The rate of liver transplantation is increasing among the elderly population; however, data is limited on the post-liver transplantation outcomes in patients ≥70 years. Given the scarcity in liver allograft resources, a meta-analysis on the outcomes of liver transplantation in patients ≥70 years is warranted.
Materials and MethodsMultiple databases were searched through March 2022 for studies that reported on the outcomes of liver-transplantation in patients ≥70 years. Meta-analysis was conducted using the random-effects model and heterogeneity was assessed using the I2 statistics.
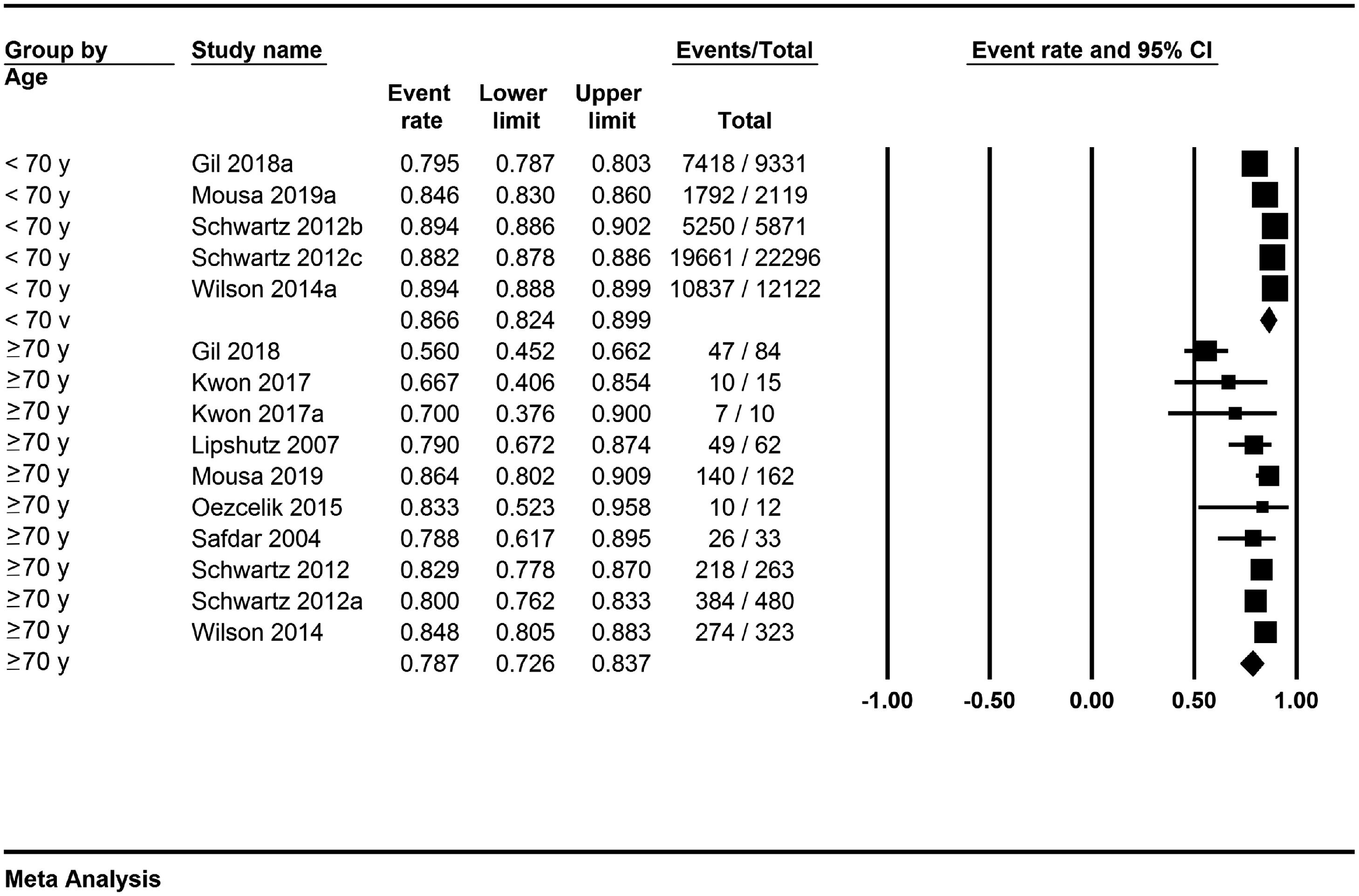
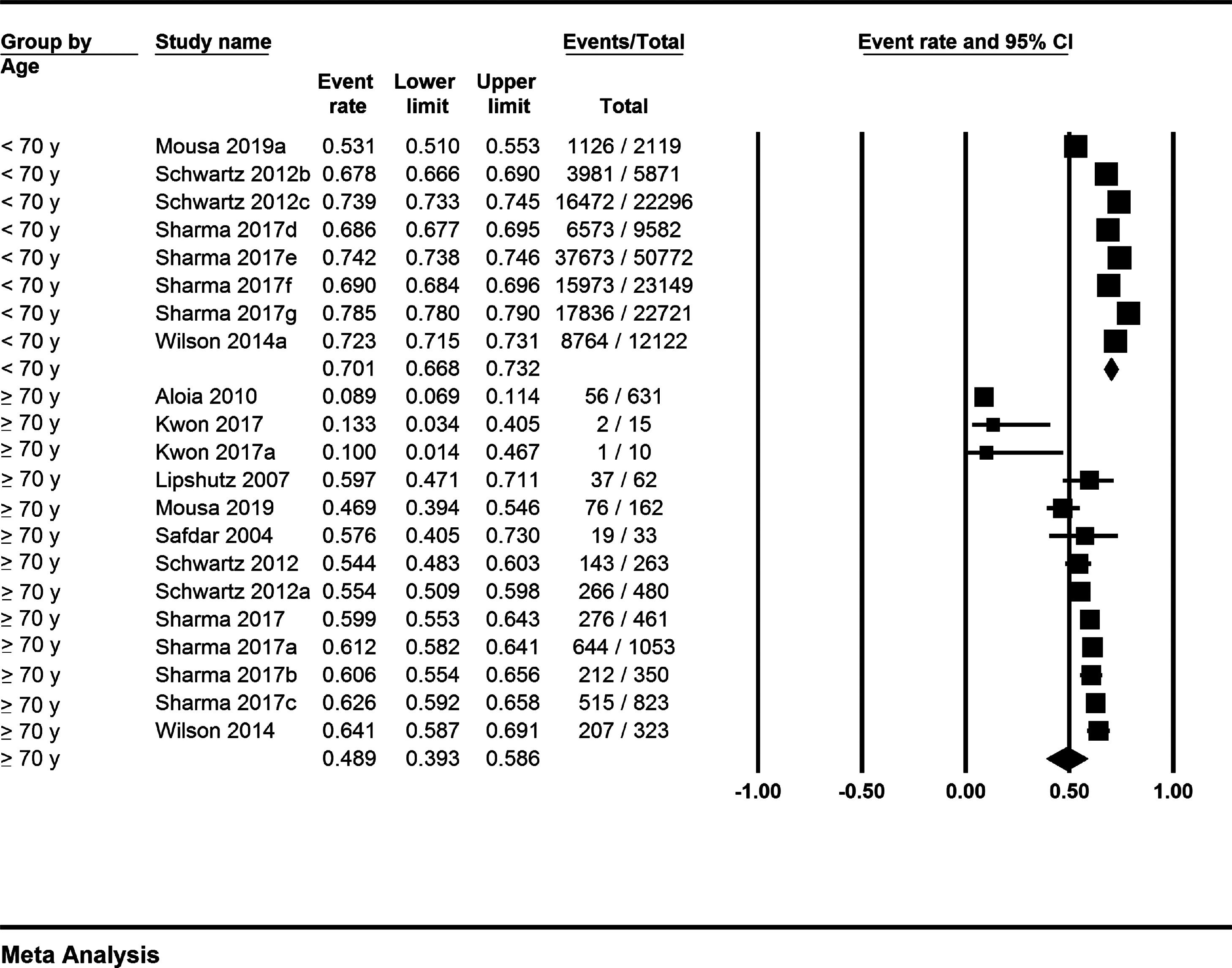
ResultsTen studies were included that analyzed 162,725 patients. The pooled rate of 1-year, 3-years and 5-years post liver transplant survival for patients ≥70 years was 78.7% (72.6–83.7; I2=74%), 61.2% (52.3–69.5; I2=87%), and 48.9% (39.3–58.6; I2=96%), respectively. The corresponding 1-year, 3-years and 5-years survival for patients <70 years were 86.6% (82.4–89.9; I2=99%), 73.2% (63–81.3; I2=99%), and 70.1% (66.8–73.2; I2=99%); respectively. Descriptive p-values of comparison were statistically significant at 1-year and 5-years (p = 0.02 and <0.001). The pooled rate of perioperative complications in patients ≥70 years was 40.7% (26.2–57; I2=93%). The pooled rate of graft failure in patients ≥70 years was 6.7% (3.3–13.1; I2=93%) and in patients <70 years was 3.7% (1–12.4; I2=99%). The pooled rate of perioperative mortality in patients ≥70 years was 16.6% (7.6–32.5; I2=99%) and in patients <70 years was 0.8% (0–33.1; I2=88%).
ConclusionPatients ≥70 years undergoing liver transplantation seem to demonstrate significantly lower 1-year and 5-year survival rates as compared to patients <70 years, albeit limited by heterogeneity.
Liver transplantation increases the probability of survival for patients with end-stage liver disease (ESLD) [1, 2]. Prevalence and incidence of ESLD have increased in the elderly, resulting in increased rates of liver transplantation among the elderly population across the globe [3–12]. Refinements in surgical techniques and improved post-operative care have made this possible. Based on the latest United States Vitals report, the life expectancy of people at age 60 is about 23.3 years and the life expectancy at 70 years of age is 15.7 years [13]. As a result of increasing life expectancy across the world, the demand for liver transplantation is expected to increase in the elderly population [2].
Although there is no one single accepted age cut-off to define ‘elderly’, data suggests that recipients greater than age 60, or 65, or 70 are particularly vulnerable to poor outcomes in the presence of other medical comorbidities [2, 14]. It is understandable that the aging of both recipients and donors presents challenges to the liver transplant community. Studies have reported on the outcomes of liver transplantation in elderly population, and data seem to suggest similar outcomes between younger transplant recipients and the carefully selected aged recipient [14]. Many centers have, therefore, increased the recipient age cut-off to patients in their late 70 s.
As per current American Association for the Study of Liver Diseases (AASLD) guidelines, chronologic age in itself should not be considered an absolute contraindication for liver transplant. The number of liver transplant recipients aged ≥70 years has increased since 2010 and therefore, the importance of knowing the risks and potential outcomes of liver transplant in this age group has become increasingly necessary [2]. However, data pertaining to relevant outcomes of liver transplant recipients over the age of 70 years has not been well summarized. The majority of the studies use age ≥65 as the cut-off to define ‘elderly’ [14]. In this systematic review and meta-analysis, we sought to consolidate the post liver transplant outcome data exclusive to liver transplant recipients 70 years of age or older.
2Methods2.1Search strategyThe published English literature was searched by authors BPM, JFG, and SI for studies that reported on the post liver transplantation outcomes in patients ≥70years of age. A comprehensive search of several databases from inception to March 2022 was performed. The databases included ClinicalTrials.gov, Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+ including epub ahead of print, in-process & other non-indexed citations), Scopus (1970+) and Web of Science (1975+). Controlled vocabulary supplemented with keywords was used to search for studies of interest. The search strategies were created using a combination of keywords and standardized index terms. Keywords included “liver transplantation”, “orthotopic liver transplantation”, “age ≥70 years”, “elderly” and “older patients”. Results were limited to English language. Details of study selection are provided in PRISMA Flow Chart – Supplementary Figure 1[15]. The full search strategy is available in Supplementary Table 3. The MOOSE checklist and PRISMA checklist were followed and details were provided in Supplementary Table 4 & Supplementary Table 5[16]. Reference lists of evaluated studies were examined to identify other studies of interest.
2.2Study selectionIn this meta-analysis, we included studies that reported on the outcomes of liver transplantation in patients ≥70 years of age. Studies were included regardless of living or deceased donor liver status, model for end-stage liver disease (MELD) score, concomitant hepatocellular carcinoma, follow-up time, geography and whether published as full manuscripts or abstracts, as long as they provided the clinical outcomes data needed for the analysis. Studies that reported on post liver transplantation outcomes in the elderly population in general, without a particular age cut-off, were reviewed for data of interest in case data exclusive to ≥70 years were provided. Additionally, if provided, data on post transplantation outcomes in patients <70 years were gathered to be used as a cohort for comparison.
Our exclusion criteria were as follows: (1) data on liver transplantation patients <70 years without associated data on patients ≥70 years, (2) studies performed in the pediatric population (Age <18 years), (3) studies with sample size <10 patients including single patient case reports, and (4) studies not published in English language. In cases of multiple publications from a single research group reporting on the same patient, same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained. The retained studies were decided by two authors (BPM, SRK) based on the publication timing (most recent) and/ or the sample size of the study (largest). In case a conclusion was not reached, potential effects of overlapping cohorts were analyzed by sensitivity analysis wherein pooled rates would be checked by removing one study at a time.
2.3Registration & protocolThis article was not registered, and a protocol was not prepared.
2.4Data abstraction and quality assessmentData on study-related outcomes from the individual studies were abstracted independently onto a standardized form by at least two authors (SRK, PY). Author BPM cross-verified the collected data for possible errors and two authors (SC, SRK) did the quality scoring independently. The Newcastle-Ottawa scale for cohort studies was used to assess the quality of studies [17]. This quality score consisted of eight questions, the details of which are provided in Supplementary Table 2.
2.5Outcomes assessedThe primary outcomes of interest were the pooled rate of post-transplant survival at 1-year, 3-years and 5-years. Secondary outcomes of interest were pooled rate of perioperative complications, pooled rate of graft failure, pooled rate of perioperative mortality, hospital length of stay and intensive care unit (ICU) length of stay.
2.6Statistical analysisWe used meta-analysis techniques to calculate the pooled estimates and 95% CIs (confidence intervals) in each case following the methods suggested by DerSimonian and Laird using the random-effects model [18]. When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis. Heterogeneity between studies was assessed by means of a χ2 test (Cochran Q statistic) and quantified with the I2 statistic [19, 20]. In this, values of <30%, 30% - 60%, 61% - 75%, and ≥75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively. Publication bias was ascertained qualitatively by visual inspection of funnel plot and quantitatively by the Egger test. Test for publication bias was deferred if the total number of studies analyzed was 10 or below. A p-value alpha of <0.05 was used to define significance between the groups compared [21]. All p-values mentioned for the secondary outcomes are to be taken descriptive only as they are presented uncorrected for multiple testing and are not based on any null-hypothesis.
All analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 3 (BioStat, Englewood, NJ).
3Results3.1Search results and population characteristicsFrom an initial pool of 7319 studies, 3994 records were screened after deduplication, 45 full-length articles were assessed. 10 studies were included in the final analysis [3–12]. The study selection flowchart is illustrated in Supplementary Figure-1. For patients ≥70 years of age, 1-year survival data and 3-years survival data were reported in 10 patient cohorts, and 5-years survival data was reported in 13 patient cohorts. For patients <70 years, five patient cohorts reported on 1-year and 3-years survival data, whereas 8 cohorts reported on 5-years survival data.
A total of 4752 patients were ≥70 years of age and 157,973 were <70 years. In the ≥70-year-old recipient cohort, 62% were males, 18.8% had hepatocellular carcinoma (HCC), 17.6% had viral hepatitis, 1.5% had non-alcoholic fatty liver disease (NAFLD) and 1.1% had alcohol related liver disease. Whereas, in the <70-year-old cohort, 68% were males, 12.1% had HCC, 28.1% had viral hepatitis, 1% had NAFLD and 1.8% had alcohol. The rest were cryptogenic and/or unknown etiology. Further details along with the population characteristics are described in Supplementary Table 1.
3.2Characteristics and quality of included studiesFive studies analyzed population-based data [3, 4, 10-12] Based on the New-Castle Ottawa assessment for study quality, seven studies were considered to be of high quality [3-6, 10-12] and three studies were considered to be of medium quality. There were no low-quality studies. The quality scoring system analysis is summarized in Supplementary Table 2.
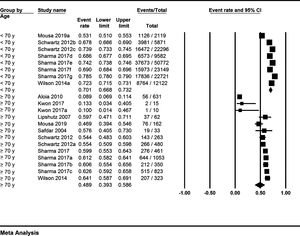
3.3Meta-analysis outcomesPooled rates were calculated from 10 studies that were included in the final analysis. The pooled rate of post liver transplant survival for patients ≥70 years at 1-year was 78.7% (95% CI 72.6–83.7; I2=74%) (Forest Plot: Fig. 1), at 3-years was 61.2% (95% CI 52.3–69.5; I2=87%) (Forest Plot: Fig. 2), and at 5-years was 48.9% (95% CI 39.3–58.6; I2=96%) (Forest Plot: Fig. 3). The corresponding 1-year, 3-years and 5-years post-transplant survival for patients <70 years were 86.6% (95% CI 82.4–89.9; I2=99%) (Forest Plot: Fig. 1), 73.2% (95% CI 63–81.3; I2=99%) (Forest Plot: Fig. 2), and 70.1% (95% CI 66.8–73.2; I2=99%) (Forest Plot: Fig. 3); respectively. Descriptive p-values of comparison were statistically significant for the post-transplant survival outcomes at 1-year and 5-years (p = 0.02, and <0.001; respectively). Results are summarized in Table 1.
Summary of pooled results.
ICU: intensive care unit, NA: data not available.
The pooled rate of perioperative complications of liver transplantation in patients ≥70 years was 40.7% (95% CI 26.2–57; I2=93%) (Forest plot: Supplementary Figure 2). The reported complications were postoperative bleeding, hepatitis C virus infection reactivation, disseminated tuberculosis, biopsy-proven acute cellular rejection, sepsis due to pneumonia, spontaneous pneumothorax, biliary stricture, and biliary leakage. Data on the perioperative complications of liver transplantation in patients <70 years were not adequately reported in the included studies for a quantitative synthesis.
The pooled rate of graft failure in patients ≥70 years was 6.7% (95% CI 3.3–13.1; I2=93%) and in patients <70 years was 3.7% (95% CI 1–12.4; I2=99%) (Forest plot: Supplementary Figure-3). The pooled rate of perioperative mortality in patients ≥70 years was 16.6% (95% CI 7.6–32.5; I2=99%) and in patients <70 years was 0.8% (95% CI 0–33.1; I2=88%) (Forest plot: Supplementary Figure 4).
The pooled mean hospital length of stay in patients ≥70 years was 30.5 (95% CI 19.6–41.2; I2=99%) days and in patients <70 years was 18.7 (95% CI 1–37.8; I2=99%) days (p = 0.3) (Forest plot: Supplementary Figure 5). The pooled ICU length of stay in patients ≥70 years was 8.9 (95% CI 1.3–16.6; I2=99%) days (Forest plot: Supplementary Figure 6). Adequate data was not provided for patients <70 years on ICU length of stay.
3.4Validation of meta-analysis results3.4.1Sensitivity analysisTo assess whether any one study had a dominant effect on the meta-analysis, we excluded one study at a time and analyzed its effect on the main summary estimate. In this analysis, no one study affected or changed the reported pooled outcomes and/ or the reported heterogeneity.
3.4.2HeterogeneityWe assessed dispersion of the calculated rates using the I2 percentage values. Overall, high heterogeneity was noted in the pooled outcomes of 1-year, 3-years and 5-years survival data for both the age groups studied. Limited number of total included studies, along with limited data points, prevented further assessment by means of sub-group analysis and/ or meta-regression analysis to evaluate the observed heterogeneity. However, it is well-known that I2 is higher when considering continuous variables as compared to categorical outcomes due to the intrinsic numeric nature of these variables, where a potentially infinite number of results determines a considerably lower change to confirm the null hypothesis that all studies have the same underlying magnitude of effect. Therefore, the high I2 values in this study should be interpreted with caution and does not really reflect varying direction of pooled effects, particularly in the instance of pooled proportions.
3.4.3Publication biasA publication bias assessment was not performed due to the fact that the total number of studies assessed were 10.
4DiscussionThe issue of liver transplantation in patients ≥70 years has rarely been addressed. Studies have reported conflicting results regarding the outcomes of liver transplantation in patients ≥70 years old. In this meta-analysis we assessed the post liver transplantation outcomes in patients ≥70 years of age, and we report a pooled 1-year survival rate of 78.7%, 3-years survival rate of 61.2% and 5-years survival rate of 48.9%. This is the first meta-analysis reporting on the pooled survival outcomes exclusive to patients ≥70 years of age undergoing liver transplantation and demonstrated key findings when the outcomes were compared to patients <70 years of age.
The post-transplant survival was lower in patients ≥70 years when compared to patients <70 years. The post-transplant 1-year survival in patients <70 years was 86.6%, 3-year survival was 73.2% and 5-year survival was 70.1%. Upon comparison, the pooled outcomes demonstrated statistical significance at 1-year and 5-years. The 3-year outcome approached statistical significance (p = 0.07). Improvements in surgical techniques, intensive care unit skills and high-end post-operative care have greatly influenced the hospital stay in this complex patient population. Our analysis of the hospital length of stay seems to demonstrate comparable pooled rates in the study groups. The pooled mean hospital length of stay for transplant recipients ≥70 years-old was 30.5 days as compared to 18.7 days in recipients <70 years-old, and although longer, it did not reach statistical significance (p = 0.3). The pooled mean ICU length of stay in ≥70 years was 8.9 days. Unfortunately, data was not available to calculate the pooled ICU length of stay in patients younger than 70 years.
A previously published meta-analysis, by Gomez Gavara et al., evaluated the outcomes of liver transplantation in elderly patients and based out of twenty-two studies reported that elderly patients have similar long-term survival and graft loss rates as young patients [14] How does one define ‘elderly’? As per society guidelines, chronological age per se should not be used as a contraindication to liver transplants. Studies published thus far have demonstrated comparable survival outcomes between carefully selected elderly patients and younger populations undergoing liver transplantation. However, the age cut-off to define ‘elderly’ has been inconsistent across studies. In the review by Gomez Gavara et al., one study defined elderly as ≥63 years, thirteen studies defined elderly as ≥65 years, seven studies had a cut-off of ≥70 years and one study reported on outcomes ≥75 years [14]. The maximum age cut-off used in the Organ Procurement and Transplantation Network/ Scientific Registry of Transplant Recipients (OPTN/ SRTR) annual report 2018 was 65 years [2]. Understandably, there is no consensus definition for elderly patients on transplant wait lists.
Nevertheless, the rate of liver transplantation is increasing and a greater number of patients 65 and older are on the transplant wait list based on the 2018 OPTN/ SRTR statistics report [2]. The supply and demand disparity has led to debate between two main principles of ethics: equity and utility. Utility-based allocation aims at saving the most life-years and deals with the concept of benefit that in turn depends on the time horizon. The time horizon is calculated by statistical modeling and depends on the duration of observation or the follow-up time. A ‘utility’ based organ allocation and transplant benefit is considered with a long-term time horizon where ageism and long-term survival predictions play the major role. Although difficult to ascertain, a balance of transplant benefit between urgency and utility is probably achieved between a time horizon of 5 and 10 years after transplant [22].
There are several strengths to our review including systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good quality studies with detailed extraction of data pertaining to age group ≥70, rigorous evaluation of study quality, and statistics to establish and/or refute the validity of the results of our meta-analysis. The majority of the studies do not address the ethical concepts of equity and utility. The primary reason being these parameters cannot be measured and therefore cannot be compared between the cohorts of interest. Studies have consistently reported their findings of post liver-transplant outcomes in patients ≥70 years in relation to their outcomes if liver transplantation is denied. Although this is important, literature seldom makes conclusions on the utility of liver transplantation in elderly patient group in comparison to the younger age group. This discussion is warranted given the scarce resource of liver allografts. In this study we have analyzed studies that reported on patients undergoing liver transplantation ≥70 years with comparison to patients <70 years and thereby hope to add data that might help answer the question of utility.
There are limitations to this study, most of which are inherent to any meta-analysis. Many retrospective studies were included in the analysis thereby inherent bias was not avoidable. Elderly patients were more frequently transplanted at high-volume centers, and therefore the results might not represent outcomes of patients in the general community. A high degree of heterogeneity was noted, and we were not able to statistically explain it or ascertain a cause for it. However, multiple different etiologies of liver disease, variability in MELD score, and variations in live vs dead donor could probably be some of the contributing factors.
Additionally, granular data on pretransplant evaluation features and important perioperative causes of mortality in the two groups were not provided in the included studies. Data was limited to calculate these outcomes in patients <70 years. Therefore, we do not know if the perioperative complications were different in two groups. Furthermore, one of the criteria on the transplant waiting list is the age of the patients, currently the age for transplant admission list is 70 and prior to this it was 65 years old. We were not able to assess the effect of this age criteria for inclusion by a sub-group analysis. However, we do not believe this would change our reported results, as the included studies classified the clinical outcomes based on 75 years as the age cut-off.
5ConclusionIn conclusion, based on this meta-analysis, patients ≥70 years undergoing liver transplantation seem to demonstrate significantly lower 1-year and 5-year survival rates as compared to patients <70 years, with comparable hospital length of stay, albeit limited by heterogeneity. Further well-conducted studies with good sample size and adequate follow up time are warranted to establish our findings.
Author contributionsBPM: conception and design, drafting of article. BPM, JFG, SI: study search, review and selection. SRK, BPM, PY: data collection and synthesis. BPM, SP, PY: statistical analysis of data and interpretation of results. All authors: interpretation of data, drafting of article, critical revision of the article for important intellectual content and final approval of the article
Data availability statementThis is a systematic review and meta-analysis of already published studies, therefore data used in this research is openly accessible from the included studies.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None