Liver transplantation can be a curative treatment for patients with hepatocellular carcinoma (HCC); however, the morbidity and mortality associated with HCC varies by socioeconomic status and race and ethnicity. Policies like Share 35 were implemented to ensure equitable access to organ transplants; however, their impacts are unclear. We aimed to characterize differences in post-liver transplant (LT) survival among patients with HCC, when considering race and ethnicity, income, and insurance type, and understand if these associations were impacted by Share 35.
Materials and MethodsWe conducted a retrospective cohort study of 30,610 adult LT recipients with HCC. Data were obtained from the UNOS database. Survival analysis was carried out using Kaplan-Meier curves, and multivariate Cox regression analysis was used to calculate hazard ratios.
ResultsMen (HR: 0.90 (95% CI: 0.85-0.95)), private insurance (HR: 0.91 (95% CI: 0.87-0.92)), and income (HR: 0.87 (95% CI: 0.83-0.92)) corresponded with higher post-LT survival, when adjusted for over 20 demographic and clinical characteristics (Table 2). African American or Black individuals were associated with lower post-LT survival (HR: 1.20 (95% CI: 1.12-1.28)), whereas. Asian (HR: 0.79 (95% CI: 0.71-0.88)) or Hispanic (HR: 0.86 (95% CI: 0.81-0.92)) individuals were associated with higher survival as compared with White individuals (Table 2). Many of these patterns held in the pre-Share 35 and Share 35 periods.
ConclusionsRacial, ethnic, and socioeconomic disparities at time of transplant, such as private insurance and income, influence post-LT survival in patients with HCC. These patterns persist despite the passage of equitable access policies, such as Share 35.
Hepatocellular carcinoma (HCC) is one of the fastest-rising causes of cancer-related deaths worldwide, with racial and ethnic minorities disproportionately affected [1]. Racial and ethnic minorities have lower rates of liver transplant (LT) referral and are less likely to be listed or transplanted [2,3]. Of those who are listed, Black patients are significantly more likely than White patients to be declined for transplantation because of drug and alcohol misuse [4,5]. Black patients have been found to have higher mortality and lower odds of early-stage HCC at diagnosis, and are less likely to receive liver transplantation for HCC as compared with White patients [4,6]. A study of the SEER database demonstrated that with early-stage HCC, even after accounting for differences in stage, use of invasive treatments, and treatment response, Black patients were found to have a 12% higher mortality rate compared to White patients [7–10]. These differences have not been explained by differences in tumor burden, severity or etiology of liver disease, or presence of other co-morbidities [11]. Studies suggest that they may be due to disparities in decreased utilization of healthcare services and access to curative therapies, particularly liver transplantation. [12–14]. As novel systemic treatments for HCC such as immune checkpoint inhibitors (ICI) have emerged, persistent racial/ethnic disparities in effectiveness of ICI have been found that warrant further study into predictors of response to better facilitate effective treatment for diverse populations [15]. Regardless of treatment options, socioeconomic disparities influence patient outcomes related to HCC.
Socioeconomic factors significantly impact post-liver transplant outcomes as well. Patients with Medicaid or Medicare as their primary insurer have poorer waitlist outcomes and higher post-transplant mortality [16]. Studies have consistently demonstrated lower overall survival among Black recipients, compared with White recipients, even after transplantation [2,17]. Differences in post-LT mortality over time have demonstrated that the Black-White disparity has worsened in recent years, with greater differences noted beyond 1-year post-LT and among patients without hepatitis C virus (HCV). Early analyses suggest that these disparities may be largely mediated by increases in alcohol-associated liver disease [18].
There are unique considerations with liver transplantation for patients with HCC. Traditionally, LT priority is based on the risk of waitlist mortality as determined by the Model for End-stage Liver Disease (MELD) score. Patients with HCC typically have lower MELD scores that are reflective of well-compensated liver disease but not of their overall prognosis. For conditions where MELD does not accurately predict mortality, exception points are used to adjust listing priority to better reflect disease severity [19]. Several different allocation systems have been proposed for LT in patients with HCC, with the Milan criteria most widely used [20]. Disparities within LT for patients in HCC have been less well studied, though have important implications for patients and policymakers.
In 2013, the United Network for Organ Sharing (UNOS) instituted a LT allocation policy known as “Share 35,” with the goal of decreasing waitlist mortality through the wider regional sharing of livers among patients with MELD score ≥35. Policies such as Share 35 were created to ensure that organs would be offered to patients with the greatest medical need; however, analyses of post-transplant outcomes suggest that structural health inequities have persisted [21].
The aim of this study was to characterize associations between socioeconomic factors, specifically race and ethnicity, income, and insurance type, and post-liver transplant outcomes among patients with HCC, and understand if these associations were impacted by Share 35.
2Materials and MethodsData from United Network for Organ Sharing / Organ Procurement Transplant Network, Standard Transplant Analysis and Research (UNOS/OPTN STAR) were used to conduct a retrospective cohort study.
A total of 30,610 patients who underwent a deceased-donor liver transplant for hepatocellular carcinoma from January 2005 to December 2020 were identified. Recipients with missing data for the income variable were excluded. The study period was selected to reflect the MELD allocation system.
The primary exposures were insurance, income, and race and ethnicity. Public insurance included Medicaid, Department of Veterans Affairs, or other government-related coverage. Income was defined as the presence of income. Race and ethnicity were categorized as follows in the UNOS database: 1) White, 2) Hispanic, 3) African American or Black, 4) Asian, 5) Other.
Covariates, obtained at the time of LT, were age, reported sex, race and ethnicity, insurance, income, citizenship status, etiology of liver disease, mortality, and associated medical history including diabetes mellitus (DM), ascites, and hepatic encephalopathy (HE), as well as MELD score, Donor Risk Index (DRI), and race/ ethnicity of donor (Table 1). Etiologies of liver disease were classified into the following categories: 1) Hepatitis C virus (HCV), 2) Alcoholic liver disease, 3) Non-alcoholic fatty liver disease (NAFLD), 4) Hepatitis B virus (HBV), 5) Other.
Characteristics of the study population, organized by time period
| Characteristic | 2005 – 2020 N=30,610 | Era 1: 2005 – 2013 N=15,056 | Era 2: 2014 – 2020 N=15,554 | p-value |
|---|---|---|---|---|
| Recipient characteristics – Demographics | ||||
| Age (year) (mean ± SD) | 59.4 ± 7.5 | 57.8 ± 7.3 | 60.9 ± 7.4 | <0.0001 |
| Male, n (%) | 23641 (77.2) | 11683 (77.6) | 11958 (76.9) | 0.14 |
| Race and/or ethnicity, n (%)* | <0.0001 | |||
| White | 20112 (65.7) | 9943 (66.0) | 10169 (65.4) | |
| African American or Black | 2832 (9.3) | 1427 (9.5) | 1405 (9.0) | |
| Hispanic | 4994 (16.3) | 2247 (14.9) | 2747 (17.7) | |
| Asian | 2231 (7.3) | 1239 (8.2) | 992 (6.4) | |
| Other | 441 (1.4) | 200 (1.3) | 241 (1.6) | |
| Public insurance, n (%) | 17204 (56.2) | 9273 (61.6) | 7931 (51.0) | <0.0001 |
| No income, n (%) | 20343 (66.5) | 9877 (65.6) | 10466 (67.3) | 0.0018 |
| Without United States citizenship, n (%) | 268 (0.9) | 136 (0.9) | 132 (0.9) | 0.61 |
| Recipient characteristics – Clinical | ||||
| Etiology of liver disease | ||||
| Hepatitis C, n (%) | 18760 (61.3) | 10169 (67.5) | 8591 (55.2) | <0.0001 |
| Alcoholic liver disease, n (%) | 3315 (10.8) | 1267 (8.4) | 2048 (13.2) | <0.0001 |
| Non-alcoholic fatty liver disease, n (%) | 3153 (10.3) | 791 (5.3) | 2362 (15.2) | <0.0001 |
| Hepatitis B, n (%) | 1828 (6.0) | 1029 (6.8) | 799 (5.1) | <0.0001 |
| Other, n (%) | 3554 (11.6) | 1800 (12.0) | 1754 (11.3) | 0.0001 |
| Body mass index (mean ± SD) | 29.0 ± 5.2 | 28.7 ± 5.1 | 29.4 ± 5.3 | <0.0001 |
| Diabetes, n (%) | 9885 (32.3) | 4274 (28.4) | 5611 (36.1) | <0.0001 |
| Portal venous thrombosis, n (%) | 4222 (13.8) | 1532 (10.2) | 2690 (17.3) | <0.0001 |
| Transjugular intrahepatic portosystemic shunt, n (%) | 2427 (7.9) | 1017 (6.8) | 1410 (9.1) | <0.0001 |
| Ascites, n (%) | 17783 (58.1) | 9154 (60.8) | 8629 (55.5) | <0.0001 |
| Hepatic encephalopathy, n (%) | 13815 (45.1) | 6939 (46.1) | 6876 (44.2) | 0.0009 |
| Dialysis, n (%) | 1483 (4.8) | 562 (3.7) | 921 (5.9) | <0.0001 |
| Death, n (%) | 8636 (28.2) | 6297 (41.8) | 2339 (15.0) | <0.0001 |
| MELD Exception Score (mean ± SD) | 26.5 ± 5.7 | 25.4 ± 5.1 | 27.6 ± 6.0 | <0.0001 |
| Calculated MELD Score (mean ± SD) | 15.4 ± 8.5 | 14.9 ± 7.9 | 15.9 ± 9.0 | <.0001 |
| Serum creatinine (mg/dL) (mean ± SD) | 1.2 ± 1.0 | 1.2 ± 1.0 | 1.2 ± 1.0 | 0.028 |
| Serum INR (mean ± SD) | 1.5 ± 0.8 | 1.5 ± 0.7 | 1.6 ± 0.8 | <0.0001 |
| Serum bilirubin (mg/dL) (mean ± SD) | 3.8 ± 6.7 | 3.8 ± 6.7 | 3.8 ± 6.7 | 0.642 |
| Serum albumin (g/dL) (mean ± SD) | 3.2 ± 0.7 | 3.2 ± 0.7 | 3.3 ± 0.7 | <0.0001 |
| Serum sodium (mEq/L) (mean ± SD) | 137.1 ± 4.5 | 137.0 ± 4.4 | 137.2 ± 4.5 | <0.0001 |
| Donor characteristics – Demographic | ||||
| Age, donor (year) (mean ± SD) | 42.7 ± 16.6 | 42.3 ± 16.8 | 43.1 ± 16.3 | <0.0001 |
| Male, donor, n (%) | 18431 (60.2) | 8965 (59.5) | 9466 (60.9) | 0.0188 |
| Race and/or ethnicity, donor* | <0.0001 | |||
| White | 19841 (64.8) | 9821 (65.2) | 10020 (64.4) | |
| African American or Black | 5288 (17.3) | 2590 (17.2) | 2698 (17.4) | |
| Hispanic | 4154 (13.6) | 2022 (13.4) | 2132 (13.7) | |
| Asian | 840 (2.7) | 438 (2.9) | 402 (2.6) | |
| Other | 487 (1.6) | 185 (1.2) | 302 (1.9) | |
| Donor characteristics – Clinical | ||||
| Body mass index, donor (mean ± SD) | 27.9 ± 6.5 | 27.4 ± 6.1 | 28.5 ± 6.8 | <0.0001 |
| Donor Risk Index (mean ± SD) | 1.6 ± 0.7 | 1.6 ± 0.7 | 1.6 ± 0.7 | 0.04 |
| Cold ischemia time (mean ± SD) | 6.4 ± 2.7 | 6.8 ± 3.0 | 6.1 ± 2.3 | <0.0001 |
The primary outcome was post-transplant survival, examined by race, ethnicity, and socioeconomic status. Follow-up was assessed from the time of liver transplantation to the last day of follow-up.
2.1Statistical analysesChi-square and T-tests were used to analyze categorical and continuous recipient and donor variables, respectively. Categorical variables were presented by number and percentage, and continuous variables were presented by mean and standard deviation. Kaplan-Meier plots were plotted for survival analysis. Predictors of survival were identified using forward stepwise multivariate Cox proportional hazard regression models, adjusted for clinical and demographic characteristics, during the pre-Share 35 (2005-2013), Share 35 (2014-2020), and entire time period (2005-2020). The p-value for entry into and removal from the model was set at 0.05.
Results were reported by hazard ratio (HR) and 95% confidence interval (CI). Data were extracted from the database and transferred into the Statistical Analysis Software (version 9.4) and Stata software (version 16.1; StataCorp), which were used for data cleaning, management, and analyses.
2.2Ethical statementInstitutional review board (IRB) approval was not required, as UNOS contains publicly available de-identified data.
3Results3.1Characteristics of the study populationDemographic and clinical characteristics are displayed in Table 1.
A total of 30,610 transplant recipients with HCC were included (2005-2013: n=15,056; 2014-2020: n=15,554). Across the entire study period, 59.4 years (SD 7.5) was the mean age and 77.2% of recipients were men. The majority of recipients were White (65.7%), with the majority of recipients classified as having HCV as the etiology of liver disease (61.3%). This was found to be consistent across the time periods analyzed (pre-Share 35 and Share 35).
Regarding medical co-morbidities of the transplant recipients, body mass index (BMI) and MELD scores were seen to increase across the time periods studied, as did the proportions of individuals on dialysis and with DM, portal venous thromboses (PVT), and transjugular intrahepatic portosystemic shunts (TIPS). In contrast, the proportion of individuals with ascites and hepatic encephalopathy at time of transplant decreased over time.
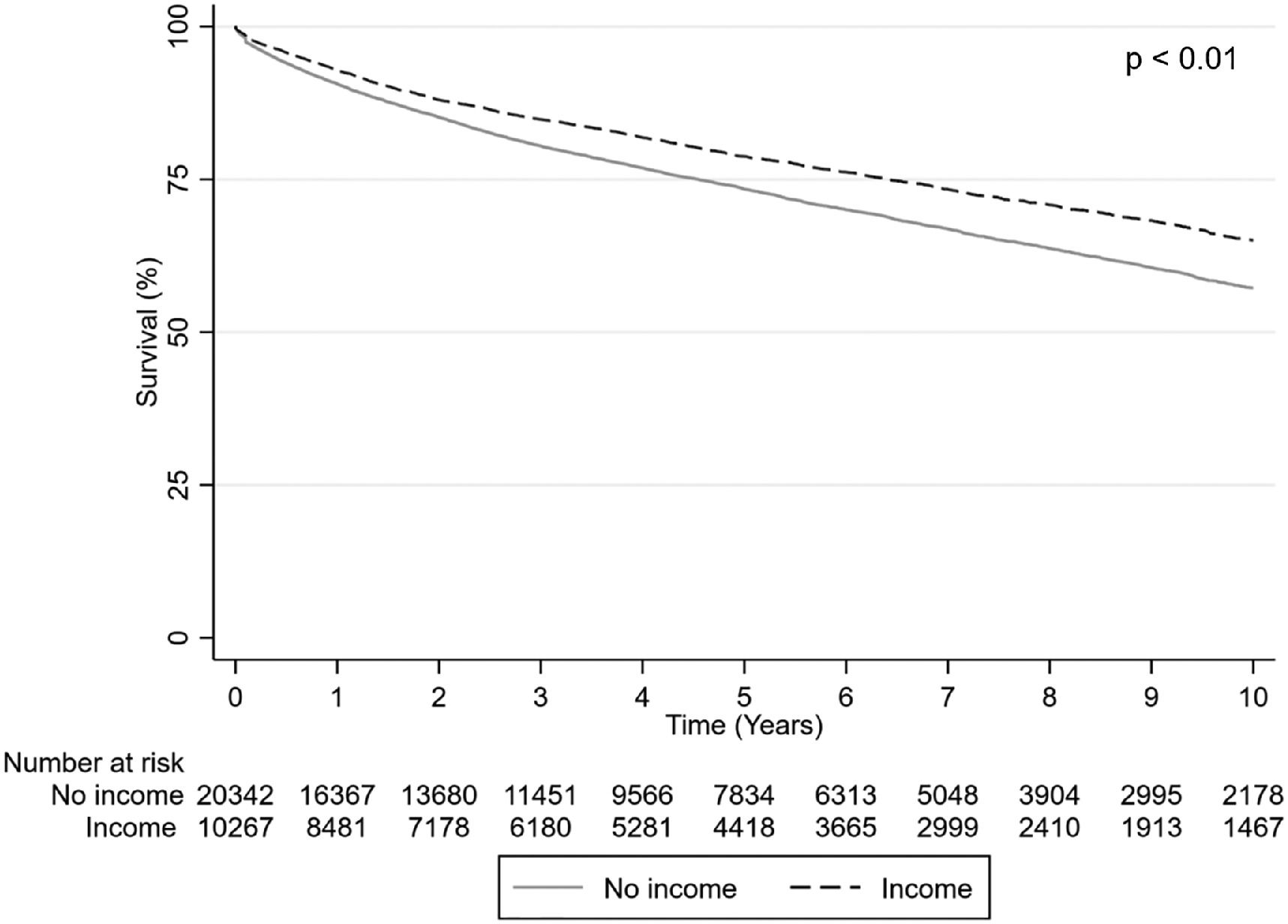
3.2OutcomesSurvival varied by time period studied, with increased survival noted in the Share 35 period as compared to the pre-Share 35 period (Fig. 1). Recipients with private insurance at the time of transplant, as compared to recipients with public insurance, were noted to have higher survival at one-, five-, and ten- years post-transplant (Fig. 2). Recipients with income were found to have higher survival at 1-, 5-, and 10-years post-transplant (Fig. 3). Race or ethnicity was also associated with variation in post-transplant survival: Asian transplant recipients were found to have the highest survival post-transplant, whereas African American or Black transplant recipients were found to have the lowest survival post-transplant, as compared to White recipients (Fig. 4).
In multivariate analysis, demographic characteristics associated with higher post-LT survival of HCC patients included men, private insurance, and income, as well as Asian and Hispanic individuals. Higher post-LT survival was associated with the liver disease etiologies of alcoholic liver disease, NAFLD, and HBV. Medical comorbidities such as DM and hepatic encephalopathy as well as higher MELD score were associated with lower post-LT survival. When analyzed by pre-Share 35 and Share 35 time periods, many of these associations held across these time periods.
The results of the multivariate analysis are shown in Table 2. All multivariate analyses were adjusted for demographic and clinical characteristics, which are noted in Table 1.
Associations between patient characteristics and mortality
In this analysis of the UNOS database, we found significant disparities in post-LT survival among HCC patients that were seen up to 10 years post-transplantation. Our findings showed higher post-LT survival in patients with the following characteristics: male, private insurance, presence of income, and Asian or Hispanic race/ethnicity. African American or Black recipients were found to have the lowest survival post-transplantation.
These racial, ethnic, and socioeconomic disparities post-transplantation parallel differences described in the literature [22]. Studies have shown that Black patients have lower odds of receiving curative treatment than White patients, and Black patients living in high-poverty neighborhoods have both lower odds of receiving curative treatment and worse overall survival [18]. Income inequality is often tied to race and ethnicity, and likely compound negative outcomes.Hispanic patients have been found to present with more decompensated liver disease and medical comorbidities but increased patient and graft survival overtime, as compared to non-Hispanic White patients [23]. We also demonstrated the documented gender disparity in liver transplantation, which has been attributed to suboptimal MELD scoring systems and biological and behavior differences between genders. [24–27]. Biologic (e.g. hormones, with estrogen as protective) and behavioral differences (e.g. substance use, engagement with healthcare system) have been described as modifiers of the differences between males and females with HCC [27]. We note the goal to correct gender bias in MELD scoring, with development of MELD 3.0, which we believe represents a positive step in acknowledging and correcting limitations in allocation systems.
Private insurance and income were found to be protective factors for survival. They encompass socioeconomic status and stand as proxy variables for other social determinants of health, which have been well-known to impact health outcomes, including HCC [28]. Demographic characteristics can impact survival just as severely as known medical co-morbidities.
Our results were also notable for observational differences in age, public insurance, and income between the time periods studied. The aging of patients may reflect the increase in life expectancy, as well as advancements in medical care that may have strengthened the candidacy of older patients. We acknowledge the decrease in public insurance and increase in lack of income: These are more difficult to singularly interpret but likely reflective of changing political and economic climates in the United States (U.S.). For instance, the increase in patients without income mirrors documented changes within the U.S. economy [29]. These changes are important to note when considering their influence on the outcomes studied.
We also studied the clinical characteristics of transplant recipients across time periods, with differences likely reflective of clinical trends over the past two decades. Patient encounters have become more clinically complex, as patients present with numerous medical comorbidities. This was seen in our analysis, with a higher proportion of patients with DM and PVTs in the Share 35 era. The accessibility of medical advancements such as TIPS and dialysis has also increased, and is likely representative of other changes in medical treatments that have influenced recipient candidacy and health. The Share 35 era was also notable for lower cold ischemia times with expected improved survival. Medical and surgical advancements in the detection and treatment of HCC, such as direct anti-viral therapies (DAT) and ICI, have likely contributed to the lower proportion of HCC transplant recipients with decompensated liver disease (ascites and hepatic encephalopathy) in the Share 35 period.
Our analysis showed that there was increased post-transplant survival in the Share 35 period as compared with the pre-Share 35 period. This is again likely reflective of scientific advancements in earlier recognition and management of liver disease, and is concordant with prior studies demonstrating improved post-transplant survival after Share 35 for all LT indications [30]. As expected, the presence of comorbidities such as DM and HE and higher MELD score at time of transplant was associated with lower post-transplant survival: Sicker patients did not fare as well.
However, while the implementation of Share 35 has allowed for greater regional sharing of livers for patients with MELD≥35, its impact may have been less significant in patients with HCC. Previous studies did not find significant changes in wait-time for patients after implementation of the policy, though higher rates of wait-list mortality were observed in patients with HCC after passage of the policy [31]. This may be reflective of not only the clinical differences in patients with HCC but also the disparities across racial, ethnic, and socioeconomic groups that carried through the passage of Share 35, affecting the care and outcomes of patients with HCC. Disparities inherent in the referral and listing (pre-transplant) process, while not explicitly studied here, likely affect outcomes. The disparities in post-transplant survival seen in our analysis are novel, suggesting that both disease severity at time of transplantation and systemic barriers contribute to challenges with equitable post-transplant follow-up and outcomes for patients with HCC.
Addressing socioeconomic disparities is imperative in ensuring improved post-transplantation outcomes. Consistent follow-up with liver center specialists and care coordination within an interdisciplinary team are crucial to providing the necessary support to prevent adverse outcomes post-transplantation. These supports may be more easily accessible for patients with reliable income and insurance, and efforts to build and offer these supports to individuals with limited income or insurance should be prioritized. As discussed earlier, studies have demonstrated that Black patients living in high-poverty neighborhoods have lower odds of receiving curative treatment and worse survival, even among those who are insured [22]. In high-poverty communities, there may be poorer access to hospitals, clinics, and subspecialists. Insured patients living in these areas may have other barriers, such as transportation, need for child care, and food insecurities, which result in delayed care as well, especially if out-of-pocket costs for care remain high. Following transplantation, these barriers may contribute to delays in appointments essential for surveillance and detection of HCC recurrence as well as management of immunosuppression, leading to worse post-transplant outcomes [32]. Prioritizing and funding systems that build access to care, including but not limited to community health outreach, provision of transportation, and health literacy programs, are essential to sustainably improve outcomes for patients. Clinical providers should remain cognizant of the structural disparities that impact their patients and outcomes in order to ensure equitable care, and continuing medical education should work to bridge these gaps.
Interventions to reduce disparities in HCC detection and create more equitable access to healthcare and liver transplantation are needed. Treatment delays have been found to be associated with younger age, Hispanic ethnicity or Black race, earlier tumor stage, treatment at academic centers, receipt of non-curative therapy, and region [33]. In one study examining the survival of HCC patients within safety net hospitals without an on-site liver transplant program, despite adequate referrals, overall survival and rate of liver transplantation were found to be significantly decreased as compared with programs with on-site liver transplant programs [34,35]. Referral alone does not ensure equal outcomes. However, efforts to improve screening policies and facilitate earlier referrals have been seen to lead to earlier detection, treatment, and improved survival [36,37]. Telemedicine may provide an alternative access route to transplant evaluation [38]; however, disparities in telehealth utilization still remain, most notably with older, non-Hispanic African American or Black populations and those with public insurance [39]. This remains concerning, given our findings demonstrating that these characteristics are associated with poorer survival post-transplantation. Listing and receipt of LT alone is not sufficient to ensure improved long-term survival for patients with limited access to healthcare, and draws attention to the need for continued efforts in making post-transplant care equitable.
Emerging studies suggest that socioeconomic factors may also mediate disparities on an epigenetic level, resulting in immunologic changes that may drive cancer biology and the progression of the disease [40]. The cumulative impact of chronic stress of racism and poverty in the U.S. may differentially drive tumor growth [41]. This is especially striking in contrast to recent European studies where socioeconomic status was not shown to affect outcomes for liver transplantation for HCC [42]. While the authors hypothesize that these findings may be in part be due to access to free universal healthcare, further studies of cancer biology across varied geographic regions with different social structures are needed to parse out potential epigenetic changes which could mediate immunologic changes driving the progression of the disease.
This study contains a variety of limitations. This was a retrospective analysis of a large, heterogeneous database, which likely included variable reporting of demographic and clinical variables. Grouping of variables by general categories, such as five categories for race/ ethnicity and binary categories for income and insurance, can contribute to misclassification bias with over-simplification of results. The variables, documented at time of transplant, also do not capture potential changes over the study period; for example, insurance and income are variables that may have evolved over the study period, and peri-operative support, resources, and standard of care likely changed over this time period as well. However, these variables are not well-documented in large datasets like UNOS. Moreover, the variables studied are also incomplete markers of socioeconomic status: While income is an accepted indicator of socioeconomic status, it is binary in this study, and additional indicators, such as level of education, place of residence, employment, and social support, are not explicitly captured here. We also acknowledge that clinical characteristics relating to HCC, such as tumor size and number, alpha fetoprotein tumor marker levels, were not analyzed, given database constraints.
Future work would benefit from inclusion of more nuanced data, including indicators of socioeconomic status (e.g. level of education, profession, social supports) and social determinants of health. Understanding how best to accurately measure and analyze social determinants of health over time will be challenging. We propose that these would include measures of economic stability (e.g. employment, income, debt), environment (e.g. ease of transportation and geography), education (e.g. literacy, language), food security, and health access. Compilation of these variables, whether through linkage of existing datasets or additional collection, into one, unified dataset, such as UNOS should be considered, with the goal of identifying which variables may be modifiable or unmodifiable. Unmodifiable variables could then be included in future allocation models.
Additional statistical analyses focused on quantifying concurrent policy changes (e.g. the introduction of the Affordable Care Act in 2010) and further examining the close – and nearly inseparable – relationship between socioeconomic status and race and ethnicity in the U.S. would benefit future interpretations of the data.
5ConclusionsIn conclusion, our study highlights that racial, ethnic, and socioeconomic disparities observed at the time of transplantation influence post-LT survival in patients with HCC. These patterns persist despite the passage of equitable access policies, such as Share 35. We have suggested a variety of community and clinical-based interventions to address these findings, as well as additional foci of data collection and analyses for future studies. Moving forward, a deeper understanding of the structural causes of these disparities is needed to design effective interventions to promote health equity and improve outcomes for patients with HCC post-transplantation.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to acknowledge UNOS, as the contractor for OPTN, for the administration of the database used. The interpretation and reporting of this data are the responsibility of the authors.