Acute-on-chronic liver failure (ACLF) is associated with reduced short-term survival, and liver transplantation is frequently the only therapeutic option. Nonetheless, the post-transplantation prognosis seems to be worse in ACLF patients.
Materials and MethodsThe databases of two university centers were retrospectively evaluated, and adult patients with cirrhosis who underwent transplantation between 2013 and 2020 were included. One-year survival of patients with ACLF was compared to that of patients without ACLF. Variables associated with mortality were identified.
ResultsA total of 428 patients were evaluated, and 303 met the inclusion criteria; 57.1% were male, the mean age was 57.1 ± 10.2 years, 75 patients had ACLF, and 228 did not. The main etiologies of ACLF were NASH (36.6%), alcoholic liver disease (13.9%), primary biliary cholangitis (8.6%) and autoimmune hepatitis (7.9%). Mechanical ventilation, renal replacement therapy, the use of vasopressors and the requirement of blood product transfusion during liver transplantation were significantly more frequent in ACLF patients. Among those recipients without and with ACLF, survival at 1, 3 and 5 years was 91.2% vs. 74.7%, 89.1% vs. 72.6% and 88.3% vs. 72.6%, respectively (p=0.001). Among pre-transplantation variables, only the presence of ACLF was independently associated with survival (HR 3.2, 95% CI: 1.46-7.11). Post-transplantation variables independently associated with survival were renal replacement therapy (HR 2.8, 95% CI: 1.1-6.8) and fungal infections (HR 3.26, 95% CI: 1.07-9.9).
ConclusionsACLF is an independent predictor of one-year post-transplantation survival. Importantly, transplant recipients with ACLF require the use of more resources than patients without ACLF.
Chronic liver diseases are notably common and in the long term frequently fatal, causing a significant use of resources. Their incidence has increased in parallel with a rise in alcohol consumption, the overuse of drugs and the epidemics of diabetes, obesity and fatty liver disease [1].
Acute-on-chronic liver failure (ACLF) is an increasingly well-recognized entity related to an unusually increased exposure to pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs) or virulence factors in the context of acute decompensation in patients with chronic liver disease. These molecules lead to an uncontrolled release of cytokines and tissue damage leading to organ failure. ACLF is associated with high mortality that increases with the number of failed organs [1–3].
The most common causes of ACLF are bacterial infections, severe alcoholic hepatitis, variceal bleeding, hepatotoxic drugs and the reactivation of viral infections or autoimmune hepatitis. Despite the greater knowledge of its pathophysiology and characterization in recent years, an identifiable cause is absent in up to 40% of cases [4].
There are several definitions of ACLF, one of the most commonly used is the one proposed by the European Foundation for the Study of Chronic Liver Failure (EF-CLIF), which is based on the results of the CANONIC study that established three grades according to the severity of the failure of six organs/systems: the brain, kidneys, and liver and the circulatory, respiratory and coagulation systems [4,5].
Notably, ACLF can be graded based on the number of failed organs (grades 1, 2 and 3). Thus, a higher grade is associated with higher mortality. Hence, the 28-day mortality rate is 21% for grade 1, 57% for grade 2 and 87% for grade 3 [2].
In properly selected candidates, liver transplantation (LT) is a plausible alternative that dramatically improves survival in ACLF patients. Notably, ACLF prognosis is not accurately predicted by the Model for End-stage Liver Disease (MELD). Thus, it has been suggested that organ allocation should be performed employing different criteria [6–8].
Although LT significantly improves survival in ACLF recipients, their posttransplantation prognosis seems to be worse than that of recipients without ACLF, being even lower in those patients with ACLF grade 3. This is an important fact considering that the futility of LT is a critical issue, especially in regions with a low donation rate, such as Latin America. Thus, potential recipients must be carefully selected to maximize recipient survival and avoid transplantation futility [8,9]. The post-transplantation prognosis of patients with ACLF is an important issue, and the transplant center experience related to ACLF must be evaluated to properly use the available resources and maximize graft survival.
Thus, this study aims to evaluate the effect of the diagnosis of ACLF just before LT on long-term post-transplantation survival in two university centers in Chile.
2Materials and Methods2.1PatientsThis study was a retrospective evaluation of a cohort including patients enrolled in the liver transplant program of two centers in Latin America: The Pontificia Universidad Católica de Chile and the Clinical Hospital of the Universidad de Chile. We evaluated patients older than 18 years who were listed for liver transplantation from 2013 to 2020.
Patients who were under 18 years old; patients who were undergoing retransplantation; and patients with acute liver failure, an absence of cirrhosis or a noncirrhotic etiology (i.e., neuroendocrine or vascular diseases), and a lack of complete data were excluded.
Data were collected retrospectively from the clinical records of the transplant programs of both centers, including age, sex, underlying liver disease, MELD-Na score, Child-Pugh score, presence of contraindications for transplantation, and presence or absence of ACLF and ACLF grade. The results of posttransplantation evaluations of graft complications and infections and long-term survival after liver transplantation were also recorded. We also estimated the CLIF-C, Donor Risk Index and BAR to evaluate their potential role in predicting the posttransplantation prognosis of patients with ACLF [4,10,11].
2.2Diagnostic criteria for ACLF2.2.1Definition of organ failureACLF grade 1 (ACLF-1): defined by the presence of renal failure (creatinine > 2 mg/dl) or the presence of one organ failure associated with renal dysfunction (plasma creatinine between 1.5 and 1.9 mg/dl) and/or hepatic encephalopathy grade I-II of the West Haven classification or hepatic encephalopathy grade III-IV and plasma creatinine between 1.5 and 1.9 mg/dl.
ACLF grade 2 (ACLF-2): defined as the presence of the failure of 2 organs.
ACLF grade 3 (ACLF-3): defined as the presence of the failure of 3 or more organs.
The primary aim of our study was to assess the impact of the presence of ACLF on posttransplantation survival.
The secondary objectives were to evaluate the impact of the presence of ACLF on the frequency of graft complications (including vascular complications), the requirement of mechanical ventilation, renal replacement therapy, the use of blood product transfusion and the frequency of infectious complications.
2.3Statistical analysisWe reported continuous variables as the mean and standard deviation (SD). Nonnormal variables were reported as the median and interquartile range (IQR). Differences between normally distributed continuous variables were evaluated using the T-test and Mann‒Whitney U test for those without a normal distribution. Normal distribution was evaluated employing the Kolmogorov‒Smirnov test.
Qualitative variables were described using percentages and were compared using the chi-square test. The main outcome was posttransplantation survival. Kaplan‒Meier curves were generated to compare survival between patients with and without ACLF. A comparison among patients without ACLF and ACLF grade 1, 2 and 3 was also made. These comparisons were performed using the log-rank test. A p-value ≤ 0.05 was considered significant. Secondary outcomes were the requirement of mechanical ventilation or renal replacement therapy (pre- and post-liver transplantation), the use of vasopressor drugs, blood product transfusion during transplantation surgery, biliary complications, vascular complications, infections and acute rejection.
A multivariate logistic regression model was used to estimate the variables independently associated with survival by analyzing pre- and posttransplantation variables separately. Data were processed using SPSS version 24.0 (IBM Corp.).
2.4Ethical statementWritten informed consent was obtained from each patient included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of the School of Medicine, Pontificia Universidad Católica de Chile. All data were processed anonymously (ID 210429009).
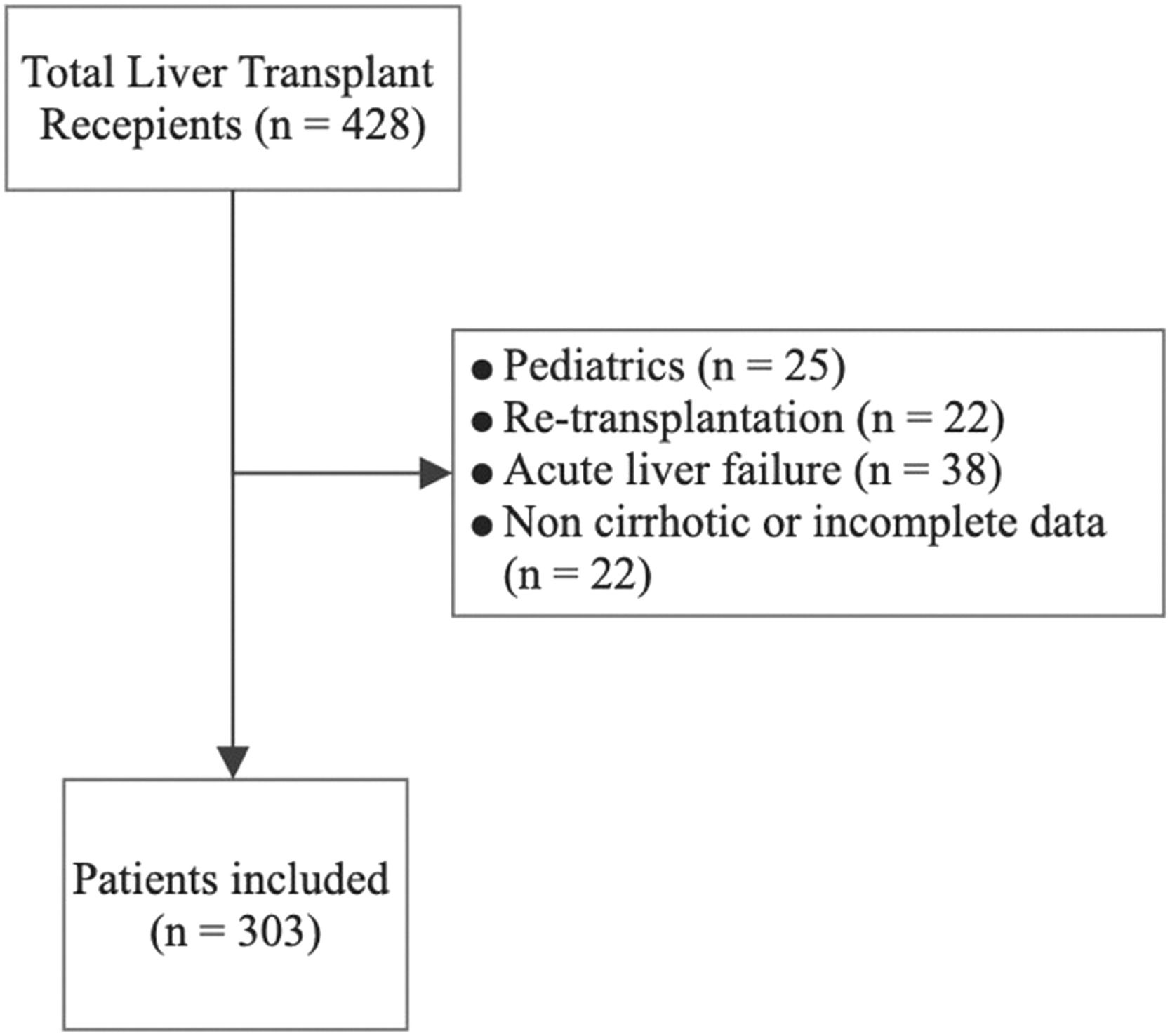
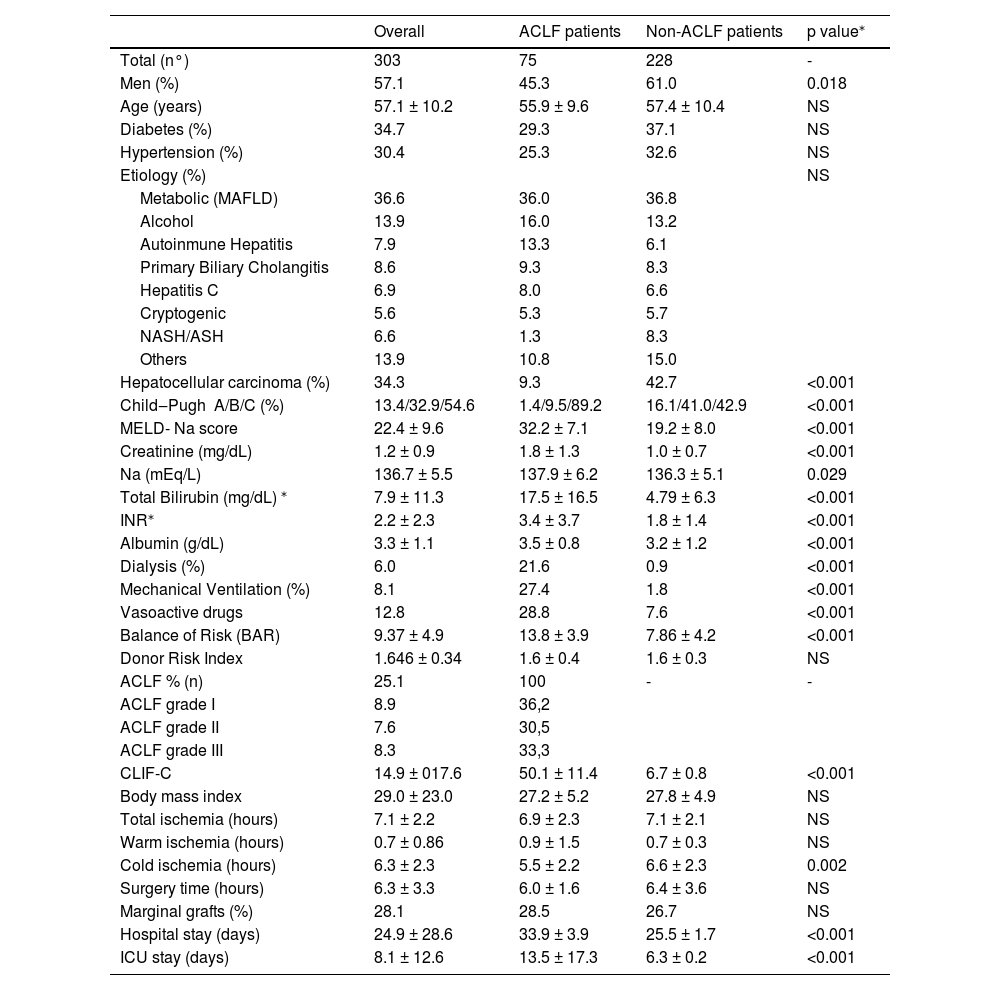
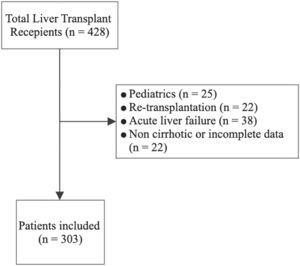
3ResultsFrom an initial cohort of 428 patients (all of them transplanted with a deceased donor), we excluded 25 patients who were under 18 years old, 22 patients who underwent retransplantation, 38 patients with acute liver failure and 40 patients with other noncirrhotic etiologies or incomplete information. Finally, we included 303 patients in the analysis (Fig. 1); among them, 75 patients had ACLF, and 228 did not. The mean follow-up was 1219 ± 808 days. The mean age was 57.1 ± 10.2 years, 57.1% of patients were male, and the main etiologies were NASH (36.6%), alcoholic liver disease (13.9%), primary biliary cholangitis (8.6%), autoimmune hepatitis (7.9%) and chronic hepatitis C (6.9%). Mean BMI was 27.6 ± 4.9 (p<0.001) and it was higher in NASH patients vs. non-NASH patients (29.3 ± 5.5 vs. 26.6 ± 4.3, p<0.001). The percentage of patients with ACLF was 25.1%, among whom ACLF grade 1 was found in 36.2%, grade 2 in 30.5% and grade 3 in 33.3% (Table 1). The causes of decompensation were infections in 37.3% (spontaneous bacterial peritonitis 22.7%, urinary tract infection 10.7% and pneumonia 4.0%); cryptogenic in 32%; variceal hemorrhage in 6.7%; autoimmune hepatitis reactivation in 4.0% and others in 20%. The frequency of renal replacement therapy, mechanical ventilation, and the use of vasoactive drugs was 6.0%, 8.1% and 12.8%, respectively. MELD-Na score was 22.4 ± 9.6, creatinine was 1.2 ± 0.9 mg/dL, Na was 136.7 ± 5.5 mEq/L, total bilirubin was 7.9 ± 11.3 mg/dL, INR was 2.2 ± 2.3, and albumin was 3.3 ± 1.1 g/dL (Table 1). Overall posttransplantation survival at 1, 3 and 5 years in our cohort was 87.1%, 85.0% and 84.4%, respectively.
Patient characteristics.
| Overall | ACLF patients | Non-ACLF patients | p value⁎ | |
|---|---|---|---|---|
| Total (n°) | 303 | 75 | 228 | - |
| Men (%) | 57.1 | 45.3 | 61.0 | 0.018 |
| Age (years) | 57.1 ± 10.2 | 55.9 ± 9.6 | 57.4 ± 10.4 | NS |
| Diabetes (%) | 34.7 | 29.3 | 37.1 | NS |
| Hypertension (%) | 30.4 | 25.3 | 32.6 | NS |
| Etiology (%) | NS | |||
| Metabolic (MAFLD) | 36.6 | 36.0 | 36.8 | |
| Alcohol | 13.9 | 16.0 | 13.2 | |
| Autoinmune Hepatitis | 7.9 | 13.3 | 6.1 | |
| Primary Biliary Cholangitis | 8.6 | 9.3 | 8.3 | |
| Hepatitis C | 6.9 | 8.0 | 6.6 | |
| Cryptogenic | 5.6 | 5.3 | 5.7 | |
| NASH/ASH | 6.6 | 1.3 | 8.3 | |
| Others | 13.9 | 10.8 | 15.0 | |
| Hepatocellular carcinoma (%) | 34.3 | 9.3 | 42.7 | <0.001 |
| Child‒Pugh A/B/C (%) | 13.4/32.9/54.6 | 1.4/9.5/89.2 | 16.1/41.0/42.9 | <0.001 |
| MELD- Na score | 22.4 ± 9.6 | 32.2 ± 7.1 | 19.2 ± 8.0 | <0.001 |
| Creatinine (mg/dL) | 1.2 ± 0.9 | 1.8 ± 1.3 | 1.0 ± 0.7 | <0.001 |
| Na (mEq/L) | 136.7 ± 5.5 | 137.9 ± 6.2 | 136.3 ± 5.1 | 0.029 |
| Total Bilirubin (mg/dL) ⁎ | 7.9 ± 11.3 | 17.5 ± 16.5 | 4.79 ± 6.3 | <0.001 |
| INR⁎ | 2.2 ± 2.3 | 3.4 ± 3.7 | 1.8 ± 1.4 | <0.001 |
| Albumin (g/dL) | 3.3 ± 1.1 | 3.5 ± 0.8 | 3.2 ± 1.2 | <0.001 |
| Dialysis (%) | 6.0 | 21.6 | 0.9 | <0.001 |
| Mechanical Ventilation (%) | 8.1 | 27.4 | 1.8 | <0.001 |
| Vasoactive drugs | 12.8 | 28.8 | 7.6 | <0.001 |
| Balance of Risk (BAR) | 9.37 ± 4.9 | 13.8 ± 3.9 | 7.86 ± 4.2 | <0.001 |
| Donor Risk Index | 1.646 ± 0.34 | 1.6 ± 0.4 | 1.6 ± 0.3 | NS |
| ACLF % (n) | 25.1 | 100 | - | - |
| ACLF grade I | 8.9 | 36,2 | ||
| ACLF grade II | 7.6 | 30,5 | ||
| ACLF grade III | 8.3 | 33,3 | ||
| CLIF-C | 14.9 ± 017.6 | 50.1 ± 11.4 | 6.7 ± 0.8 | <0.001 |
| Body mass index | 29.0 ± 23.0 | 27.2 ± 5.2 | 27.8 ± 4.9 | NS |
| Total ischemia (hours) | 7.1 ± 2.2 | 6.9 ± 2.3 | 7.1 ± 2.1 | NS |
| Warm ischemia (hours) | 0.7 ± 0.86 | 0.9 ± 1.5 | 0.7 ± 0.3 | NS |
| Cold ischemia (hours) | 6.3 ± 2.3 | 5.5 ± 2.2 | 6.6 ± 2.3 | 0.002 |
| Surgery time (hours) | 6.3 ± 3.3 | 6.0 ± 1.6 | 6.4 ± 3.6 | NS |
| Marginal grafts (%) | 28.1 | 28.5 | 26.7 | NS |
| Hospital stay (days) | 24.9 ± 28.6 | 33.9 ± 3.9 | 25.5 ± 1.7 | <0.001 |
| ICU stay (days) | 8.1 ± 12.6 | 13.5 ± 17.3 | 6.3 ± 0.2 | <0.001 |
The results of ACLF and non-ACLF patient comparisons were as follows: MELD-Na score 32.2 ± 7.1 vs. 19.2 ± 8.0, p<0.001; creatinine (mg/dL) 1.88 ± 1.3 vs. 1.0 ± 0.7, p<0.001; Na (mEq/L) 137.9 ± 6.2 vs. 136.3 ± 5.1, p=0.029; total bilirubin (mg/dL) 17.58 ± 16.5 vs. 4.7 ± 6.3, p<0.001; INR 3.4 ± 3.7 vs. 1.8 ± 1.4, p<0.001 and CLIF-C 50.1 ± 11.4 vs. 6.7 ± 0.8, p<0.001. We also found differences between ACLF and non-ACLF patients when comparing the requirement of mechanical ventilation (27.4% vs. 1.4%, p < 0.001), renal replacement therapy (21.6% vs. 0.5%, p < 0.001) and vasopressor drugs (28.8% vs. 6.7%, p < 0.001) (Table 1). Hospital and ICU stays (days) were significantly longer in ACLF patients: 33.9 ± 3.9 vs. 25.5 ± 1.7, p<0.001 and 13.5 ± 6.3 ± 0.2, p<0.001, respectively.
There were no differences in the total ischemia times between patients with and without ACLF however cold ischemia times were longer in non-ACLF patients (Table 1).
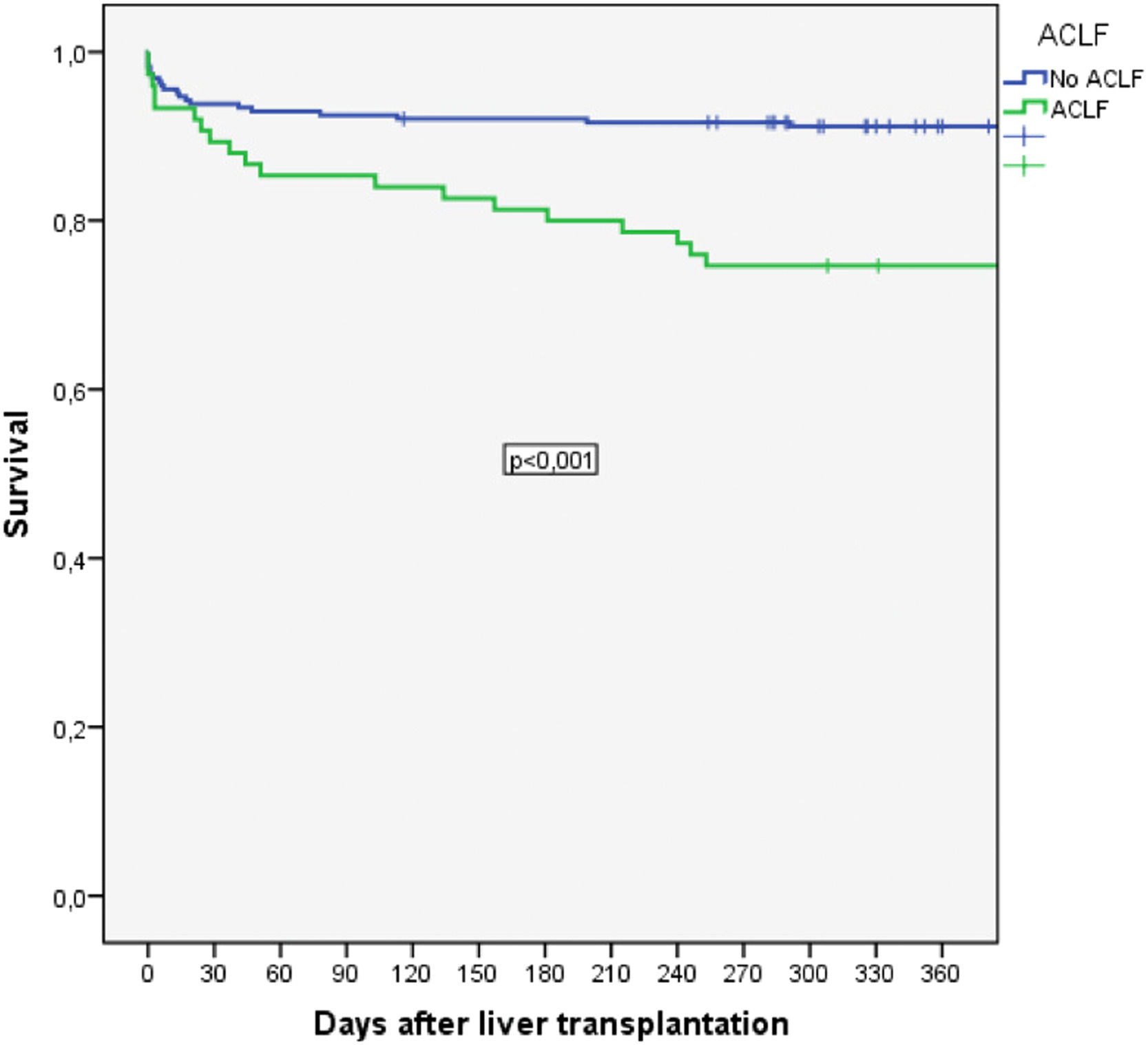
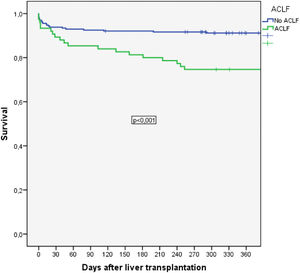
Among patients with ACLF, long-term post-transplantation mortality was significantly increased compared to that of patients without ACLF. Thus, 30-day mortality was 24.1% vs. 33.3%, p=NS, and 90-day survival was 23.6% vs. 36.0%, p=NS. Posttransplantation survival at 1, 3 and 5 years was 74.7% vs. 91.2%, 72.6% vs. 89.1% and 72.6% vs. 88.3%, respectively (p=0.001) (Fig. 2A).
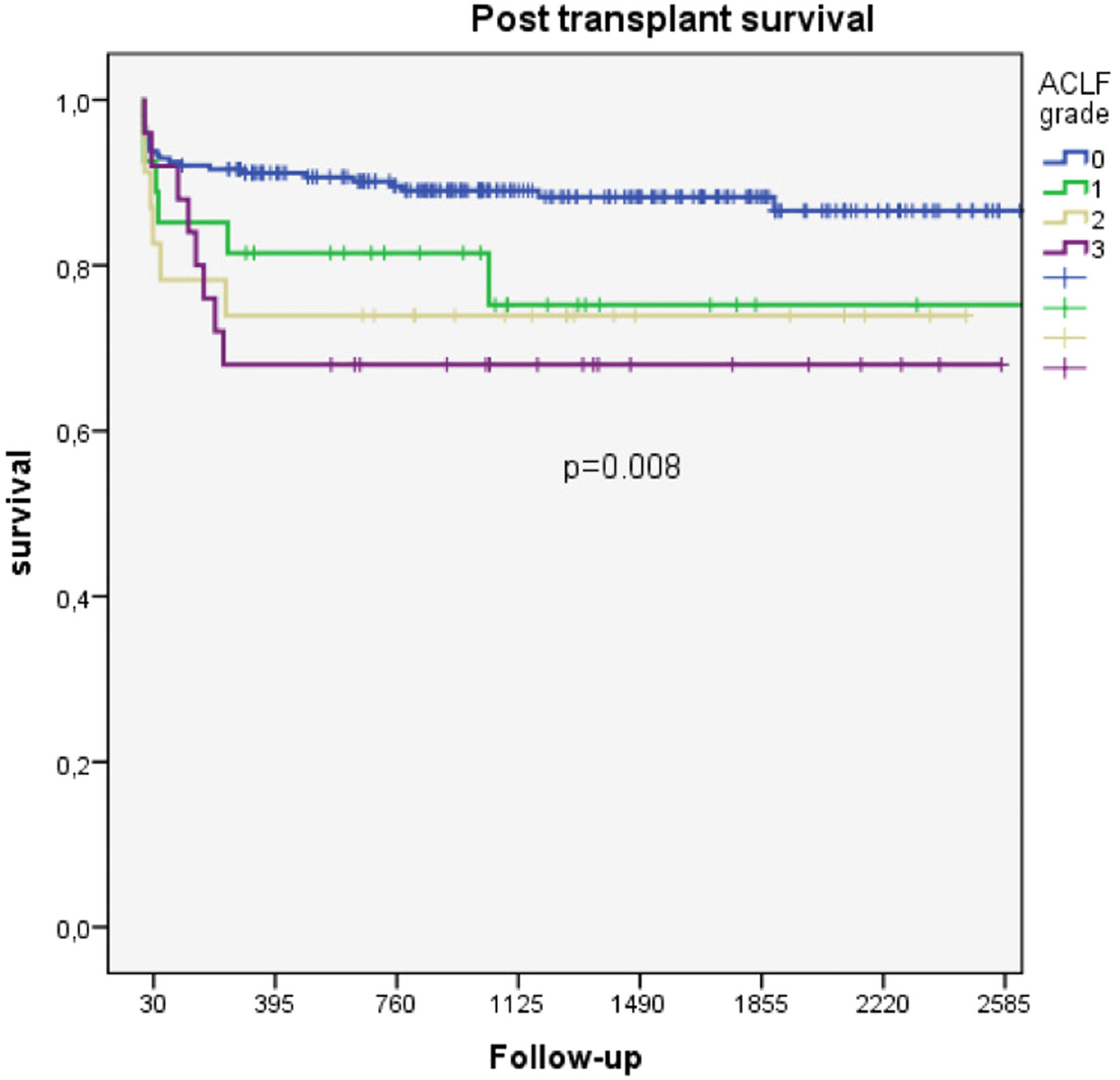
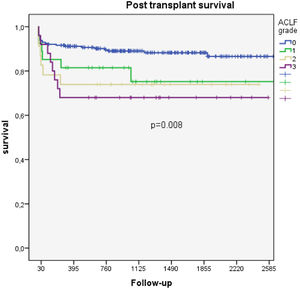
Among patients with ACLF, there were significant differences in survival according to the severity of organ failure. Thus, the 1- and 2-year survival rates for those with ACLF grades 1, 2 and 3 were 81.5% vs. 73.9% vs. 68.0% and 75.2% vs. 73.9% vs. 68.0%, respectively (p=0.009) (Fig. 2B).
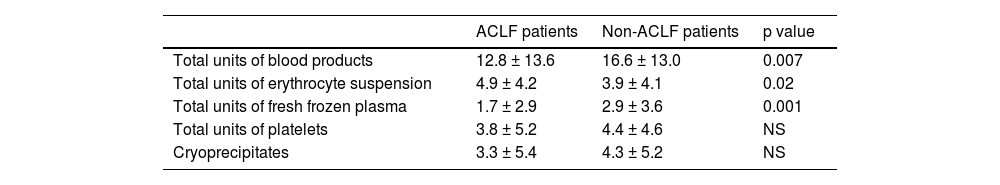
We evaluated the transfusion of blood products during surgery. Its use was remarkably higher in patients with ACLF. Thus, the mean of administered blood products for non ACLF patients was 11.6 ± 13.6 units vs. 16.6 ± 13.0 units for ACLF patients, p<0.007. The transfusion of erythrocyte suspension and fresh frozen plasma was also higher in the ACLF group (Table 2).
Blood products administered during surgery to ACLF and non-ACLF patients.
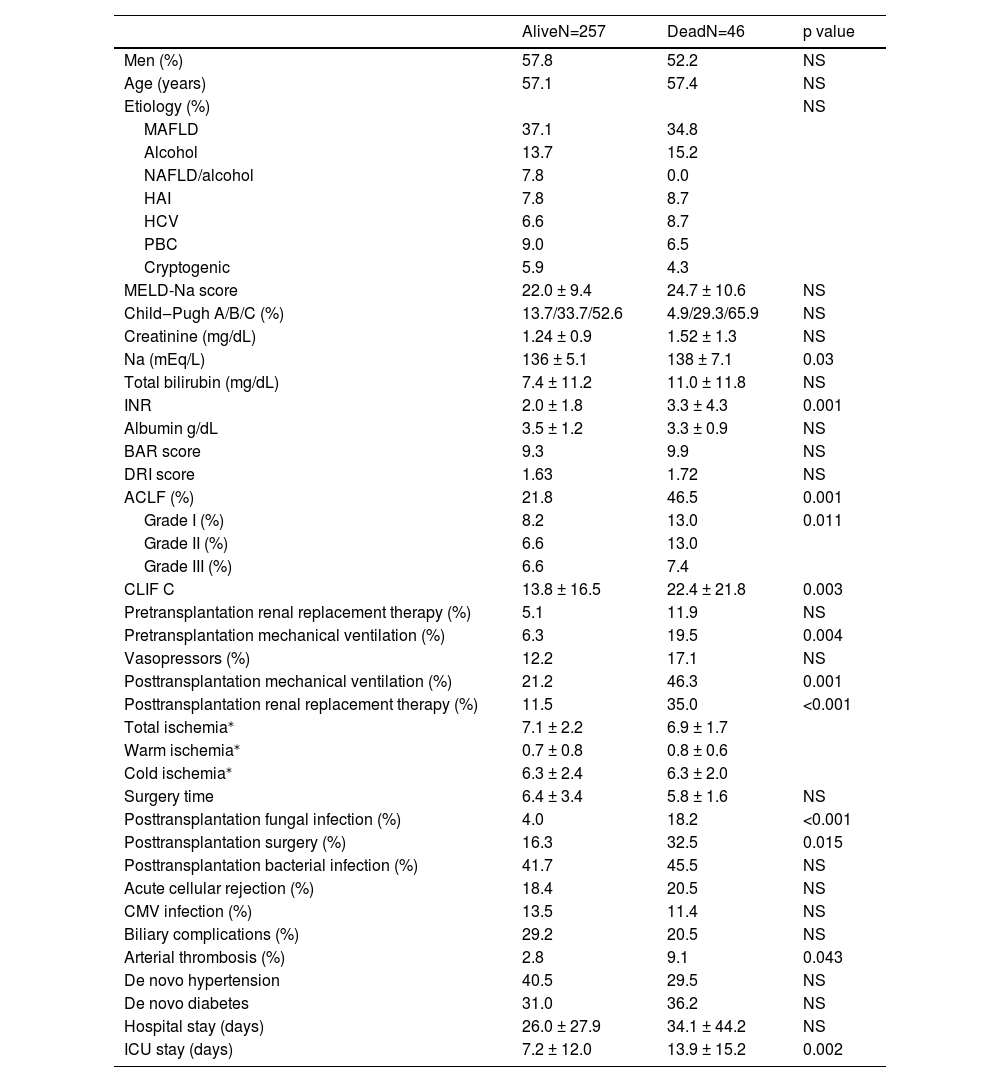
Several variables seemed to be associated with survival in the univariate analysis, such as the diagnosis of ACLF, sodium, INR, pretransplantation mechanical ventilation, posttransplantation renal replacement therapy, arterial thrombosis and fungal infection. Posttransplantation surgery was associated with survival, probably because in several cases, it was performed after arterial thrombosis. Interestingly, the MELD-Na and Child-Pugh scores were not associated with posttransplantation survival (Table 3).
Variables associated with survival. CMV: cytomegalovirus. NAFLD: Nonalcoholic fatty liver disease.
| AliveN=257 | DeadN=46 | p value | |
|---|---|---|---|
| Men (%) | 57.8 | 52.2 | NS |
| Age (years) | 57.1 | 57.4 | NS |
| Etiology (%) | NS | ||
| MAFLD | 37.1 | 34.8 | |
| Alcohol | 13.7 | 15.2 | |
| NAFLD/alcohol | 7.8 | 0.0 | |
| HAI | 7.8 | 8.7 | |
| HCV | 6.6 | 8.7 | |
| PBC | 9.0 | 6.5 | |
| Cryptogenic | 5.9 | 4.3 | |
| MELD-Na score | 22.0 ± 9.4 | 24.7 ± 10.6 | NS |
| Child‒Pugh A/B/C (%) | 13.7/33.7/52.6 | 4.9/29.3/65.9 | NS |
| Creatinine (mg/dL) | 1.24 ± 0.9 | 1.52 ± 1.3 | NS |
| Na (mEq/L) | 136 ± 5.1 | 138 ± 7.1 | 0.03 |
| Total bilirubin (mg/dL) | 7.4 ± 11.2 | 11.0 ± 11.8 | NS |
| INR | 2.0 ± 1.8 | 3.3 ± 4.3 | 0.001 |
| Albumin g/dL | 3.5 ± 1.2 | 3.3 ± 0.9 | NS |
| BAR score | 9.3 | 9.9 | NS |
| DRI score | 1.63 | 1.72 | NS |
| ACLF (%) | 21.8 | 46.5 | 0.001 |
| Grade I (%) | 8.2 | 13.0 | 0.011 |
| Grade II (%) | 6.6 | 13.0 | |
| Grade III (%) | 6.6 | 7.4 | |
| CLIF C | 13.8 ± 16.5 | 22.4 ± 21.8 | 0.003 |
| Pretransplantation renal replacement therapy (%) | 5.1 | 11.9 | NS |
| Pretransplantation mechanical ventilation (%) | 6.3 | 19.5 | 0.004 |
| Vasopressors (%) | 12.2 | 17.1 | NS |
| Posttransplantation mechanical ventilation (%) | 21.2 | 46.3 | 0.001 |
| Posttransplantation renal replacement therapy (%) | 11.5 | 35.0 | <0.001 |
| Total ischemia⁎ | 7.1 ± 2.2 | 6.9 ± 1.7 | |
| Warm ischemia⁎ | 0.7 ± 0.8 | 0.8 ± 0.6 | |
| Cold ischemia⁎ | 6.3 ± 2.4 | 6.3 ± 2.0 | |
| Surgery time | 6.4 ± 3.4 | 5.8 ± 1.6 | NS |
| Posttransplantation fungal infection (%) | 4.0 | 18.2 | <0.001 |
| Posttransplantation surgery (%) | 16.3 | 32.5 | 0.015 |
| Posttransplantation bacterial infection (%) | 41.7 | 45.5 | NS |
| Acute cellular rejection (%) | 18.4 | 20.5 | NS |
| CMV infection (%) | 13.5 | 11.4 | NS |
| Biliary complications (%) | 29.2 | 20.5 | NS |
| Arterial thrombosis (%) | 2.8 | 9.1 | 0.043 |
| De novo hypertension | 40.5 | 29.5 | NS |
| De novo diabetes | 31.0 | 36.2 | NS |
| Hospital stay (days) | 26.0 ± 27.9 | 34.1 ± 44.2 | NS |
| ICU stay (days) | 7.2 ± 12.0 | 13.9 ± 15.2 | 0.002 |
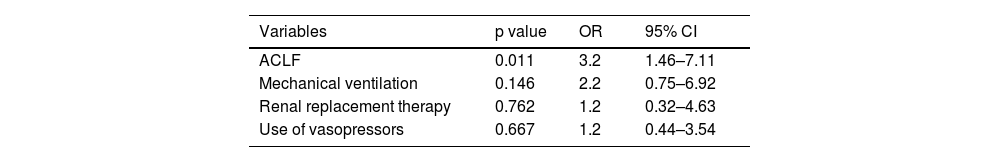
We decided to perform two multivariate analyses for pre- and posttransplantation variables considering that pretransplantation variables are potentially modifiable or can change the decision to perform LT. Thus, we included the most clinically significant variables associated with survival in the univariate analysis. Interestingly, the only variable independently related to survival was ACLF (OR 3.2, 95% CI 1.46-7.11, p=0.011). Although mechanical ventilation, renal replacement therapy and the use of vasopressor showed a trend toward significance, their results were not statistically significant (Table 4).
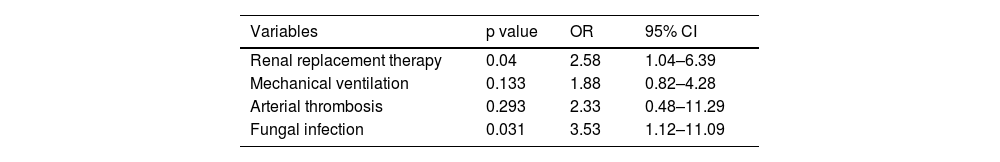
Among posttransplantation variables, the multivariate analysis showed that the variables independently associated with survival were renal replacement therapy (OR 3.093, 95% CI 1.26-7.593, p=0.014) and fungal infections (OR 3.672, 95% CI 1.153-11.693, p=0.028) (Table 5).
4DiscussionThe presence of ACLF implies a significant reduction in short-term survival. In fact, 28-day mortality ranges from 18.6% for patients with single kidney failure to 88.9% for patients with the failure of four or more organs [4,5]. Frequently, medical management is insufficient, and liver transplantation can be the only strategy to improve survival in the most severe and properly selected cases [12].
Interestingly, it seems that the severity of ACLF is related not only to the number of failing organs but also to the type of decompensating insult. Thus, patients with nonhepatic insults appear to have a worse prognosis than those with liver-related insults. Shi et al. showed that the 90-day mortality of patients with nonhepatic insults was higher than that of patients with hepatic insults (68.3% vs. 58.9%, p=0.035) [13]. This information could help to identify the patients who will eventually obtain a higher transplantation benefit.
On the other hand, to avoid futility, identifying those patients who are too sick to undergo transplantation is a very important goal, although still elusive. From our point of view, the reduced posttransplantation survival observed in ACLF grade 3 patients is not low enough to consider LT as a futile procedure considering that this survival is comparable to that observed in patients with acute liver failure. Thus, further studies are needed to identify variables associated with very poor survival. However, a significant limitation to performing LT is that very poor candidates for whom post-transplantation survival is expected to be very short are not listed based on the criteria of the transplant team. Thus, considering the available information at this point, it has been suggested that prioritization of the most severely ill ACLF patients could be appropriate [9].
As in our study, other authors have shown that the most severe forms of ACLF seem to be associated with worse posttransplantation survival. Sundaram et al. showed that posttransplantation survival was reduced in patients with ACLF, especially in those with ACLF grade 3. Thus, one-year survival was 91.9%, 89.1%, 88.1% and 81.8% for patients without ACLF, ACLF grade-1, ACLF grade-2 and ACLF grade-3, respectively [9].
These findings have been corroborated in a recent European study that included 234 liver recipients previously diagnosed with ACLF. Overall, one-year survival was 81%, although no differences were found among patients with various ACLF grades. Variables independently related to one-year survival were pre-LT arterial lactate >4 mmol/Lt, renal replacement therapy and recent infection associated with a multidrug resistant organism [15]. Thus, organ failure remains an important determinant of post-LT prognosis.
Our study has the peculiarity of having a different ACLF etiological profile than most of the series previously published, as the most common etiology of cirrhosis in our study was NAFLD (36.6%), followed by alcoholic liver disease (13.9%) and autoimmune hepatitis (7.9%). Notably, the frequency of HCV infection was low (6.9%). This is not surprising to us considering that obesity is highly prevalent in our country, as reported in the last national health report [14]. 74.2% of the Chilean population is overweight or obese. Moreover, NASH is the leading cause of liver transplantation in our centers (approximately 38%). Hence, our results are interesting considering the epidemiological characteristics of the cohort.
In the present study, as in Sundaram´s study, we found that ACLF is independently related to posttransplantation survival. 1-year posttransplantation survival can be as low as 68.0% in ACLF grade 3 patients.
Notably, the only pretransplantation variable independently associated with posttransplantation mortality was the diagnosis of ACLF at the time of LT. This is interesting considering that several variables can be considered candidates to explain reduced survival. We think that ACLF encompasses several characteristics that are associated with worse posttransplantation survival, such as circulatory failure, ventilatory failure, susceptibility to infections and, in some cases, sarcopenia and frailty. Notably, our patients had ACLF when LT was performed. Thus, those who developed ACLF that was resolved before LT were not considered as having ACLF in our study. Therefore, we selected a group of very high-risk patients with severe ongoing organ failure. Thus, we are not surprised by this finding. Nonetheless, it is interesting that although the requirements of renal replacement therapy, mechanical ventilation and/or vasopressor drugs are clear indicators of severe organ failure, only the diagnosis of ACLF was able to capture the real effect of organ failure on posttransplantation survival (Table 3). This finding is different from that found by Belli et al., who could not identify ACLF as an independent variable associated with posttransplantation survival, although other indicators of severe organ failure appeared to be associated (i.e., pre-LT arterial lactate levels >4 mmol/L and renal replacement therapy) [15].
Thus, it is feasible that other variables can also be independently related to survival. We think that in the future, larger studies should include more variables in a multivariate analysis to identify different associations.
We observed a gradual increase in posttransplantation mortality that was associated with an increase in the severity of organ dysfunction. Thus, the one-year posttransplantation survival of patients without ACLF and with ACLF grade 1, grade 2 and grade 3 was 91.2%, 81.5%, 73.9%, and 68.0%, respectively (p=0.042). We think that these data are valuable considering that although the posttransplantation survival of ACLF grade 3 patients is relatively low, it could be considered similar to that of patients with acute liver failure [16,17].
We identified posttransplantation variables associated with reduced survival, such as mechanical ventilation, reoperation, renal replacement therapy, arterial thrombosis and fungal infections. Among them, only renal replacement therapy and fungal infections were independently associated with mortality. This is not a surprising finding considering that renal failure has been consistently found to be related to posttransplantation mortality in the liver but also in heart, lung and intestine transplantations [18]. In addition, invasive fungal infections have an ominous prognosis in patients who have undergone organ transplantation [19]. However, the specific posttransplantation interventions to avoid the occurrence of these complications have limited efficacy, and several factors are related to the condition of the patient before transplantation and surgical technical issues.
In our study, the causes of decompensation were mostly infections and cryptogenic causes as has been described in a previous series [1]. Notably, in our cohort, there was a very low frequency of hepatic decompensating events. Only 4.0% of patients had an autoimmune hepatitis flare, and none had alcoholic hepatitis. This can be partially explained because only recently has the listing of patients with alcoholic hepatitis been allowed in Chile and because of the low prevalence of hepatitis B virus infection.
As expected, we found a higher administration of total units of blood products (due to the use of more erythrocyte suspension and fresh frozen plasma units) during surgery in ACLF patients. This can be explained by more severe coagulopathy secondary to very severe liver failure and the effect of the decompensating event, especially infections.
Our study has some limitations. First, it is a retrospective study. However, most of the data were prospectively recorded, so we only had to consult our records. Second, only two centers were included in our study. However, they are the most active transplantation programs in our country, so our results properly reflect the real-life experience in the two most important centers. These centers represent approximately 80% of liver transplantation activity in Chile, so it seems to be a representative sample. In addition, in our opinion, it likely represents an experience similar to that of several liver transplantation centers in Latin America.
We also consider that our study has some strengths. First, it is the largest study in Latin America specifically designed to evaluate posttransplantation survival among ACLF patients. Thus, we believe that it contributes valuable information that is similar to that already published for other populations. Second, the etiological characteristics of our cohort (most of the cases of cirrhosis were secondary to NASH) provide very valuable information that is possibly not previously published. Notably, BMI was higher in patients with NASH although this diagnosis was not related to survival. Third, although our cohort was not particularly large, we were able to obtain results in line with those previously reported. Fourth, we were able to find a gradual decrease in survival based on the severity of organ failure. Thus, each transplantation team can evaluate whether the posttransplantation survival is appropriate based on its clinical judgment.
There is a paucity of studies from Latin America about the outcomes of ACLF patients after LT. In fact, to the best of our knowledge, this is the first study of the region specifically designed to address this issue. We believe that it would be valuable to validate the knowledge obtained in the current study in different populations with different epidemiological characteristics, transplantation programs and donation rates.
5ConclusionsIn conclusion, we found that the presence of ACLF is strongly related to posttransplantation survival, especially in the most severe cases. There is a gradual decrease in survival with increasing severity of ACLF; thus, posttransplantation survival is worse in patients with ACLF grades 2 and 3. There are posttransplantation variables related to survival that are difficult to modify, although we think that the proper selection of potentially listed patients could improve these results. Finally, this is the first study in Latin America to evaluate the posttransplantation survival of ACLF patients and to identify related factors.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsCarlos Benítez: Study design, data analysis, writing of the manuscript; Jorge Arnold: Data analysis, writing of the manuscript; Verónica Cambindo: Data analysis, Acquisition of data; Fernanda Schoenfeld: Acquisition of data; Alejandra Cancino: Acquisition of data; Samuel Ibáñez: Acquisition of data; Catalina Grandy: Acquisition of data; Paola Hunfan: Acquisition of data; Jorge González: Acquisition of data; Catalina Guerra: Acquisition of data; Esteban Godoy: Acquisition of data; Verónica Araneda: Acquisition of data. Constanza Mollo: Acquisition of data; Jaime Poniachik: Critical analysis of the manuscript; Alvaro Urzúa: Critical analysis of the manuscript; Máximo Cattaneo: Critical analysis of the manuscript; Juan Pablo Roblero: Critical analysis of the manuscript; Ilan Oppenheimer: Acquisition of data; Vicente Pizarro: Acquisition of data.