Intrahepatic cholestasis of pregnancy (ICP) is often accompanied by fetal and maternal complications.
Materials and MethodsRetrospective review of the clinical course of women with ICP and their neonates treated at our medical center over a 10-year period. Special attention was paid to the maternal and neonatal response to 2 different modes of ursodeoxycholic acid (UDCA) administration.
ResultsNeonates of mothers with high total bile acid levels had a poorer composite neonatal outcome. Twenty-seven women who presented at an advanced stage of their pregnancies did not receive UDCA. UDCA was administered in 2 modes: either a full dose at admission (76 women) or a gradually increasing dose until the desired dosage was reached (25 women). The mean gestational age at delivery for the 94 neonates that were exposed to full UDCA dose was the lowest (36±2.3 weeks for the full dose, 37±1.4 weeks for the 30 neonates from the gradually increasing dose, 38±1.6 weeks for the 29 neonates from the no treatment group, p<0.001). The group of neonates that were exposed to full UDCA dose had the highest rate of unfavorable composite neonatal outcome (53% for full dose, 30% for gradually increasing dose, 24% for the no treatment group, p=0.006).
ConclusionsCompared to the administration of a full UDCA dose, the administration of a gradually increasing dose of UDCA may be associated with a greater gestational age at delivery and fewer events of unfavorable composite neonatal outcomes. These novel findings should be retested prospectively in a large cohort of patients.
The most common cause of elevated liver enzymes during the second and third trimesters of pregnancy is Intrahepatic Cholestasis of Pregnancy (ICP) [1,2]. The incidence of ICP varies throughout the globe, ranging from 0.1 to 1.5% in mothers from central and western Europe and North America to 1.5–4.0% in South America, especially in Chile [2]. The variability in the prevalence of ICP depends on genetic, geographical, and environmental factors [1–4].
ICP is often accompanied by fetal and maternal complications [5–13]. Independent predictors of fetal and maternal outcome in patients with ICP have been suggested and include genetic background, the serum levels of liver enzymes, and the fasting total bile acids (TBA). High TBA levels (> 40 μmol/L) were found to be associated with meconium staining of the amniotic fluid (MSAF), low APGAR scores, premature birth, and intra uterine fetal death (IUFD) [10–16].
In recent years, the introduction of Ursodeoxycholic Acid (UDCA) at a dose of 15 mg/kg/day has become the common practice in pregnant women with ICP [17–19]. UDCA administration has been associated with reduced incidence of fetal complications, improved maternal symptoms, and possible reduction of maternal postpartum blood loss [17–19].
The aims of our study were to describe the maternal and neonatal outcomes of women with ICP treated at our center and their neonates, and their association with serum TBA levels at presentation. Moreover, we aimed to describe the effects of UDCA administration on the maternal and neonatal outcomes of those patients. UDCA administration was performed in two modes: either in a full dose at admission or via a protocol of gradually increasing the dose until the desired dosage was reached.
2Patients and Methods2.1Setting and populationWe describe a historical cohort study. The study population was composed of all ICP patients who were hospitalized at one of the two medical centers of Hadassah Medical Organization (HMO) during a ten-year period (from November 2007 until December 2017). During this period, 1405 patients were tested for serum TBA levels at our biochemistry laboratory. Fasting serum TBA levels were measured using TBA assay kit (Diazyme Laboratories, CA, USA). The reference range for bile acids depends on the assay technique used and whether the patient fasted before blood sampling. Some studies use an upper limit of normal between 10 and 14 micromoles/L for an enzymatic assay, but this may be reduced to between 6 and 10 micromoles/L in fasting women [10].
The study cohort included women who suffered from pruritus during the second or third trimester of pregnancy and were found to have elevated AST and/or ALT levels and elevated 12 h fasting TBA levels (> 6 μmol/L)[10]. We excluded women with no reliable diagnosis of ICP; women who did not give birth at one of the HMO centers; women found to have normal AST and/or normal ALT levels; women suffering from any viral or bacterial infection (including active infection with either HIV and/or hepatitis B, and/or hepatitis C virus); women reporting psychoactive substances or alcohol use prior to hospitalization; women suffering from any active liver disease (like chronic autoimmune hepatitis, primary biliary cholangitis, Wilson's disease); and patients in whom pruritus was secondary to an active skin disorder.
Obstetrical care was provided to all patients with ICP in accordance with accepted standard of care measures [10]. UDCA administration was not provided to all our ICP patients in a uniform manner. Three modes of UDCA administration were identified among the ICP patients during the study period: no UDCA treatment, full recommended UDCA dose from the first day of hospitalization [17–19]; or gradually increasing UDCA dose [20]. Gradual UDCA administration was as follows: day 1: 100–200 mg; day 2: 200–300mg; day 3: 300–400 mg. The doses of UDCA reported on the first, second and third days represent the total dose administered to the patients on each day of their hospitalization. From the fourth day, gradual increments of the UDCA dose (but no more than 300–400 mg per increment) were introduced until the desired dose was achieved. The decision on whether to administer UCDA and the mode and dosage of UDCA prescribed was made independently by the attending obstetricians providing patient care in the two medical centers. At that time, no research was envisioned.
The following parameters were extracted from the maternal clinical files: gestational age at ICP diagnosis; number of fetuses (single, twin or triplets); mode and dosage of UDCA; presence of maternal co-morbidities (diabetes, gestational diabetes, chronic high blood pressure); presence of proteinuria (>300 mg daily); presence of pre-eclampsia; presence of thrombophilia (congenital or acquired); administration of medications such as aspirin and/or one of the low molecular weight heparins; smoking; preterm labor (spontaneous or iatrogenic); cesarean or operative delivery; occurrence of postpartum hemorrhage, premature rupture of membranes (PROM), or chorioamnionitis.
Maternal aminotrasferases levels were measured at admission and every few days during the follow-up period until delivery. Two representative measurements of each aminotrasferase taken during the follow-up period for each patient are presented in the appropriate tables.
The following clinical parameters were extracted from the neonatal files and included: 5-minute APGAR score; umbilical cord blood pH; and neonatal complications including IUFD, intra uterine growth restriction (IUGR), fetal heart abnormality, MSAF, hyperbilirubinemia, respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), admission to the neonatal intensive care unit (NICU), and the need for mechanical ventilation.
To compare the maternal and neonatal outcomes in the three study groups, composite neonatal and composite maternal outcomes were defined. Composite maternal outcome included any of the following: gestational diabetes and pregnancy-induced hypertension, cesarean section, postpartum hemorrhage, and preterm delivery (<37 weeks).
Composite neonatal outcome included the presence of any of the following: 5 min APGAR score < 7, NICU admission, sepsis, IUGR, fetal heart tracing abnormality, hyperbilirubinemia requiring phototherapy, MSAF, RDS, TTN, mechanical ventilation, and stillbirth.
2.2Statistical analysisDescriptive statistics are reported as mean and standard deviation for parametric variables and percent for categorical data. One-way analysis of variance with the F-test was used to compare means between the study groups, and the Chi-square test was used for comparing categorical data. A p-value below 0.05 was considered statistically significant.
2.3Ethical considerationsThe research proposal was approved by the Helsinki Committee of the HMO (No: 0275-16-HMO). Due to the retrospective nature of the present research, no personal informed consent was required.
3ResultsThe study population included 143 women suffering from ICP who were hospitalized and subsequently gave birth in our center to 171 neonates. All these women suffered from generalized itching and elevated AST and ALT levels.
Demographic and clinical data of the ICP patients are presented in Table 1. Seven women suffered from various medical conditions that were diagnosed prior to the present pregnancy: three with mild fatty liver disease, two with hypertension, one with diabetes mellitus, and one was a smoker. Admission TBA levels in the women with clinical diagnosis of ICP were as follows: less than 10 μmol/L (n=64), 10–20 μmol/L (n=39), 20–40 μmol/L (n=24) and ≥40 μmol/L (n=16) women.
Demographic and laboratory tests in intrahepatic cholestasis of pregnancy (ICP) patients by admission total bile acids (TBA) level.
| TBA level(μmol/L) | <10 | 10 - 20 | 20 - 40 | ≥40 | p-value |
|---|---|---|---|---|---|
| Women with ICP | 64 (44.7%) | 39 (27.3%) | 24 (16.8%) | 16 (11.2%) | |
| Parity | 1.4 (1.7) | 0.9 (1.0) | 1.0 (1.4) | 1.7 (2) | 0.16 |
| Gravidity | 3.0 (2.4) | 2.2 (1.4) | 2.2 (1.6) | 3.4 (2.4) | 0.08 |
| Mean maternal age | 29.5 (6.2) | 29.3 (6.5) | 28.3 (5.5) | 35.0 (6) | 0.005 |
| Multiple pregnancies | 5 (8%) | 10 (26%) | 6 (25%) | 4 (25%) | 0.01 |
| Medical history | |||||
| PIH | 5 (8%) | 4 (10%) | 1 (4%) | 1 (7%) | 0.86 |
| Gestational DM | 9 (16%) | 2 (6%) | 2 (9%) | 0 | 0.2 |
| GBS carrier | 5 (8%) | 7 (18%) | 4 (17%) | 1 (6%) | 0.74 |
| Previous ICP | 14 (21%) | 10 (26%) | 3 (12%) | 4 (25%) | 0.62 |
| Liver transaminases | |||||
| AST at admission | 83 (69) | 112 (88) | 84 (56) | 94 (79) | 0.14 |
| ALT at admission | 122 (87) | 167 (134) | 106 (85) | 134 (136) | 0.06 |
| 2nd AST | 98 (94) | 112 (90) | 106 (84) | 137 (101) | 0.35 |
| 2nd ALT | 132 (112) | 139 (111) | 147 (101) | 194 (174) | 0.20 |
| 3rd AST | 73 (53) | 61(50) | 101 (108) | 92 (97) | 0.16 |
| 3rd ALT | 112 (94) | 84 (82) | 136 (101) | 166 (198) | 0.06 |
Continuous parameters are presented as mean (± standard deviation). PIH, pregnancy-induced hypertension; DM, diabetes mellitus; GBS, group B streptococcal; AST, aspartate transaminase (expressed in IU/L); ALT, alanine transaminase (expressed in IU/L).
Women with the highest TBA levels were older than those with lower TBA levels (<40 μmol/L) (p=0.005). Women with the lowest TBA levels (< 10 μmol/L) had significantly more singleton pregnancies compared with those with higher TBA levels (> 10 μmol/L). Except for these findings, there were no differences in parity, gravidity, or medical history among the four patient groups. Moreover, no significant correlation was found between the admission TBA levels with the admission AST and ALT levels (Table 1).
In 27 women (18.9 %), UDCA was not administered at all. In these women, the presentation of pruritus occurred in a more advanced stage of the pregnancy than among women who were given UDCA, [36 ±2.5 vs. 31.5 ±3.4 weeks of gestation respectively, p<0.001]. As UDCA requires more than two weeks of administration to be fully effective [21], it was decided that these women would not be treated with UDCA.
The no treatment group women gave birth shortly after being diagnosed with ICP [1.8 ±2.1 weeks vs. 4.6 ±3.2 weeks in those that received UDCA, p<0.001]. Of the 27 women not given UDCA, 11 (41%), presented with TBA levels of more than 10 μmol/L. Two women (2/11), were found to have TBA levels > 40 μmol/L. Both women delivered immediately after presentation, at gestational weeks 34 and 37 (Table 2 and Appendix Table 1).
Maternal outcomes in intrahepatic cholestasis of pregnancy (ICP) patients by admission total bile acids (TBA) level.
| TBA level (μmol/L) | <10 | 10–20 | 20–40 | ≥40 | p-value |
|---|---|---|---|---|---|
| Women with ICP | 64 | 39 | 24 | 16 | |
| IVF treatment | 4 (6.3%) | 7 (18%) | 4 (17%) | 0 | 0.32 |
| GA at onset of pruritus | 33.6 (3.4) | 32 (3.7) | 32 (4.2) | 31 (5.1) | 0.07 |
| Mode of UDCA administration† | |||||
| Full dose at admission | 27 (47%) | 26 (70%) | 11 (58%) | 12 (80%) | 0.16 |
| Gradual dosing | 14 (24%) | 7 (19%) | 3 (16%) | 1 (7%) | |
| No UDCA administration‡ | 16 (28%) | 4 (11%) | 5 (26%) | 2 (13%) | |
| Duration of UDCA administration (weeks) | |||||
| Full dose at admission | 2.9 (2.6) | 3.1 (2.1) | 2.2 (2.4) | 3.2 (3.9) | 0.6 |
| Gradual dosing | 4.3 (2.8) | 3.6 (3.1) | 3.3 (2.5) | 8.0& | 0.5 |
| Pregnancy complications | |||||
| Preeclampsia | 3 (5%) | 5 (13%) | 1 (4%) | 1 (6%) | 0.41 |
| Oligohydramnios | 1 (1.5%) | 1 (3%) | 1 (4%) | 0 (0) | 0.6 |
| Premature contractions | 8 (12%) | 7 (18%) | 5 (20%) | 5 (25%) | 0.47 |
| Spontaneous preterm delivery | 4 (7%) | 5 (13%) | 3 (16%) | 3 (19%) | 0.1 |
| PROM | 2 (3%) | 3 (8%) | 1 (4%) | 3 (18%) | 0.12 |
| postpartum hemorrhage | 0 | 4 (11%) | 3 (16%) | 0 | 0.1 |
| Mode of delivery§ | 0.26 | ||||
| Cesarean section | 20 (31%) | 8 (21%) | 8 (32%) | 7 (44%) | |
| OPV birth | 2 (3%) | 6 (15%) | 2 (8%) | 0 | |
| NVD | 41 (64%) | 24 (62%) | 14 (58%) | 8 (50%) | |
| Composite maternal outcome | 46 (72%) | 20 (51%) | 18 (75%) | 12 (75%) | 0.1 |
Continuous parameters are presented as mean (± standard deviation). IVF, in vitro fertilization; GA, gestational age; PROM, premature rupture of membranes; OPV, operative vaginal; NVD, normal vaginal delivery. The composite maternal outcome includes any gestational diabetes, pregnancy-induced hypertension, cesarean section, postpartum hemorrhage, or premature contractions.
Maternal outcomes among our patient population are presented in Table 2. The gestational age at the onset of pruritus and the mode of UDCA administration did not differ between the groups of patients who received UDCA. Moreover, there were no significant differences in pregnancy complications, composite maternal outcome, and mode of delivery among the various groups of patients (Table 2). UDCA administration (yes or no) and the mode of UDCA administration (either full dose at admission or gradually increasing dose) had no impact on maternal aminotrasferases levels or on any of the maternal outcome complications that were recorded, and nor on composite maternal outcome (Appendix Tables 1 and 2).
Neonatal outcomes are presented in Tables 3 and 4 and Appendix Table 3. As opposed to maternal outcome, composite neonatal outcome was relatively poor. Two cases of stillbirth were recorded. While the first case occurred in a patient with high TBA levels (> 40 μmol/L), the latter occurred in a patient with very low TBA levels (< 10 μmol/L) (table 3). Neonates born to mothers with higher TBA levels had a lower gestational age of delivery, lower birth weight and higher rates of NICU admission, higher rates of mechanical ventilation and poorer composite outcome (Table 3 and Appendix Table 3). No differences were observed in 5-minute APGAR scores, MSAF, oligohyramnios, IUGR, abnormal fetal cardiotocography, hyperbilirubinemia, RDS, sepsis, TTN, and stillbirth (Table 3 and Appendix Table 3).
Neonatal outcomes by maternal total bile acids (TBA) level at admission.
| TBA level (μmol/L) | <10 | 10–20 | 20–40 | ≥40 | p-value |
|---|---|---|---|---|---|
| No. of newborns | 69 | 50 | 30 | 22 | |
| APGAR score (< 7) | 1 (1.5%) | 0 | 0 | 1 (5%) | 0.4 |
| Birth weight (Kg) | 2. 9 (0.56) | 2.6 (0.57) | 2.4 (0.63) | 2.5 (0.62) | 0.01 |
| pH of umbilical blood | 7.3 (0.06) | 7.3 (0.07) | 7.2 (0.1) | 7.3 (0.9) | <0.001 |
| MSAF | 6 (9%) | 2 (4%) | 6 (20%) | 1 (5%) | 0.1 |
| Oligohydramnios | 1 (1.5%) | 2 (4%) | 1 (3%) | 0 | 0.7 |
| IUGR | 3 (4%) | 4 (8%) | 2 (7%) | 0 | 0.6 |
| FHTA | 10 (15%) | 7 (14%) | 2 (7%) | 2 (9%) | 0.7 |
| NICU admission | 5 (7%) | 13 (26%) | 9 (30%) | 8 (36%) | 0.002 |
| Hyperbilirubinemia | 11 (16%) | 10 (20%) | 4 (13%) | 6 (27%) | 0.5 |
| RDS | 0 | 4 (8%) | 1 (3%) | 1 (5%) | 0.07 |
| MV | 3 (4%) | 9 (18%) | 9 (30%) | 14 (18%) | <0.001 |
| Sepsis | 3 (4%) | 0 | 2 (7%) | 2 (9%) | 0.14 |
| TTN | 1 (1.5%) | 3 (6%) | 3 (10%) | 1 (5%) | 0.2 |
| Stillbirth | 1 (1.5%) | 0 | 0 | 1 (5%) | 0.4 |
| Composite neonatal outcome | 22 (32%) | 25 (50%) | 14 (46%) | 14 (64%) | 0.04 |
Continuous parameters are presented as mean (± standard deviation).APGAR score was measured at 5 min. MSAF, meconium staining of the amniotic fluid; IUGR, intra uterine growth restriction; FHTA, fetal heart tracing abnormality; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome; MV, Mechanical ventilation; TTN, transient tachypnea of the newborn. The composite neonatal outcome includes any of: APGAR at 5 min< 7, NICU admission, sepsis, IUGR, FHTA, hyperbilirubinemia requiring phototherapy, MSAF, RDS, TTN, MV, and stillbirth.
Statistically significant p values are in bold.
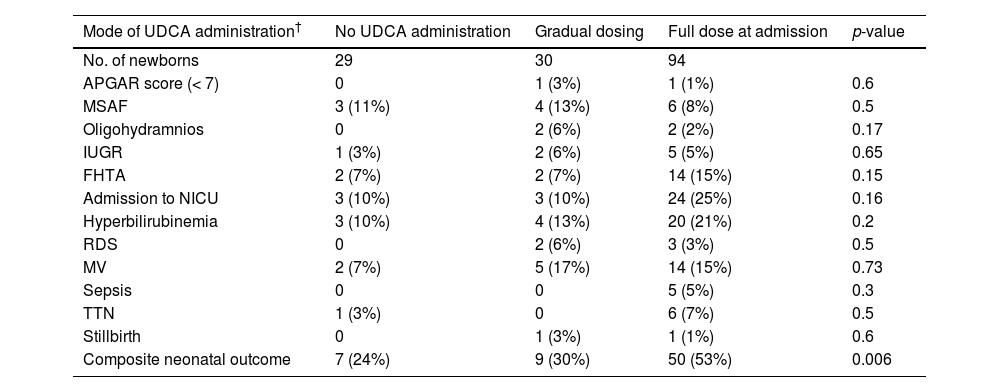
Neonatal outcomes in intrahepatic cholestasis of pregnancy (ICP) patients by the mode of Ursodeoxycholic acid (UDCA) administration.
| Mode of UDCA administration† | No UDCA administration | Gradual dosing | Full dose at admission | p-value |
|---|---|---|---|---|
| No. of newborns | 29 | 30 | 94 | |
| APGAR score (< 7) | 0 | 1 (3%) | 1 (1%) | 0.6 |
| MSAF | 3 (11%) | 4 (13%) | 6 (8%) | 0.5 |
| Oligohydramnios | 0 | 2 (6%) | 2 (2%) | 0.17 |
| IUGR | 1 (3%) | 2 (6%) | 5 (5%) | 0.65 |
| FHTA | 2 (7%) | 2 (7%) | 14 (15%) | 0.15 |
| Admission to NICU | 3 (10%) | 3 (10%) | 24 (25%) | 0.16 |
| Hyperbilirubinemia | 3 (10%) | 4 (13%) | 20 (21%) | 0.2 |
| RDS | 0 | 2 (6%) | 3 (3%) | 0.5 |
| MV | 2 (7%) | 5 (17%) | 14 (15%) | 0.73 |
| Sepsis | 0 | 0 | 5 (5%) | 0.3 |
| TTN | 1 (3%) | 0 | 6 (7%) | 0.5 |
| Stillbirth | 0 | 1 (3%) | 1 (1%) | 0.6 |
| Composite neonatal outcome | 7 (24%) | 9 (30%) | 50 (53%) | 0.006 |
Continuous parameters are presented as mean (± standard deviation). APGAR score was measured at 5 min. MSAF, meconium staining of the amniotic fluid; IUGR, intra uterine growth restriction; FHTA, fetal heart tracing abnormality; NICU - neonatal intensive care unit; RDS- respiratory distress syndrome. MV- Mechanical ventilation; TTN - transient tachypnea of the newborn. The composite neonatal outcome includes any of APGAR at 5 min< 7, NICU admission, sepsis, IUGR, FHTA, hyperbilirubinemia requiring phototherapy, MSAF, RDS, TTN, MV and stillbirth.
A novel and unique finding in our study was the finding that the mode of UDCA administration was associated with neonatal outcome. There were 25 women who received gradual UDCA treatment, accounting for 24.2% (30/124) neonates who were exposed in-utero to gradual UDCA administration.
Comparing neonates exposed to gradual vs. full dose UDCA treatment, beneficial effects were observed in the gradual administration group. These neonates had a higher gestational age at delivery (37±1.4 weeks vs. 36±2.3 weeks in the gradual vs. full dose UDCA treatment groups, respectively, p<0.001) (Appendix Table 1). Moreover, these neonates had fewer events of composite neonatal outcome (24% in the no treatment group, 30% in the gradually increasing group, and 53% in the full dose group, p=0.006) (Table 4).
Duration of exposure to UDCA was not associated with increased rates of poor neonatal outcome. Maternal TBA level had no effect on the association between UDCA administration and maternal or neonatal outcome; neither did the week of pruritus presentation.
On logistic regression, controlling for maternal age, number of fetuses, gestational age at pruritus presentation, gestational age at delivery, and TBA level, there was no significant association between UDCA treatment and poor neonatal outcome. However, using a similar model controlling for maternal age, number of fetuses, TBA level, and weeks of UDCA administration revealed that UDCA administration was associated with an OR of 3.9 towards poor neonatal outcome (95% CI 1.21–12.6, p=0.02) compared to no treatment. In this study, 32 women with gestational diabetes, gestational hypertension, or preeclampsia were included. Controlling for these conditions still demonstrated that UDCA administration, compared to no administration, was associated with an OR of 3.2 (95% CI 1.12–9.4, p=0.03) towards more poor neonatal complications.
During the study period, 12 women from our original cohort were readmitted to our medical centers with recurrent ICP during their subsequent pregnancies: 11 women were readmitted following one recurrence and one patient suffered two recurrences. The maternal and neonatal information from these 13 additional episodes of ICP was not included in the present manuscript.
4DiscussionIn the present retrospective cohort study comprising 143 women suffering from ICP who delivered 171 neonates in our medical centers in Jerusalem during a 10-year period, we observed several interesting findings.
After stratification of our patient population by TBA levels, we were unable to detect any differences in demographic parameters, gestational and medical history, and levels of liver aminotrasferases at ICP presentation, gestational age at onset of pruritus, mode and duration of delivery, and composite maternal outcome.
UDCA is considered the drug of choice for patients with ICP [17–19]. UDCA is a natural component of the human bile, accounting for 1-3% of bile acids in healthy subjects [20]. It has been reported that in patients with ICP, UDCA administration provides multiple maternal hepatoprotective effects, including improvement in bile acid secretion and anti-cholestatic and anti-apoptotic effects. UDCA administration may also change the bile acid pool, reduce the concentration of other bile acids like cholic, and reduce the expression of oxytocin receptors in the myometrium and thus could decrease the possibility of spontaneous preterm delivery in women with ICP [20,22]. Studies published from various parts of the world reported that UDCA administration was associated with improvement of some maternal parameters such as pruritus [17–19,23,24], reduction in the TBA [17] and aminotrasferases [17,23,24] concentrations, and reduction in the median estimated blood loss during delivery [23].
Recently, results of a meta-analysis of data from multiple studies [22] reported that maternal outcomes were not related to TBA concentrations, and that there were no significant differences in maternal outcomes between women treated with UDCA and women not treated with UDCA [22]. In our study, UDCA administration (yes or no) and the mode of UDCA administration (either full dose at admission or gradually increasing dose) had no impact on the rate of any of the maternal outcome complications that were recorded, and nor on the composite maternal outcome.
After stratification of the neonates into four subgroups according to the maternal TBA levels, we noted that neonates whose mothers had higher TBA levels were prone to be delivered at earlier gestational age and have lower birth weights, as well as higher rates of NICU admission, mechanical ventilation, and poorer composite neonatal outcome. Similar to our results, other investigations from different parts of the globe reported that higher maternal TBA levels (> 40 μmol/L) were associated with adverse perinatal outcomes [6,8,9,12–15,21,25,26].
Administration of UDCA to mothers with ICP was reported to have possible protective effects on the fetus. It may reduce the bile acid concentration in the fetus, probably by up-regulating placental bile acid export [27]. Moreover, it may also have beneficial effects on pathological events that are possibly involved in the processes that cause stillbirth: UDCA was reported to inhibit the uptake and the vasoconstrictor effects of taurocholate in human placenta [28] and to reduce the fetal blood concentrations of N-terminal pro-brain natriuretic peptide, which is a marker of abnormal heart function [29].
Despite the widespread recommendation for UDCA administration in women with ICP, the evidence base for its beneficial effects on perinatal outcomes has not yet been fully established. A randomized controlled trial (PITCHES) that enrolled women with ICP to receive either UDCA (305 women) or placebo (300 women) demonstrated only a reduction in the incidence of MSAF. Otherwise, no reduction of other adverse perinatal events was observed among the 322 neonates who received UDCA [23,24]. Moreover, meta-analyses that reported data on perinatal outcomes from several trials, including a total of 715 women, were inconclusive [19].
To overcome these obstacles, Ovadia and colleagues performed a systematic review of observational and randomized control studies that included individual participant data from 6974 women and their 7135 fetuses. A large proportion of this group [4726 (67.8%) women and their 5097 (71.4%)] fetuses received treatment with UDCA [22]. After analyzing the data from the randomized control studies, these authors concluded that antenatal UDCA treatment was associated with an improved composite outcome (that was defined in this study as a combination of stillbirth and preterm birth) and a significant reduction in the risk of spontaneous (but not iatrogenic) preterm birth for women with singleton pregnancies with TBA concentrations equal or above 40 μmol/L. Additional beneficial findings for antenatal UDCA treatment reported in this analysis were lower odds of MSAF and higher odds for large gestational-age babies [22].
UDCA is considered a safe and well-tolerated drug at its recommended daily doses [20]. Some patients reported gastrointestinal complaints like diarrhea and dyspepsia [20]. In a minority of patients with cholestatic liver diseases and pre-existing pruritus, initiation of UDCA treatment may cause a transient worsening of pruritus. The etiology of this phenomenon is not completely clear. It has been recommended that in patients with pre-existing cholestatic liver diseases and pruritus, UDCA treatment should be administrated gradually, with a slow increase of the dose until the desired dose is achieved [20].
Bacq and colleagues from France reported their experience with gradual introduction of UDCA in 98 women with ICP. UDCA was prescribed until delivery in all patients at a mean dose of 14 mg/kg/day for a mean duration of 30.4 days. Improvement of pruritus and a decrease in ALT levels and TBA concentrations were observed in a substantial percentage of their patients. The authors of this study reported the results of the maternal outcome for the whole group of patients without reporting whether there was any difference in the maternal and neonatal outcome between those women in whom UDCA was introduced gradually to those who received the full dose of UDCA immediately after they were diagnosed with ICP [30].
In our study, the mode of UDCA administration had an impact on only two parameters: the gestational age at delivery and the composite neonatal outcome. Neonates who were exposed to gradually increasing UDCA doses were found to have a higher gestational age at delivery and fewer events of composite neonatal outcome than those who were exposed to the full dose of UDCA dose immediately after their mothers were diagnosed with ICP.
The causes for these unique and novel observations regarding the beneficial effects of gradual UDCA administration are not known. It can be hypothesized that the slow and gradual replacement of endogenous bile acid derivates within the bile acid pool could prevent the transient accumulation of endogenous bile acid conjugates and the formation of pruritogenic substances that may harm the fetus and promote early delivery [20,22,27–29]. As a result of the greater gestational age at delivery, these neonates probably suffered fewer complications related to prematurity.
Our study has several limitations. Firstly, this is a retrospective observational study and as such is subject to selection bias. Secondly, multiple physicians took care of these patients and the documentation of the medical records regarding symptoms (like pruritus and its intensity during the hospital course) and the mode of UDCA administration was not uniform. Thirdly, the number of patients included in this study are relatively small and firm conclusions from our findings regarding the possible favorable therapeutic effect of gradual introduction of UDCA to women with ICP, should be considered with caution. Despite these limitations, our study has several strengths: the inclusion criteria were very clear, and all patients included in our study suffered from ICP. Moreover, all patients had fasting admission TBA levels, all the laboratory testing was performed during the study period by the same laboratory, using the same laboratory kits and techniques, and the data presented in this manuscript were retrieved from the archives of our institution and not from multiple sources.
5ConclusionsOur study showed that neonates born to mothers with ICP have high rates of poor composite neonatal outcomes. Neonates whose mothers had higher TBA levels had poorer composite outcomes. A novel and still unreported phenomenon was the finding that neonates that were exposed in-utero to gradual UDCA administration were found to have a higher gestational age at delivery and, as a possible secondary effect, fewer events of composite neonatal outcome than those neonates that were exposed to full UDCA dose. These findings should be retested prospectively in controlled trials.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Amir Hamud: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. Matan J. Cohen: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. Drorith Hochner-Celnikier: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. Benjamin Bar-Oz: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. Zvi Ackerman: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft, Resources.