Due to organ shortages, liver transplantation (LT) using donation-after-circulatory-death (DCD) grafts has become more common. There is limited and conflicting evidence on LT outcomes using DCD grafts compared to those using donation-after-brain death (DBD) grafts for patients with hepatocellular carcinoma (HCC). We aimed to summarize the current evidence on the outcomes of DCD-LT and DBD-LT in patients with HCC.
Materials and MethodsOnline databases were searched for studies comparing DCD-LT and DBD-LT outcomes in patients with HCC and a meta-analysis was conducted using fixed- or random-effects models.
ResultsFive studies involving 487 (33.4%) HCC DCD-LT patients and 973 (66.6%) DBD-LT patients were included. The meta-analysis showed comparable 1-year [relative risk (RR)=0.99, 95%CI:0.95 to 1.03, p=0.53] and 3-year [RR=0.99, 95%CI:0.89 to 1.09, p=0.79] recurrence-free survival. The corresponding 1-year [RR=0.98, 95%CI:0.93 to 1.03, p=0.35] and 3-year [RR=0.94, 95%CI:0.87 to 1.01, p=0.08] patient survival and 1-year [RR=0.91, 95%CI:0.71 to 1.16, p=0.43] and 3-year [RR=0.92, 95%CI:0.67 to 1.26, p=0.59] graft survival were also comparable. There were no significant differences between the two cohorts regarding the tumor characteristics, donor/recipient risk factors and the incidence of post-operative complications, including acute rejection, primary non-function, biliary complications and retransplantation.
ConclusionsBased on the current evidence, it has been found that comparable outcomes can be achieved in HCC patients using DCD-LT compared to DBD-LT, particularly when employing good quality graft, strict donor and recipient selection, and effective surgical management. The decision to utilize DCD-LT for HCC patients should be personalized, taking into consideration the risk of post-LT HCC recurrence. (PROSPERO ID: CRD42023445812).
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide [1]. Liver transplantation (LT) is the preferred treatment for early-stage HCC [2]. However, the demand for liver donors exceeds the supply. One strategy to expand the pool of available liver grafts is to use marginal livers, including livers from older donors, steatotic livers, hepatitis C positive livers, split livers, and donations after circulatory death (DCD) [3]. Several countries have approved programs utilizing DCD grafts, but the effectiveness of such programs relies on careful donor and recipient selection and acceptance of the associated risk [4]. Compared with high-quality donation after brain death (DBD) grafts, DCD grafts have been associated with inferior outcomes, including higher rates of graft failure and ischemic cholangiopathy [5,6].
In terms of LT, HCC patients with higher model for end-stage liver disease (MELD) scores typically receive organs from DBD donors, whereas those with lower MELD scores are more likely to receive organs from DCD donors [7]. There are contradictory findings on the outcomes of DCD-LT and DBD-LT in HCC patients, with some studies reporting inferior survival outcomes for DCD-LT and others reporting comparable results [8,9]. In addition, some studies indicated no disparity in HCC recurrence between DCD-LT and DBD-LT; others have highlighted significant concerns about increased recurrence in DCD-LT [8,10]. In view of the limited data available on the topic and the ongoing debate surrounding this issue, the objective of this study was to provide a comprehensive summary of the current evidence regarding outcomes of DCD-LT compared to DBD-LT, with a specific focus on patients with HCC. This objective was achieved by conducting a thorough systematic review of the existing literature and performing a meta-analysis utilizing the available data.
2Materials and methodsThis review was registered with PROSPERO in 2023 (CRD42023445812). This systematic review followed the PRISMA [11] and MOOSE [12] guidelines and adhered to a predefined PROSPERO protocol. The study utilized the PICO framework to establish the criteria for study selection. The specific criteria were as follows: (1) Population/Participants (P): adult patients of any gender or race who were undergoing LT for HCC, (2) Intervention (I): DCD-LT, (3) Comparison (C): DBD-LT. 4) Outcomes (O): The primary outcome measured was recurrence-free survival. Secondary outcomes included patient survival, graft survival, as well as risk factors related to both the donor and recipient.
Study selection, data extraction, and quality assessment were conducted independently by two reviewers (A.A and Z.S), and any disagreements were resolved through discussion. The PRISMA and MOOSE checklists can be found in the supplemental file (Figs.S1, S2). A thorough literature search was conducted using the following bibliographic databases from inception to September 2023: PubMed, Embase, PROSPERO, and Google Scholar. The search was restricted to studies involving humans and published in the English language. The search used keyword terms related to HCC, LT, and types of donations. Boolean operators AND/OR were used to expand the search and refine the results. In addition, a manual review of the reference lists of the included studies and review articles was performed to identify additional relevant studies. The detailed search strategy is provided in the supplemental file.
2.1Data extractionA standardized form was used to extract data from the included studies for evidence synthesis and assessment of study quality. The extracted data included study characteristics (author, publication year, study center and country, study period, study design, number of patients, and mean follow-up time. Donor-related characteristics included age, gender, body mass index (BMI), warm ischemia time (WIT) for DCD-LT, cold ischemia time (CIT), donor risk index (DRI), cause of death and donor liver steatosis status. Recipient-related characteristics included age, gender, BMI, MELD score, underlying etiology and cirrhosis. Perioperative data on transfusion of packed red blood cells, fresh frozen plasma, bilirubin levels on discharge, ICU stay, hospital stay and listing time were also recorded, in addition to pre-LT data on locoregional therapy (LRT), serum alpha-fetoprotein level, tumor size, tumor number, and Milan criteria status; post-LT data on complications such as acute rejection, primary non-function, biliary complications, hepatic artery thrombosis, and retransplantation. Tumor size, number, differentiation, vascular invasion, perineural invasion, Milan criteria and follow-up time were also recorded.
2.2Risk of bias assessmentTo evaluate the potential bias in the studies included in our analysis, each study was assessed using the Newcastle-Ottawa Scale (NOS) specifically designed for cohort studies [13]. NOS includes three aspects of assessment: patient selection, comparability of groups, and outcome assessment. The quality of each study was categorized as good if it scored ≥7 points.
2.3Meta-analysis and publication biasCategorical variables were presented as frequencies and percentages, and continuous variables were presented as means and standard deviations (SDs). Survival rates were presented as median survival rates at 1 and 3 years. If the survival data were not reported, the digitizing software DigitezeIt was used to estimate the survival rate from Kaplan-Meier curves. A meta-analysis was performed to compare the outcomes between DCD-LT and DBD-LT. The analysis used the standardized mean difference (SMD) and relative risks (RRs), each with a 95% confidence interval (CI), for continuous outcomes and categorical outcomes, respectively. To harmonize the meta-analysis, conversion of measurement units was applied when necessary, and if continuous data were reported as medians and ranges, methods were used to estimate the means and SDs [14]. To assess between-study heterogeneity, the I2 statistic and Cochran's Q statistic were employed. If significant heterogeneity was observed (I2>50% and p<0.05, Cochran's Q test), a random effects model was used. Otherwise, a fixed-effects model was applied [15]. Given the lower power due to the limited number of included studies (< 10), the assessment of publication bias was hindered [16]. Statistical analyses were conducted using Stata MP 17.0.
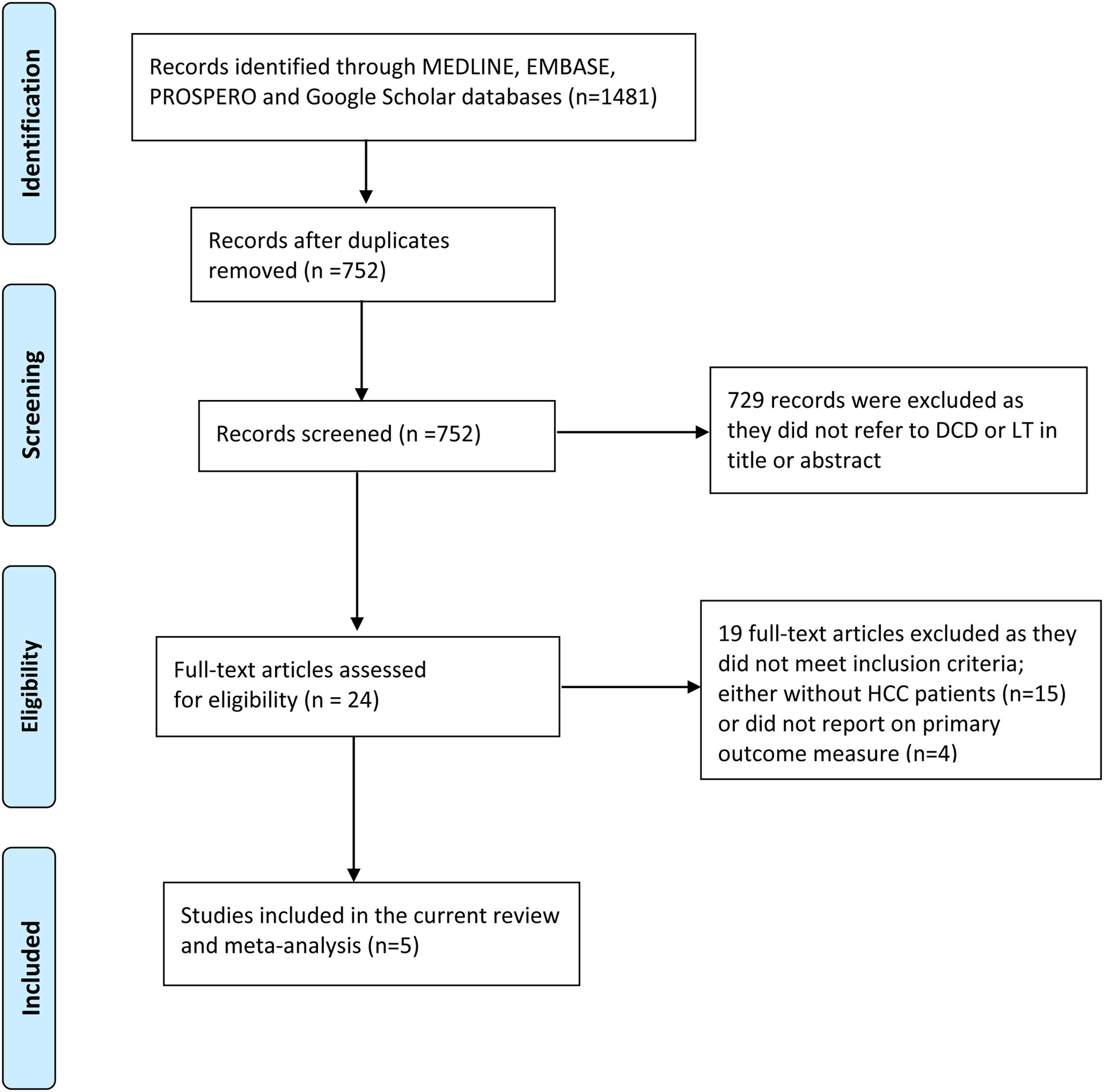
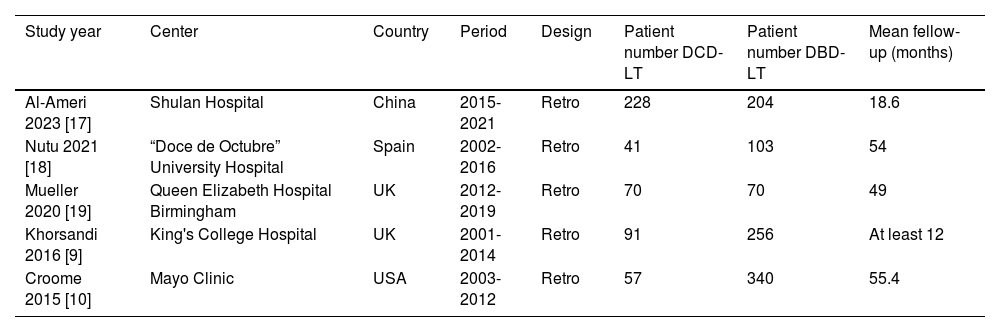
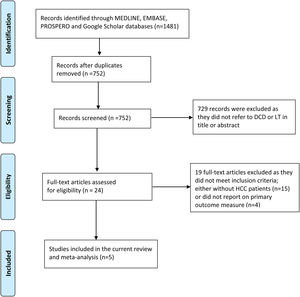
3Results3.1Description of the included studiesThe process of selecting articles for review is depicted in Fig. 1. Initially, a search of the database yielded 1481 studies. After evaluating the titles and abstracts of the 752 records, 24 articles were chosen for a full-text review. Among these articles, nine studies provided information on the outcome measures [8–10,17–22], while four studies did not report data on the primary outcome [8,20–22]. Ultimately, five studies were included in the final analysis [9,10,17–19]. Of the 1460 patients with HCC who underwent LT, 487 individuals (33.4%) underwent DCD-LT, whereas 973 individuals (66.6%) underwent DBD-LT. The detailed characteristics of the included studies are summarized in Table 1.
Main characteristics of studies included in the meta-analysis.
| Study year | Center | Country | Period | Design | Patient number DCD-LT | Patient number DBD-LT | Mean fellow-up (months) |
|---|---|---|---|---|---|---|---|
| Al-Ameri 2023 [17] | Shulan Hospital | China | 2015-2021 | Retro | 228 | 204 | 18.6 |
| Nutu 2021 [18] | “Doce de Octubre” University Hospital | Spain | 2002-2016 | Retro | 41 | 103 | 54 |
| Mueller 2020 [19] | Queen Elizabeth Hospital Birmingham | UK | 2012-2019 | Retro | 70 | 70 | 49 |
| Khorsandi 2016 [9] | King's College Hospital | UK | 2001-2014 | Retro | 91 | 256 | At least 12 |
| Croome 2015 [10] | Mayo Clinic | USA | 2003-2012 | Retro | 57 | 340 | 55.4 |
DBD-LT, donation-after-brain-death liver transplantation; DCD-LT, donation-after-circulatory-death liver transplantation; NA, not available; Retro, retrospective cohort study.
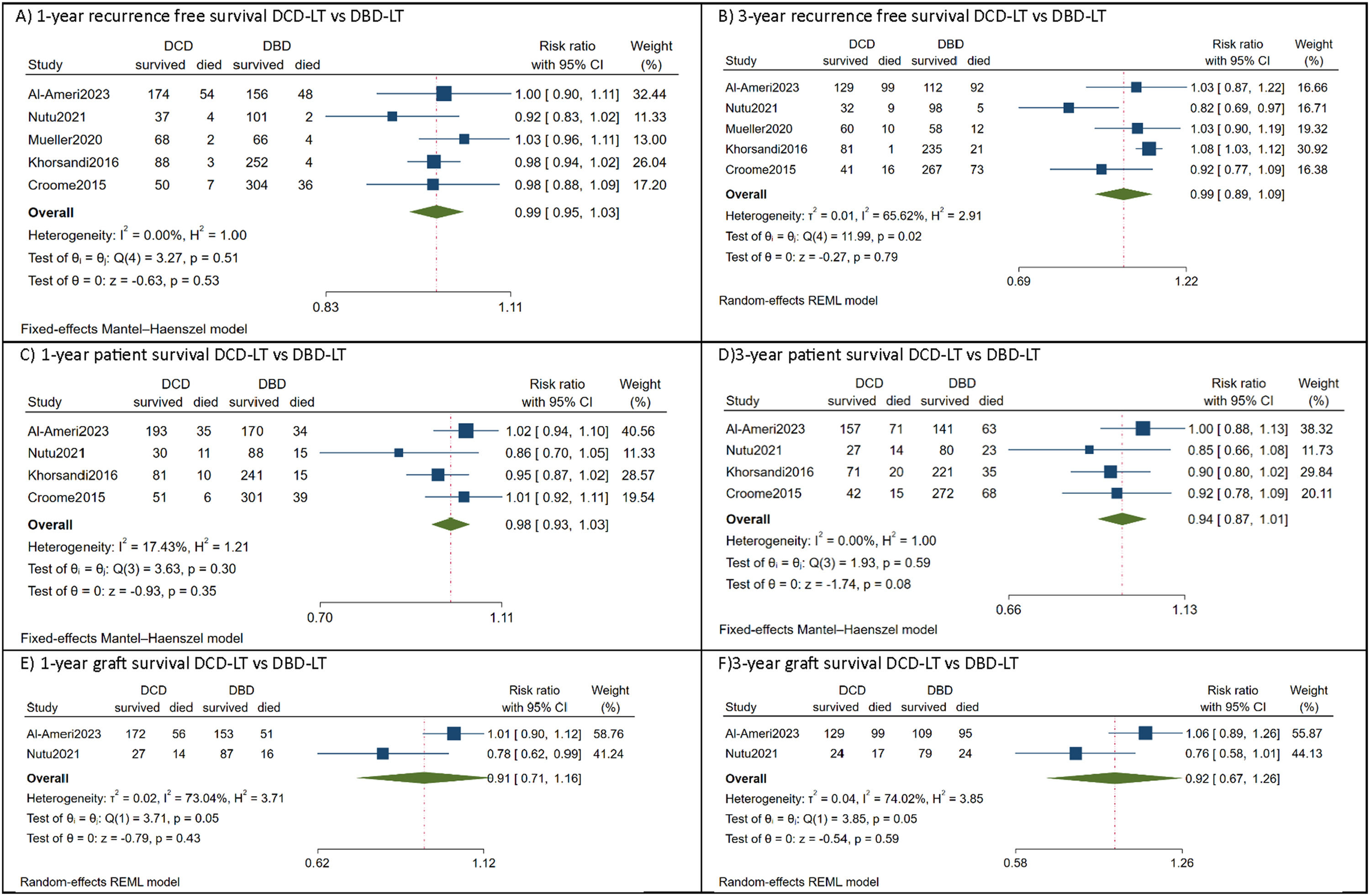
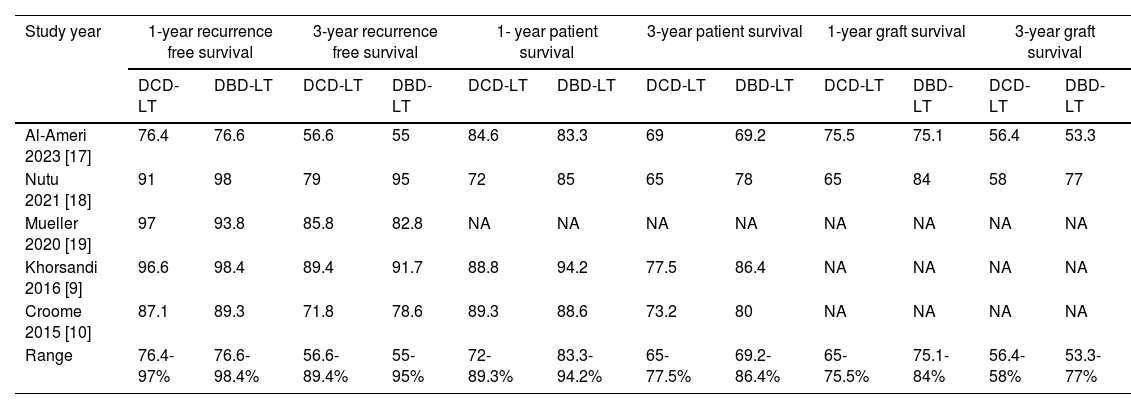
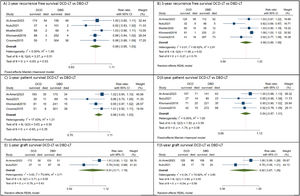
All included studies reported comparable recurrence-free survival between DCD-LT(n=487) and DBD-LT (n=973) [9,10,17–19]. In the DCD-LT group, the median recurrence-free survival at one year ranged from 76.4% to 97%, while at three years, it ranged from 56.6% to 89.4%. As for the DBD-LT group, the median RFS rate at one year varied from 76.6% to 98.4%, and at three years, it ranged from 55% to 95%. The pooled analysis showed HCC patients who received DCD-LT had equivalent recurrence-free survival at 1-year [RR=0.99, 95%CI:0.95 to 1.03, p=0.53, (I2=0, p=0.51)] and 3-year [RR=0.99, 95%CI:0.89 to 1.09, p=0.79, (I2=65.6, p=0.02)] to those who received DBD-LT (Fig. 2 a, b).
Forest plot depicting the risk estimates for 1-year recurrence free survival (A), 3-year recurrence free survival (B), 1-year patient survival (C), 3-year patient survival (D), 1-year graft survival (E), and 3-year graft survival.
CI, confidence interval; DBD-LT, donation-after-brain-death liver transplantation; DCD-LT, donation-after-circulatory-death liver transplantation.
Four studies compared patient survival outcomes between DCD-LT (n=417) and DBD-LT (n=903) HCC patients [9,10,17,18]. Among these studies, equivalent patient survival rates between the DCD-LT and DBD-LT groups were reported, except in the study by Croome et al. [10] which showed lower patient survival in the DCD-LT group. The median 1- and 3-year patient survival rates were 72-89.3% and 65-77.5%, respectively, for the DCD-LT group and the corresponding rates for the DBD-LT group were 83.3-94.2% and 69.2-86.4%, respectively. Likewise, the meta-analysis indicated there was no significant difference in patient survival between DCD-LT and DBD-LT at 1-year [RR=0.98, 95%CI:0.93 to 1.03, p=0.35, (I2=17, p=0.30)] and 3-year [RR=0.94, 95%CI:0.87 to 1.01, p=0.08, (I2=0, p=0.59)] (Fig. 2 c, d).
Two studies compared graft survival outcomes of HCC patients between DCD-LT (n=269) and DBD-LT (n=307) [17,18]. One study reported equivalent graft survival between the two groups [17], while the other study found lower graft survival in DCD-LT group [18]. For the DCD-LT group, the median 1-year and 3-year graft survival rates were 65-75.5% and 56.4-58%, respectively, while the DBD-LT group had median 1-year and 3-year graft survival rates of 75.1-84% and 53.3-77%, respectively. Again, the pooled analysis showed no significant difference in graft survival at 1-year [RR=0.91, 95%CI:0.71 to 1.16, p=0.43, (I2=73, p=0.05)] and 3-year [RR=0.92, 95%CI:0.67 to 1.26, p=0.59, (I2=74, p=0.05)] between DCD-LT and DBD-LT groups (Fig. 2 e, f). The summary of survival rates among the included studies is shown in Table 2.
Summary of survival rates for DCD-LT versus DBD-LT.
| Study year | 1-year recurrence free survival | 3-year recurrence free survival | 1- year patient survival | 3-year patient survival | 1-year graft survival | 3-year graft survival | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCD-LT | DBD-LT | DCD-LT | DBD-LT | DCD-LT | DBD-LT | DCD-LT | DBD-LT | DCD-LT | DBD-LT | DCD-LT | DBD-LT | |
| Al-Ameri 2023 [17] | 76.4 | 76.6 | 56.6 | 55 | 84.6 | 83.3 | 69 | 69.2 | 75.5 | 75.1 | 56.4 | 53.3 |
| Nutu 2021 [18] | 91 | 98 | 79 | 95 | 72 | 85 | 65 | 78 | 65 | 84 | 58 | 77 |
| Mueller 2020 [19] | 97 | 93.8 | 85.8 | 82.8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Khorsandi 2016 [9] | 96.6 | 98.4 | 89.4 | 91.7 | 88.8 | 94.2 | 77.5 | 86.4 | NA | NA | NA | NA |
| Croome 2015 [10] | 87.1 | 89.3 | 71.8 | 78.6 | 89.3 | 88.6 | 73.2 | 80 | NA | NA | NA | NA |
| Range | 76.4-97% | 76.6- 98.4% | 56.6-89.4% | 55-95% | 72-89.3% | 83.3-94.2% | 65-77.5% | 69.2-86.4% | 65-75.5% | 75.1-84% | 56.4-58% | 53.3-77% |
DBD-LT, donation-after-brain-death liver transplantation; DCD-LT, donation-after-circulatory-death liver transplantation; NA, not available.
The pooled estimates of donor characteristics are summarized in Table 3. Compared to DBD-LT, DDRI was significantly higher in DCD-LT [SMD=0.85, 95% CI: 0.63 to 1.07, p<0.001, (I2=49, p=0.16)] [10,19]. More DCD-LT patients received donor livers without steatosis [RR=1.39, 95% CI: 1.18 to 1.63, p<0.001, (I2=48.4, p=0.16)] [9,18]. There were trends toward younger age [SMD=-0.35, 95% CI: -0.69 to 0, p=0.05, (I2 =87.7, p<0.001] [9,10,17–19] and shorter CIT [SMD= -0.24, 95% CI: -0.49 to 0, p=0.06, (I2 =76, p<0.001)] [9,10,17–19] in DCD-LT group. There were no statistical differences in the variables of donor gender, BMI and donor cause of death. The extracted data are available in the supplementary file (Table S1).
Pooled analysis of donor and recipient characteristics.
| Outcome | No. of patients | DCD-LT vs. DBD-LT (patient no.; pooled mean) or (%; events /total) | Estimate effect (95%CI) | p-value | I2(%), p-value |
|---|---|---|---|---|---|
| Donor characteristics | |||||
| Age (year) [9,10,17–19] | 1460 | (487;43.8) vs. (973;49.1) | SMD= -0.24 (-0.49 to 0) | 0.06 | 76, p<0.001 |
| Gender(male) [9,10,17,18] | 1320 | (81.5%; 340/417) vs. (71.1%; 642/903) | RR=1.14(0.94 to 1.38) | 0.18 | 88.6, p<0.001 |
| BMI [10,17–19] | 1113 | (396; 23.1) vs. (717; 23.3) | SMD= -0.14(-0.58 to 0.31) | 0.55 | 89.9, p<0.001 |
| Donor WIT for DCD-LT (min) [10,19] | 127 | NA | 20.6 (9.8 to 31.4) | <0.001 | 0, p=0.62 |
| CIT (hr) [9,10,17–19] | 1451 | (487; 7) vs. (973;7.5) | SMD=-0.35(-0.69 to 0) | 0.05 | 87.7, p<0.001 |
| DRI [10,19] | 537 | (127;2) vs. (410; 1.6) | SMD=0.85 (0.63 to1.07) | <0.001 | 49, p=0.16 |
| Cause of death (stroke vs. others) [9,10] | 744 | (34.5%,51/148) vs. (52.7%, 314/596) | RR=0.59 (0.30 to 1.16) | 0.13 | 82, p=0.02 |
| Liver steatosis (no vs. yes) [9,18] | 491 | (66.7%; 88/132) vs. (35.4%;172/359) | RR=1.39(1.18 to 1.63) | <0.001 | 48.4, p=0.16 |
| Recipient characteristics | |||||
| Age (years) [9,10,18,19] | 1460 | (487; *) vs. (973; 54.5) | SMD=0.06 (-0.12 to 0.24) | 0.49 | 54.5, p= 0.07 |
| Gender (years) [9,10,17,18] | 1320 | (86.3%; 360/417) vs. (81.8%; 739/903) | RR=1.06(0.93 to 1.20) | 0.40 | 77.7, p=0.01 |
| BMI [10,17,18] | 973 | (326;25.8) vs. (647; 25.6) | SMD=0.07(-0.07 to 0.23) | 0.33 | 0, p=0.67 |
| MELD [9,10,17–19] | 1460 | (487;15.6) vs. (973; 13.5) | SMD= 0.03(-0.28 to 0.35) | 0.83 | 85, p<0.001 |
| Etiology (viral vs. other) [9,10,17,18] | 1320 | (81.8%; 341/417) vs. (70.3%; 635/903) | RR=1.03(0.97 to 1.10) | 0.31 | 38.4, p=0.18 |
| Cirrhosis (yes vs. no) [9,17,18] | 923 | (81.9%; 295/360) vs. (75.8%; 427/563) | RR=1.01 (0.95 to 1.08) | 0.77 | 0, p=0.39 |
| PRBC transfusion (U) [17–19] | 716 | (339;1.8) vs. (377;3.7) | SMD= 0.11(-0.50 to 0.72) | 0.72 | 92.3, p<0.001 |
| FFP transfusion(U) [18,19] | 284 | (111;7) vs. (173; 5.9) | SMD= 0.45(-0.42 to 1.32) | 0.31 | 91.7, p<0.001 |
| Bilirubin on discharge (mg/dL) [17,18] | (269;16.8) vs. (307;18.7) | SMD= 0.04(-0.41 to 0.49) | 0.86 | 79.7, p=0.03 | |
| Length of ICU stay [18,19] | 284 | (111;4.1) vs. (173;3) | SMD=0.39 (0.14 to 0.63) | <0.001 | 0, p=0.86 |
| Hospital stay (days) [18,19] | 284 | (111;12.4) vs. (173;12.1) | SMD= 0.23(-0.01 to 0.48) | 0.06 | 21, p=0.26 |
| Listing time (months) [9,10,18,19] | 1028 | (259; 3.5) vs. (769; 4) | SMD= -0.17(-0.43 to 0.09) | 0.19 | 66.9, p=0.03 |
| Pre-LT characteristics | |||||
| Pre-LT locoregional therapy [9,10,17–19] | 1460 | (75.8%;369/487) vs. (69.5%;676/973) | RR= 1.04(0.97 to 1.11) | 0.26 | 0, p=0.53 |
| Pre-LT AFP >400 (ng/ml) [10,17] | 802 | (60.9%; 157/258) vs. (27.9%; 152/544) | RR= 1.04 (0.91 to 1.20) | 0.55 | 0, p=0.79 |
| Pre-LT tumor size (cm) [10,18,19] | 681 | (168;3) vs. (513;3) | SMD=0.04 (-0.14 to 0.22) | 0.68 | 0, p=0.40 |
| Pre-LT tumor number [10,18,19] | 681 | (168;1.5) vs. (513;1.4) | SMD= 0.06(-0.24 to 0.36) | 0.70 | 60.6, p=0.08 |
| Pre-LT within Milan criteria [10,18,19] | 681 | (85.1%; 143/168) vs. (80.7%; 414/513) | RR= 1.06 (0.98 to 1.14) | 0.18 | 7, p=0.34 |
| Post-LT characteristics | |||||
| Complications (yes vs. no) [17,18] | 576 | (26.4%; 71/269) vs. (26.1%; 80/307) | RR= 2.69(0.49 to 14.80) | 0.26 | 88.7, p<0.001 |
| Acute rejection (yes vs. no) [18,19] | 284 | (11.4%; 27/111) vs. (23.9%; 44/173) | RR= 1.24 (0.56 to 2.73) | 0.60 | 61.8, p=0.11 |
| Primary non-function (yes vs. no) [17,18] | 576 | (1.1%; 3/269) vs. (2%; 6/307) | RR= 0.76(0.18 to 3.21) | 0.70 | 0, p=0.81 |
| Biliary complications (yes vs. no) [17,18] | 576 | (7.4%; 20/269) vs. (5.9%; 18/307) | RR= 1.69(0.61 to 4.64) | 0.31 | 64.5, p=0.09 |
| Hepatic artery thrombosis (yes vs. no) [17,18] | 576 | (3.3%; 9/269) vs. (1.3%; 4/307) | RR= 3.06 (0.94 to 9.99) | 0.06 | 0, p=0.78 |
| Retransplantation (yes vs. no) [17,18] | 579 | (4.1%; 11/269) vs. (2%; 6/307) | RR = 3.12(0.29 to 33.96) | 0.35 | 74.8, p=0.05 |
| Post-LT tumor size (cm) [9,10,17,18] | 1320 | (417;4.2) vs. (903;4.6) | SMD= -0.05(-0.178 to 0.07) | 0.39 | 0, p=0.99 |
| Post-LT tumor number [9,10,17,18] | 1320 | (417; 2.3) vs. (903; 2) | SMD= 0.11(-0.01 to 0.24) | 0.08 | 0, p=0.83 |
| Differentiation (poor vs. well-moderate) [9,10] | 744 | (6.1%; 9/148) vs. (5.5%; 33/596) | RR = 0.80 (0.12 to 5.32) | 0.82 | 51.3, p=0.15 |
| Vascular invasion (yes vs. no) [9,10,18,19] | 1028 | (29%; 75/259) vs. (24.3%; 187/769) | RR = 0.98(0.78 to 1.23) | 0.86 | 0, p=0.48 |
| Perineural invasion (yes vs. no) [10,18] | 541 | (3.1%; 3/98) vs. (0.5%; 2/443) | RR=6.45(1.20 to 34.82) | 0.03 | 0, p=0.91 |
| Post-LT within Milan criteria [9,17,18] | 923 | (75.3%; 271/360) vs. (79.8%; 449/563) | RR= 1.00 (0.93 to 1.07) | 1.00 | 54.5, p=0.11 |
| Follow up (months) [10,17–19] | 1113 | (396;35) vs. (717; 36.2) | SMD= 0.05 (-0.16 to 0.27) | 0.64 | 57.1, p=0.07 |
AFP, serum alpha-fetoprotein; BMI, body mass index; CI, confidence interval; CIT, cold ischemia time; DBD-LT, donation-after-brain-death liver transplantation; DCD-LT, donation-after-circulatory-death liver transplantation; DRI, donor risk index; FFP, fresh frozen plasma; HCC, hepatocellular carcinoma; ICU, intensive care unit; LT, liver transplantation; SMD, standardize mean difference; OR, odds ratio; MELD, model for end-stage liver disease; NA, not available; PRBC, packed red blood cells; U, unit; WIT, warm ischemia time; * Convergence not achieved during tau2 estimation.
The pooled estimates of recipient characteristics are summarized in Table 3. Compared with DBD-LT, the DCD-LT group had a significantly longer length of ICU stay [SMD=0.39,95% CI:0.14 to 0.63, p<0.001, (I2=0, p=0.86)] [18,19]. Additionally, the incidence of perineural invasion was higher in the DCD-LT group [RR=6.45, 95% CI:1.20 to 34.82, p=0.03, (I2=0, p=0.91)] [10,18]. Trends were observed toward longer hospital stay in the DCD-LT group [SMD= 0.23, 95% CI: -0.01 to 0.48, p=0.06, (I2=21, p=0.26)] and higher incidence of hepatic artery thrombosis in the DCD-LT group [RR= 3.06, 95% CI: 0.94 to 9.99, p= 0.06, (I2=0, p=0.78)]. No significant differences were observed between DCD-LT and DBD-LT groups in terms of recipient age, gender, BMI, MELD score, underlying etiology and cirrhosis. Furthermore, perioperative requirements for transfusion of packed red blood cells, fresh frozen plasma, bilirubin levels on discharge, and listing time, pre-LT variables (LRT, serum alpha-fetoprotein level, tumor size, tumor number, Milan criteria status) and post-LT data (complications such as acute rejection, primary non-function, biliary complications, and retransplantation) were also not statistically different. Additionally, post-LT tumor number, differentiation, vascular invasion, perineural invasion, Milan criteria status and follow-up time showed no significant differences between the two groups. The extracted data are available in the supplementary file (Table S1).
3.5Critical appraisal of the included studiesTable S2 presents a critical assessment of the potential bias in the included studies using the NOS Scale. All the included studies achieved a score of ≥7 and were deemed to be of good quality. The selection of study participants was deemed appropriate. Primary outcome comparisons were possible because all studies included DBD-LT and DCD-LT groups. Nonetheless, the studies were considered representative of the general population of HCC patients requiring LT, and it was clear that the patients were drawn from a transplant waitlist, except for one study [17]. Two studies reported that none of the patients had previously undergone LT [17,18]. All studies used statistical controls to account for potential confounders, such as donor or recipient characteristics. These studies generally adequately reported the outcomes. However, the follow-up period was less than three years in only one study [17,20,21].
4DiscussionThis study is the first systematic review and meta-analysis to assess the outcomes of HCC patients who underwent DCD-LT and DBD-LT at various centers worldwide. The evidence showed that DCD-LT for HCC patients yields survival outcomes comparable to DBD-LT. The findings of this study can be attributed to several key factors, with the primary factor being the utilization of high-quality DCD grafts. The interconnected factors encompassed a comprehensive understanding of tumor biology-related characteristics, the appropriate selection of donors and recipients, and the implementation of effective surgical practices.
Initially, concerns were raised about the use of DCD-LT for HCC candidates due to the potential risk of higher HCC recurrence [8]. This concern was based on the biological possibility that ischemia-reperfusion injury (IRI) could stimulate the growth of micrometastases and enhance tumor cell adhesion, as observed in experimental studies [23]. However, subsequent clinical studies have consistently reported comparable rates of tumor recurrence-free survival between DCD-LT and DBD-LT [9,10,17–19,24,25]. This could be explained by the fact that well-selected DCD grafts with good quality do not exhibit significant IRI compared to DBD grafts. Nonetheless, there is a risk of IRI with more marginal DCD grafts. In a large-scale study involving nearly 10,000 LTs for HCC patients between 2004 and 2011, several donor-related factors were consistently associated with a higher risk of recurrence. These factors include BMI ≥ 35 kg/m², age > 60 years, liver steatosis, and prolonged WIT [26]. These factors increase the liver's vulnerability to IRI, which has been demonstrated to promote tumor progression. To minimize the risk of IRI and subsequent risk of HCC recurrence after LT, it is crucial to carefully control these factors and adhere to strict selection criteria for donors. Our meta-analysis consistently supported the findings that DCD-LT and DBD-LT have comparable rates of tumor recurrence-free survival for HCC patients by adherent to the strict selection criteria for the donors, as shown by the age no more than 45 years, BMI of less than 25 kg/m², using graft without liver steatosis and shorter WIT and CIT. By adhering to these criteria, the risk of IRI can be minimized, ultimately reducing the likelihood of HCC recurrence after LT.
Furthermore, our meta-analysis found that a significant proportion of patients who underwent DCD-LT met the strict Milan criteria (75%), i.e., 1 nodule <5cm or up to 3 nodules <3cm, no vascular invasion or extrahepatic spread. The analysis revealed the average number of tumors was 2, and the average tumor size was 4 cm among the patients. Additionally, vascular invasion was observed in 29% of cases, whereas poor differentiation was observed in only 6% of cases. Importantly, these factors did not differ significantly from those observed in patients who underwent DBD-LT. These findings demonstrate the effective management of traditional tumor-related risk factors for HCC recurrence in DCD-LT, which aligns with the well-established principles of tumor biology. The findings also support the HCC listing criteria, which are based on these principles and play a crucial role in ensuring comparable outcomes between DCD-LT and DBD-LT.
Interestingly, the wait times for DBD-LT and DCD-LT were similar, typically less than four months. The incidence of receiving pre-LT LRT was comparable between the two groups. Importantly, previous studies have shown that LRT does not negatively impact patient survival, as tumor biology is a more significant determinant of outcomes [9]. Therefore, patients with decompensated liver (lower MELD score as indicated in our analysis, less than 16), fulfilling the Milan criteria and those who show a partial response or stable disease after LRT may benefit from accepting DCD grafts to avoid being removed from the transplant waitlist. Additionally, in cases where a complete pathologic response is not achieved, delaying transplantation by three months after waitlist inclusion has been suggested to reduce the risk of early HCC recurrence [27,28].
It is worth noting that all the studies included in our analysis were conducted in high-volume centers, which resulted in comparable rates of major intraoperative events such as packed red blood cell transfusion, fresh frozen plasma transfusion, and bilirubin levels on discharge when compared to DBD-LT. Similarly, the rates of complications, including acute rejection, primary non-function, biliary complications, and retransplantation, did not show significant differences between DCD-LT and DBD-LT. However, it is important to consider the higher incidence of hepatic artery thrombosis in DCD-LT (as indicated in our analysis with RR of 3.06, p=0.06), likely due to warm ischemic damage and the hypercoagulable state associated with cancer itself. As previous studies have shown, hepatic artery thrombosis is more common after DCD-LT, potentially due to reduced blood flow caused by in situ blood stasis in the arterial tree during warm ischemia [29]. To mitigate this risk, the use of thrombolytic agents should be encouraged. In some countries, the use of thrombolytic agents in DCD-LT procedures has been considered an accepted practice [5]. By employing these agents, the chances of hepatic artery thrombosis can be reduced, ultimately ensuring further improvement in the outcomes of DCD-LT.
Our research is of utmost importance as it aims to determine the most effective decision-making approach for high-risk and low-risk HCC patients who are eligible for DCD-LT. By refining DCD-LT for high-risk HCC patients, we can minimize potential risks and maximize the benefits for low-risk HCC patients, leading to favorable outcomes. In recent years, predictive models such as the UK DCD Risk Index have been developed to assess DCD-LT outcomes by considering various factors related to both the donor and the recipient [30]. These factors include donor age, BMI, WIT, CIT, recipient age, MELD score, and retransplantation status. Higher scores on these models indicate a higher risk profile. Patients with scores above 10, known as the futile group, have been found to have less than a 40% one-year graft survival rate, making DCD-LT not recommended for this group. However, the applicability and effectiveness of these predictive models have not yet been evaluated in specific HCC patient cohorts that take into account tumor biology and risk factors for HCC recurrence, which is highly recommended. Similarly, several risk prediction models for HCC recurrence have not been assessed based on the type of graft used [31]. In a recent study involving 7,563 HCC patients who underwent DCD-LT, the high-risk group was defined as patients with AFP levels exceeding 100 ng/mL, a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score above 4, and the preoperative detection of contrast-enhanced multiple liver lesions [20]. Another study highlighted poorer outcomes associated with IRI and a subsequent high risk of HCC recurrence in patients with a WIT exceeding 50 min, positive PET scans, AFP concentration surpassing 400 ng/mL, and tumors outside the Milan criteria [32]. Moreover, a study involving 391 HCC patients demonstrated that CIT exceeding 10 h and WIT exceeding 50 min were independent risk factors for HCC recurrence, in addition to tumors surpassing the Milan criteria, AFP concentration exceeding 200 ng/mL, micro and macrovascular invasion, and poor tumor differentiation. Stratifying patients based on vascular invasion revealed that WIT and CIT remained significant risk factors in the subgroup with vascular invasion [33]. Therefore, understanding the predictive role of these factors would provide valuable insights into minimizing the risk of IRI and subsequent HCC recurrence after LT, allowing personalized patient management.
There are several limitations to consider in this study. Firstly, all the studies included in our analysis were observational cohort studies, which inherently have limitations in terms of reliability and establishing causality. The lack of randomized controlled trials limits the strength of the evidence generated from these studies. Secondly, it is important to note that the studies included in our analysis were primarily conducted in single-center settings, which may limit the generalizability of our findings to broader populations. Thirdly, given the limited access to complete data records from each of the included studies, it was not possible to extract certain baseline characteristics necessary for conducting an individual patient meta-analysis. For example, there was incomplete reporting on post-operative complications across all the included studies, which may introduce bias and potentially impact the final results and conclusions of our study. Moreover, the effects of specific immunosuppression regimens and the use of machine perfusion as an alternative preservation strategy have not been adequately explored in the included studies. Machine perfusion is increasingly being adopted, and it is recommended that future studies incorporate this technology to investigate its specific effects on HCC patients undergoing DCD-LT. This approach will contribute to an evolving understanding of the benefits and considerations associated with the use of machine perfusion in LT for HCC patients. These factors can significantly influence the outcomes of LT and should be considered in future research. Further investigation is needed to address these limitations and provide a more comprehensive understanding of the optimal decision-making pathway for HCC patients eligible for DCD-LT.
5ConclusionsTo the best of our knowledge, this study represents the first comprehensive analysis that provides reliable evidence on the outcomes of DCD-LT versus DBD-LT in HCC patients. Based on the evidence from our analysis, DCD-LT for HCC patients yields outcomes that are comparable to DBD-LT and do not have a significant impact on recurrence-free survival. These findings hold true when high-quality DCD grafts are utilized, and various factors, including donor/recipient related factors, tumor biology factors and effective surgical management, are controlled to minimize the risk of IRI and subsequent HCC recurrence after LT. The decision to utilize DCD-LT for HCC patients should be personalized, taking into consideration the individual's risk of post-LT HCC recurrence.
Data availabilityAll data in this study are available as part of the article, and no additional source data are needed.
CRediT authorship contribution statementAbdulahad Abdulrab Mohammed Al-Ameri: Conceptualization, Visualization, Formal analysis, Writing – review & editing. Shusen Zheng: Supervision, Writing – review & editing.
This work was supported by the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022002A).