 mg/L vs. 163.84 mg/L, P < 0.0001), longer PT time (13.30 s vs. 13.90 s, P = 0.002) and INR (1.00 vs. 1.08, P = 0.002) in SF group (Supplementary Table 6).
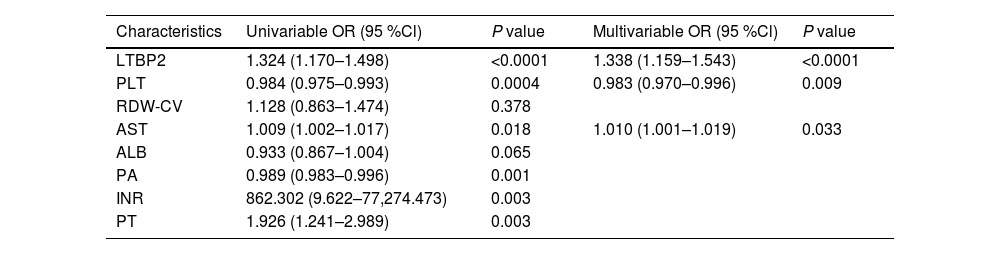
To find the factors most associated with the development of significant liver fibrosis in patients, we analyzed the laboratory parameters using univariate and multivariate analysis in this study (Table 1). In univariate analysis of the training cohort, the increased LTBP2 and AST levels, the decreased PLT and PA levels, longer PT time, and INR were associated with the development of significant fibrosis. Next, we used the data for multivariate logistic regression analysis for the factors of developing significant fibrosis (Table 1). Three variables, including serum LTBP2 levels (odds ratio 1.338, 95 % CI 1.159–1.543, P < 0.0001), PLT levels (0.983, 95 % CI 0.970–0.996, P = 0.009), and AST levels (1.010, 95 % CI 1.001–1.019, P = 0.033) were demonstrated as the independent prediction risk factors.
Risk factors related to NSF and SF groups in the training cohort.
| Characteristics | Univariable OR (95 %Cl) | P value | Multivariable OR (95 %Cl) | P value |
|---|---|---|---|---|
| LTBP2 | 1.324 (1.170–1.498) | <0.0001 | 1.338 (1.159–1.543) | <0.0001 |
| PLT | 0.984 (0.975–0.993) | 0.0004 | 0.983 (0.970–0.996) | 0.009 |
| RDW-CV | 1.128 (0.863–1.474) | 0.378 | ||
| AST | 1.009 (1.002–1.017) | 0.018 | 1.010 (1.001–1.019) | 0.033 |
| ALB | 0.933 (0.867–1.004) | 0.065 | ||
| PA | 0.989 (0.983–0.996) | 0.001 | ||
| INR | 862.302 (9.622–77,274.473) | 0.003 | ||
| PT | 1.926 (1.241–2.989) | 0.003 |
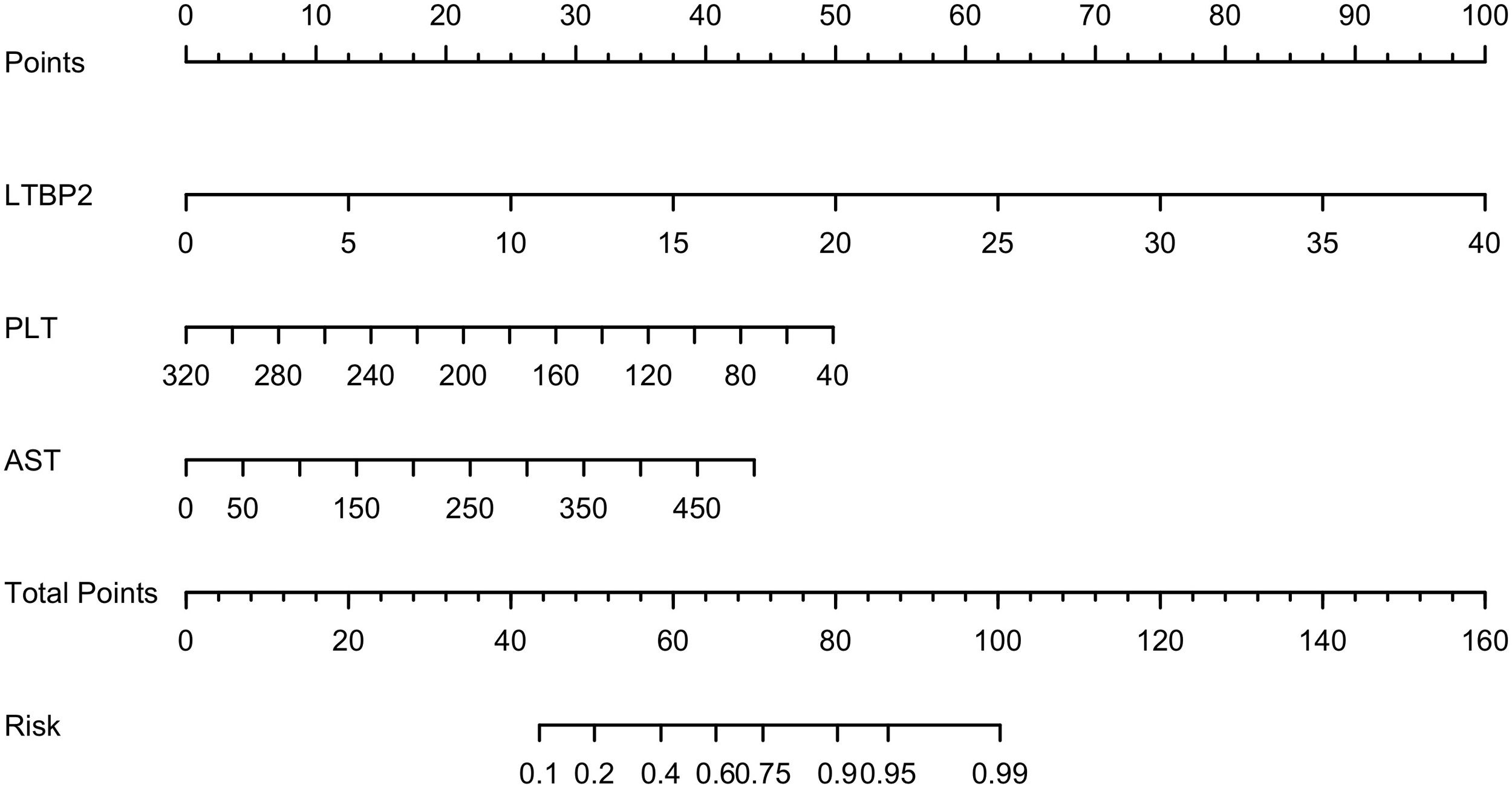
Based on the results of multiple logistic regression, we constructed a nomogram to predict the probability of SF occurrence in patients and named it the LPA model (Fig. 6). The maximum cut-off point value of the Youden index was 60 points, and the sensitivity, specificity, positive predictive value, and negative predictive value of the model were 0.767, 0.873, 0.805, and 0.846, respectively. In addition, we uploaded the model to a web page for clinicians to easily use (https://model-of-lf.shinyapps.io/LPA-MODEL/).
The nomogram of the LPA model. A Nomogram to evaluate the risk factors for patients developing significant fibrosis. In the nomogram, all relevant patient values have been positioned and drawn up along each variable axis and a point axis to determine the score for each variable value calculation. The score of the sum is located on the total axis, and a line is drawn down to determine the probability of the patient having an adverse outcome.
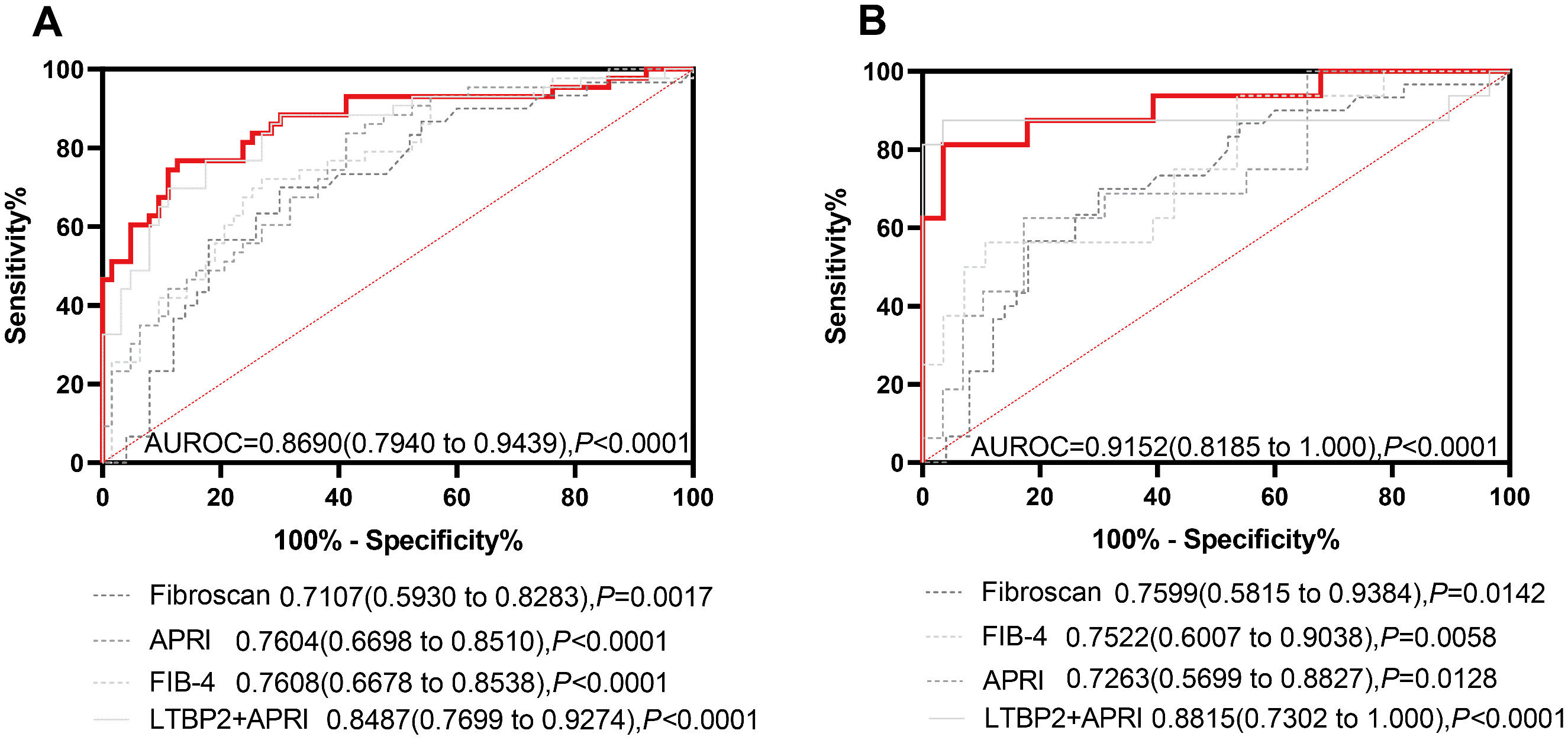
As is shown in the nomogram, three factors bring varying degrees of risk to patients. High levels of LTBP2 bring the highest risk to patients, followed by high levels of AST and decreased PLT. An overall score scale board is listed below to calculate the likelihood of SF developing by adding the scores for the three risk factors (Fig. 6). The area under the curve (AUC) for the nomogram was 0.8690 (95 % CI: 0.7940 to 0.9439, P < 0.0001) (Fig. 7A). With 1000 cycles of bootstrap resampling, the calibration curve of the model indicated good agreement between the predicted probability and the observed probability (Supplementary Fig. 7A). In addition, the probability of patients' SF occurring in the validation cohort was calculated using the nomogram model. The nomogram of the validation cohort displayed an area under the curve (AUC) of 0.9152 (95 % CI: 0.8185 to 1.000, P < 0.0001) (Fig. 7B) with a good calibration curve in estimating the risk (Supplementary Fig. 7B). The diagnostic performance of LPA model was then compared with APRI (0.7604, 95 % CI: 0.6698 to 0.8510, P < 0.0001), FIB-4 (0.7608, 95 % CI: 0.6678 to 0.8538, P < 0.0001) and Fibroscan (0.7107, 95 % CI: 0.5930 to 0.8283, P = 0.0017), and the LPA model was found to have advantages (Fig. 7). APRI index includes AST and PLT. Therefore, we compared the LPA model with LTBP2 combined APRI (0.8487, 95 % CI: 0.7699 to 0.9274, P < 0.0001 (Fig. 7), and the results showed that the LPA model still had advantages. Decision curve analysis (DCA) further evaluates the nomogram model and shows the nomogram model presented a greater net benefit with a wider range of threshold probabilities for predicting the risk of people developing SF (Supplementary Fig. 8).
Receiver operating characteristic curve of the performance of LPA model, APRI, FIB-4, Fibroscan, and LTBP2+APRI in the training cohort and validation cohort. A. ROC curve to assess discrimination performance in the training cohort. B. ROC curve for assessing discrimination performance in the validation cohort. The red lines represent the LPA model.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In this study, we performed RNA sequencing of liver tissue from 66 naive hepatitis B patients and obtained a comprehensive analysis of molecular abnormalities associated with fibrosis development. We focused on studying gene expression profiles between SF and NSF samples and identified DEGs that could provide new insights into LF. With the NSF as a control, there are 1833 upregulated and 608 downregulated genes in significant fibrosis. Pathway analysis revealed that biological processes associated with fibrosis are highly activated, suggesting a potential application of omics approaches in identifying patients with SF. Using Cytoscape software, we have identified 31 high-confidence hub genes that may play a role in liver fibrosis.
HSCs are the most important effector cells involved in hepatic fibrosis. They are the primary sources of ECM secretion and play a crucial role in the occurrence and development of liver fibrosis [3,28]. We analyzed one HSC transcriptome GSE68001, from the GEO datasets. These genes were analyzed for HSC activation, and 16 genes related to liver fibrosis and HSC activation were obtained. Spearman correlations were constructed using gene expression values and histological scores, and genes with R > 0.6 were considered highly associated with fibrosis. AEBP1, COL1A1, COL3A1, COL1A2, COL5A1, LOXL1, KRT7, LTBP2, COL16A1, THBS2 and NOTCH3 are considered to be the most associated with HSC activated and have priority in differentiating the stages of liver fibrosis.
When HSCs were activated in vitro with TGF-β for 24h, AEBP1, COL1A1, COL1A2, COL5A1, LOXL1, LTBP2, COL16A1 and THBS2 were significantly upregulated. COL1A1, COL1A2, COL5A1, and COL16A1 are key structural components in which members of the collagen family participate in ECM. To date, LOXL1 [22,29] and THBS2 [25,26] have been widely studied in LF. AEBP1 is a widely expressed protein in tissues rich in ECM, such as adipose tissue, large blood vessel walls, skin, and other organs [30]. Previous studies have shown that ACLP [31] is highly expressed in the lung tissues of patients with pulmonary fibrosis and mice. A study[23] has shown that miR-372-3p upregulates AEBP1 in patients with non-alcoholic steatohepatitis with worsening fibrosis, but more detailed mechanisms are lacking. Recently, research has shown that AEBP1 silencing alleviates renal fibrosis in vivo and in vitro via inhibiting the β-catenin signaling pathway [32]. Studying AEBP1’s mechanism in LF could serve as a therapeutic target and biomarker. The role of LTBP2 in LF has not been validated experimentally. In the present study, we validated a strong positive association between liver LTBP2 levels and the progression of fibrosis.
Next, we searched for other causes of liver fibrosis. Through transcriptome analysis, we identified that LTBP2 was most significantly upregulated in the livers of patients with significant fibrosis, not limited to the causes. Indeed, the intrahepatic LTBP2 level is strongly associated with fibrogenesis markers (COL1A1 and ACTA2). The AUROC of LTBP2 mRNA levels for significant fibrosis was superior to the FIB-4 index and APRI index. Subsequently, we also demonstrated the effectiveness of serum LTBP2 as a non-invasive biomarker for the identification of SF. The nomogram of the LPA model, including serum LTBP2 level, PLT, and AST was identified to predict the probability of SF occurrence in patients. The nomogram shows how the three independent predictors affect the prognosis. The AUROC for the nomogram was 0.8690 (95 % CI: 0.7940 to 0.9439, P < 0.0001), and validation in the validation cohort was 0.9152 (95 % CI: 0.8185 to 1.000, P < 0.0001). Meanwhile, we have verified the accuracy and clinical applicability of the model through calibration curves and DCA. The LPA model might provide even better diagnostic ability for significant fibrosis, and we posted the model to the web with the hope of making it available to more clinicians in the future.
LTBP2 is a multi-domain exocrine protein present in the ECM. It is an important molecule in the TGF-β complex, participates in forming microfibrils, and plays a role in cell adhesion, belonging to the LTBP fibrin family.
Previous studies have shown that LTBP2 may be associated with fibrosis or tissue remodeling in multiple organs, including the skin [33], heart [34], and lungs [35]. A study of dilated cardiomyopathy (DCM) showed that LTBP2 was upregulated in fibrotic tissues of DCM rats, and silencing LTBP2 reduced oxidative stress damage, myocardial fibrosis, and myocardial remodeling in DCM rats by downregulating the NF-κB signaling pathway [36]. Increased LTBP2 expression was also found in Bleomycin-induced mouse models of pulmonary fibrosis, and silencing LTBP2 prevents fibroblast differentiation into myofibroblasts and subsequent pulmonary fibrosis by inhibiting the phosphorylation of NF-κB signaling and nuclear trans-localization [35]. Studies have shown that LTBP2 expression is upregulated in human thyroid cancer cell lines and that LTBP2 knockdown inhibits the proliferation, invasion, and EMT phenotype of thyroid cancer cells and can reduce thyroid cancer growth in nude mice [37]. Other studies have shown that LTBP2 expression is increased in gastric cancer and that LTBP2 silencing effectively inhibits the proliferation, migration, and epithelial-mesenchymal transformation of gastric cancer cells [38]. In our study, KEGG and GO pathway enrichment analyses revealed that LTBP2 was enriched in collagen-containing ECM and ECM structural constituent pathways. Currently, no study has reported its role and function in LF. Based on our results, we speculate that LTBP2 may be a novel biomarker and therapeutic target for LF. Studies have demonstrated that the MAPK signaling pathway is closely associated with liver fibrosis [39]. TGF-β is a vital activator of the MAPK signaling pathway [40]. Our results reveal that TGF-β up-regulates the expression of LTBP2. Whether LTBP2 plays a role in liver fibrosis via the MAPK signaling pathway demands further investigation. This will constitute the focus of our future research on the mechanism of LTBP2 in liver fibrosis.
And there are some limitations to our study. First, although there is little literature showing related influencing factors in the form of nomograms in liver fibrosis, our study cohort is not large enough. These results may not fully summarize the characteristics that affect patient outcomes. Second, all samples were from a single center, and external hospitals other than our hospital could not be used to verify our conclusions. Third, the use of serum markers like LTBP2 has limitations, such as potential interference from other factors that affect liver function. Fourth, further in vivo and in vitro experiments are needed to test the role of LTBP2 in LF therapy.
5ConclusionsThis study suggests that LTBP2 may play a key role in LF development based on whole-genome expression profiles of HBV-associated LF patients combined with HSCs data. It was also identified as an etiologically independent factor in the LF database due to other causes. In addition, our study developed the nomogram model with LTBP2, PLT, and AST as variables to predict the probability of significant fibrosis. The LPA model showed better diagnostic performance in patients and may help clinicians in the future.
Author contributionsM-X L and L C designed the study. All authors were involved in sample collection, generation, collection, compilation and analysis of data. M-X L, S T and X-Y L wrote the manuscript; L C, Y Z and Q L revised the manuscript. All the authors have read the manuscript and approved the final manuscript.