This study aims to explore the association between Klotho and Non-Alcoholic Fatty Liver Disease (NAFLD), a condition affecting millions worldwide. Klotho may have a protective effect against NAFLD mechanisms like inflammation, oxidative stress, and fibrosis. The study will use FLI and FIB-4 score to diagnose NAFLD in a large population for investigating the link between Klotho and NAFLD.
Materials and MethodsThe study aimed to explore the association between Klotho and NAFLD by measuring the α-Klotho protein levels in the participants' blood using ELISA. Patients with underlying chronic liver diseases were excluded. The severity of NAFLD was evaluated using FLI and FIB-4, and logistic regression models were used to analyze the data obtained from NHANES. Subgroup analyses were conducted to study Klotho's effect on hepatic steatosis and fibrosis in diverse subpopulations.
ResultsThe study found that low levels of α-Klotho were associated with NAFLD, with ORs ranging from 0.72 to 0.83. However, high levels of α-Klotho were associated with NAFLD-related fibrosis. The Q4 group showed significant results in individuals aged 51 years or younger and in females. Non-Hispanic White ethnicity, education level of high school or above, non-smoking, non-hypertension, and non-diabetic groups showed negative correlations.
ConclusionsOur study suggests a potential correlation between α-Klotho levels in the blood and NAFLD in adult patients, especially among younger individuals, females and Non-Hispanic Whites. Elevated α-Klotho levels may have therapeutic benefits in treating NAFLD. Further research is required to validate these findings, but they provide new insights for managing this condition.
Nonalcoholic Fatty Liver Disease (NAFLD) is a common liver disease worldwide, encompassing both Non-Alcoholic Fatty Liver (NAFL) and Non-Alcoholic Steatohepatitis (NASH), which can progress to cirrhosis. A recent study estimated that 30% of the global population suffers from NAFLD, making it a major public health concern [1–3]. In the United States and Europe-4 countries (United Kingdom, Italy, France, and Germany), there are over 64 million and 52 million people with NAFLD, respectively. This has resulted in a substantial burden on healthcare systems, and has caused considerable individual healthcare costs, such as $103 billion a year in the United States and €35 billion a year in Europe-4 countries. NAFLD is no longer considered as a standalone disorder, but rather a multisystem disease that is strongly linked to obesity, inflammation, and lipid peroxidation. It is closely associated with other conditions such as CVD, T2DM, CKD, and osteoporosis [4].
At the end of the 20th century, Kuro-o et al. made an important discovery regarding a gene located on human chromosome 13. This gene, named 'klotho', has been found to be associated with lifespan and can contribute to premature aging. Three types of Klotho can be detected in the human body: the full-length transmembrane α-Klotho, soluble α-Klotho, and secreted α-Klotho [5,6]. Studies have also shown that klotho is associated with CVD [7], T2DM [8], and CKD [9]. Moreover, low-expressed klotho has been demonstrated to be associated with oxidative stress and inflammation [10]. Research on the relationship between Klotho and NAFLD is limited. It is currently known that variations in the Klotho gene may be associated with liver damage in children with NAFLD [11], and that Klotho can inhibit liver cancer progression and prevent obesity by regulating energy metabolism and negatively modulating the Wnt/β-catenin signaling pathway [12]. These findings suggest that Klotho has potential value in the treatment of NAFLD and liver fibrosis, but its underlying mechanism requires further investigation. Additionally, one study reported that Klotho is increased in liver cirrhosis [13], but no study has yet found a relationship between Klotho and NAFLD in a large population.
This study utilized FLI and FIB-4 to stage NAFLD and determine any significant association with Klotho. NHANES data were analyzed. Based on previous research, it is hypothesized that Klotho may protect against NAFLD. The objective of this study is to investigate the potential relationship between Klotho and NAFLD by analyzing its correlation with FLI and FIB-4 within the NHANES dataset. In summary, this study focuses on exploring the role of Klotho in NAFLD through analysis of NHANES data using FLI and FIB-4 indices.
2Methods2.1Study populationThe study population for this survey was the National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), which aimed to assess the health and nutritional status of Americans and evaluate its association with health promotion and disease prevention. This survey collected demographic information, physical examinations, biochemical and nutritional indicators, and lifestyle questionnaires using a complex sampling method to represent the population across the United States. All survey methods and analytic guidelines are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
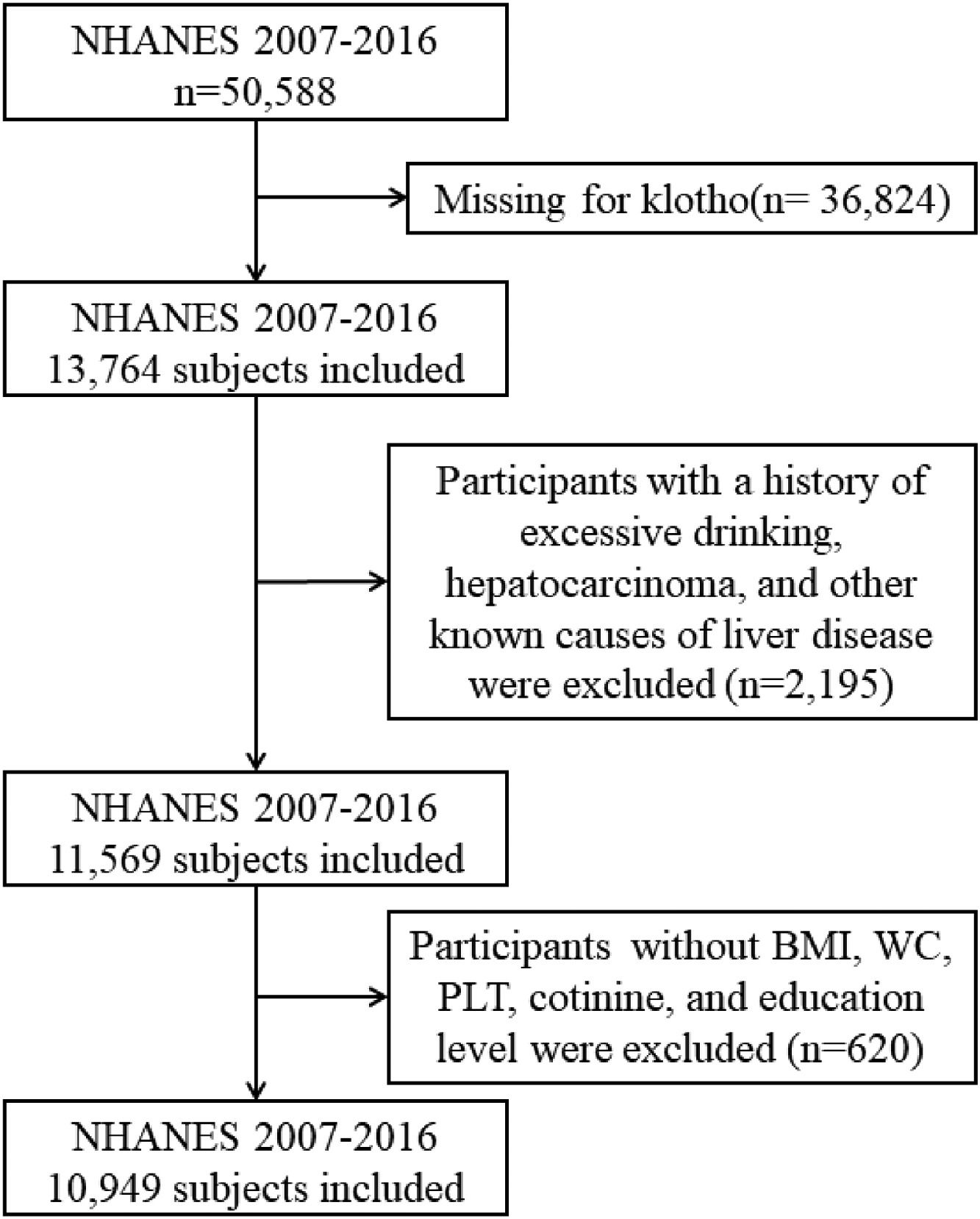
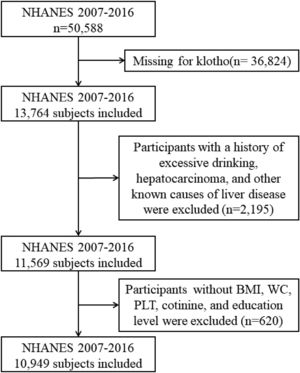
We conducted a cross-sectional study using aggregated data from the NHANES conducted between 2007 and 2016, which included information about the α-Klotho and NAFLD. The α-Klotho protein levels in the participants' blood were quantified by utilizing the highly sensitive ELISA technology [14]. This study encompassed all 50,588 participants in the survey cycles; however, 36,824 of them did not provide any data on α-Klotho. Participants who exhibited any indication of other causes of chronic liver disease such as viral hepatitis infection (defined as a positive HCV RNA, HCV-antibody or HBsAg test), autoimmune hepatitis, liver cancer, and excessive alcohol consumption were excluded, along with those without body mass index (BMI), waist circumference (WC), platelet count (PLT), cotinine, and education level. Finally, we included 10,949 subjects in our study. The overall workflow is illustrated in Fig. 1. Furthermore, the study gathered information on daily alcohol intake through two 24 h recalls. The threshold for excessive alcohol consumption was set at an average of over 20 g/day for males and over 10 g/day for females [15]. In cases where both recalls were completed, the average intake was used; if only one recall was available, that data point was used.
2.2Diagnostic criteria and definitionsBased on the number of participants, we categorized the concentration of Klotho into four groups. We collected the following laboratory data: Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Triglycerides (TG), Albumin, Gamma Glutamyl Transferase (GGT), Platelet count, Body Mass Index (BMI), and Waist Circumference (WC). We assessed the stage of NAFLD by using the Fatty Liver Index (FLI), which is calculated by TG, GGT, and WC, with a cut-off of 60 to define NAFLD [16]. Simultaneously, we also calculated the Fibrosis-4 (FIB-4) score to assess liver fibrosis. The critical value was set at 2.0 for those aged 65 years and above, and at 1.3 for those below the age of 65 [17,18]. The formulas of FLI and FIB-4 are as follows [16,19]:
With x=0.953×ln(TG)+0.139×BMI+0.718×ln(GGT)+0.053×WC−15.745
2.3CovariatesFrom past research [20–23], we have selected variables that may influence outcomes and collected the following information: age, gender, race/ethnicity, education level, poverty-to-income ratio (PIR), diabetes, and hypertension. Race/ethnicity was categorized as Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race. Education level was divided into two groups: [24], we classified smoking status into smokers and non-smokers in the subgroup analysis. Diabetes was determined by using the following criteria: (1) hemoglobin A1C concentration ≥ 6.5% or a fasting plasma glucose level ≥ 126 mg/dL, and (2) a positive answer to the question: ‘The next questions are about specific medical conditions. {Other than during pregnancy, {have you/has SP}/{Have you/Has SP}} ever been told by a doctor or health professional that {you have/{he/she/SP} has} diabetes or sugar diabetes?’ or ‘{Is SP/Are you} now taking insulin’ Hypertension was determined by a positive answer to the question: ‘{Have you/Has SP} ever been told by a doctor or other health professional that {you/s/he} had hypertension, also called high blood pressure?’, ‘{Were you/Was SP} told on 2 or more different visits that {you/s/he} had hypertension, also called high blood pressure?’ or ‘Because of {your/SP's} (high blood pressure/hypertension), {have you/has s/he} ever been told to . . . take prescribed medicine?’. The details of data can be publicity obtained at http://www.cdc.gov/nchs/nhanes/.
2.4Statistical analysisWeighted linear regression models (continuous variables) and weighted chi-square tests (categorical variables) were performed to calculate differences between the groups. We then used multivariable logistic regression models to describe the associations between serum α-Klotho and significant NAFLD or fibrosis, with the lowest category as the reference. Odds ratios (ORs) and 95% confidential intervals (CIs) were calculated. To investigate the impact of Klotho on hepatic steatosis and fibrosis across diverse subpopulations, subgroup analyses were performed based on age, gender, race, education, smoking habits, hypertension and diabetes status. To obtain unbiased national estimates, we created a 10-year MEC exam weight, calculated by one-five of the value of the Full Sample 2 Year MEC Exam Weight (WTMEC2YR×1/5). All analyses were conducted using R (http://www.R-project.org; version 4.2.2). A p-value of <0.05 was considered statistically significant.
2.5Ethical statementsThe data required for this survey are all from published NHANES data, without raw data collection, thus ethics committee approval is not necessary. The included data sources, which were approved by the local ethics committee, complied with local law, and all participants signed informed consent.
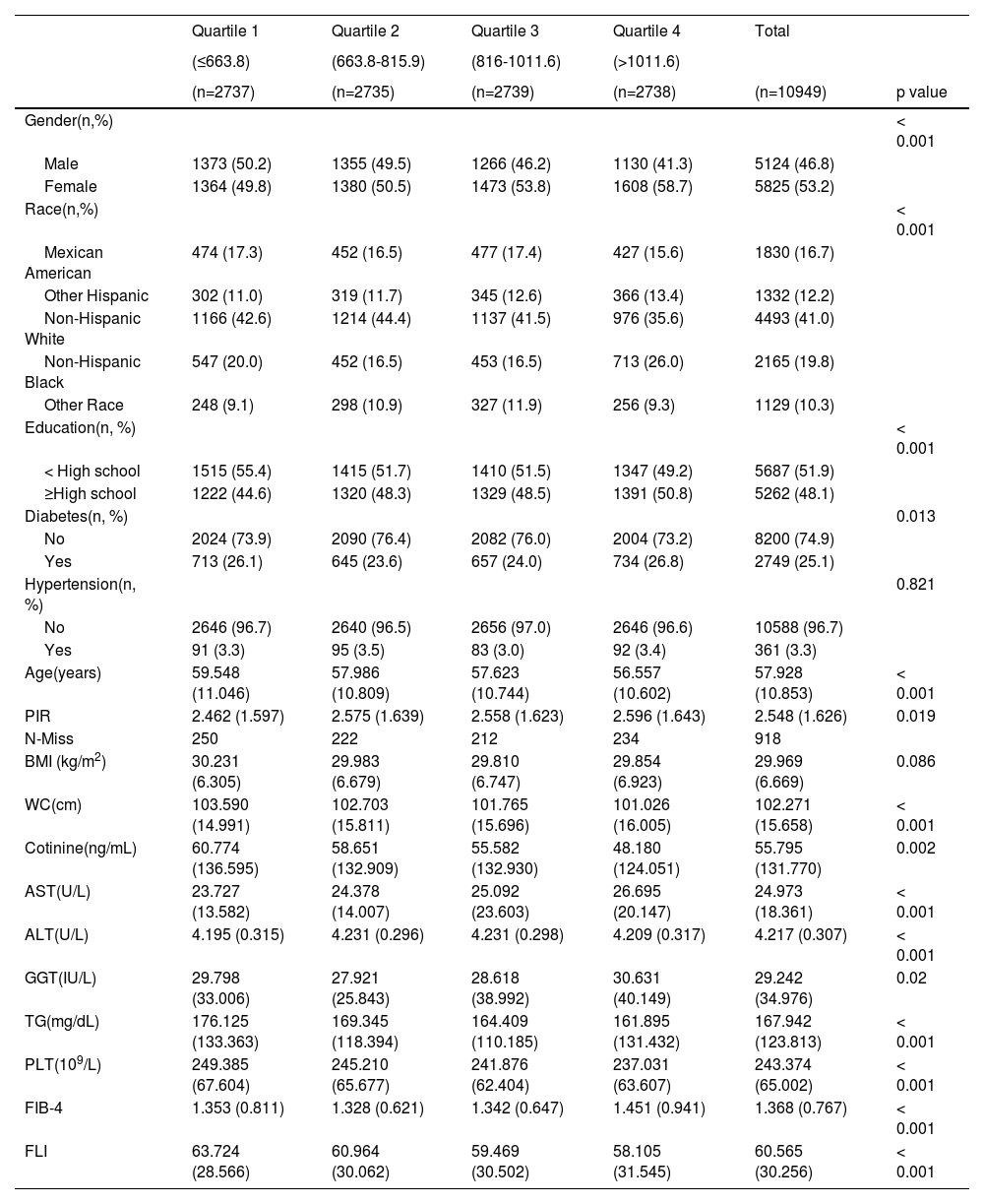
3Results3.1Baseline characteristics of included populationUltimately, a total of 10,949 individuals were included in this study in accordance with the inclusion criteria. The characteristics of the study participants according to the level of klotho were presented in Table 1. There were significant disparities in most demographic characteristics, biochemical indices, anthropometric indices, and NAFLD indices among the 4 levels of klotho. In this study, the mean age was 57.9 years, and a total of 46.8% of the participants were male. The majority race was Non-Hispanic White, and a total of 25.1% and 3.3% of the participants had diabetes and hypertension, respectively. From Table 1, females and participants who had completed education beyond high school were more likely to have a higher level of klotho between the 4 groups.
The characteristics of the study participants according to the level of klotho.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Total | ||
|---|---|---|---|---|---|---|
| (≤663.8) | (663.8-815.9) | (816-1011.6) | (>1011.6) | |||
| (n=2737) | (n=2735) | (n=2739) | (n=2738) | (n=10949) | p value | |
| Gender(n,%) | < 0.001 | |||||
| Male | 1373 (50.2) | 1355 (49.5) | 1266 (46.2) | 1130 (41.3) | 5124 (46.8) | |
| Female | 1364 (49.8) | 1380 (50.5) | 1473 (53.8) | 1608 (58.7) | 5825 (53.2) | |
| Race(n,%) | < 0.001 | |||||
| Mexican American | 474 (17.3) | 452 (16.5) | 477 (17.4) | 427 (15.6) | 1830 (16.7) | |
| Other Hispanic | 302 (11.0) | 319 (11.7) | 345 (12.6) | 366 (13.4) | 1332 (12.2) | |
| Non-Hispanic White | 1166 (42.6) | 1214 (44.4) | 1137 (41.5) | 976 (35.6) | 4493 (41.0) | |
| Non-Hispanic Black | 547 (20.0) | 452 (16.5) | 453 (16.5) | 713 (26.0) | 2165 (19.8) | |
| Other Race | 248 (9.1) | 298 (10.9) | 327 (11.9) | 256 (9.3) | 1129 (10.3) | |
| Education(n, %) | < 0.001 | |||||
| < High school | 1515 (55.4) | 1415 (51.7) | 1410 (51.5) | 1347 (49.2) | 5687 (51.9) | |
| ≥High school | 1222 (44.6) | 1320 (48.3) | 1329 (48.5) | 1391 (50.8) | 5262 (48.1) | |
| Diabetes(n, %) | 0.013 | |||||
| No | 2024 (73.9) | 2090 (76.4) | 2082 (76.0) | 2004 (73.2) | 8200 (74.9) | |
| Yes | 713 (26.1) | 645 (23.6) | 657 (24.0) | 734 (26.8) | 2749 (25.1) | |
| Hypertension(n, %) | 0.821 | |||||
| No | 2646 (96.7) | 2640 (96.5) | 2656 (97.0) | 2646 (96.6) | 10588 (96.7) | |
| Yes | 91 (3.3) | 95 (3.5) | 83 (3.0) | 92 (3.4) | 361 (3.3) | |
| Age(years) | 59.548 (11.046) | 57.986 (10.809) | 57.623 (10.744) | 56.557 (10.602) | 57.928 (10.853) | < 0.001 |
| PIR | 2.462 (1.597) | 2.575 (1.639) | 2.558 (1.623) | 2.596 (1.643) | 2.548 (1.626) | 0.019 |
| N-Miss | 250 | 222 | 212 | 234 | 918 | |
| BMI (kg/m2) | 30.231 (6.305) | 29.983 (6.679) | 29.810 (6.747) | 29.854 (6.923) | 29.969 (6.669) | 0.086 |
| WC(cm) | 103.590 (14.991) | 102.703 (15.811) | 101.765 (15.696) | 101.026 (16.005) | 102.271 (15.658) | < 0.001 |
| Cotinine(ng/mL) | 60.774 (136.595) | 58.651 (132.909) | 55.582 (132.930) | 48.180 (124.051) | 55.795 (131.770) | 0.002 |
| AST(U/L) | 23.727 (13.582) | 24.378 (14.007) | 25.092 (23.603) | 26.695 (20.147) | 24.973 (18.361) | < 0.001 |
| ALT(U/L) | 4.195 (0.315) | 4.231 (0.296) | 4.231 (0.298) | 4.209 (0.317) | 4.217 (0.307) | < 0.001 |
| GGT(IU/L) | 29.798 (33.006) | 27.921 (25.843) | 28.618 (38.992) | 30.631 (40.149) | 29.242 (34.976) | 0.02 |
| TG(mg/dL) | 176.125 (133.363) | 169.345 (118.394) | 164.409 (110.185) | 161.895 (131.432) | 167.942 (123.813) | < 0.001 |
| PLT(109/L) | 249.385 (67.604) | 245.210 (65.677) | 241.876 (62.404) | 237.031 (63.607) | 243.374 (65.002) | < 0.001 |
| FIB-4 | 1.353 (0.811) | 1.328 (0.621) | 1.342 (0.647) | 1.451 (0.941) | 1.368 (0.767) | < 0.001 |
| FLI | 63.724 (28.566) | 60.964 (30.062) | 59.469 (30.502) | 58.105 (31.545) | 60.565 (30.256) | < 0.001 |
Data were summarized as mean ± SE for continuous variables or as a numerical proportion for categorical variables; p-values were calculated by χ2tests. n: number; %: percent; PIR: poverty-income ratio; BMI: body mass index; WC: waist circumference; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; TG: triglycerides; PLT: platelet count; FIB-4: fibrosis-4; FLI: fatty liver index.
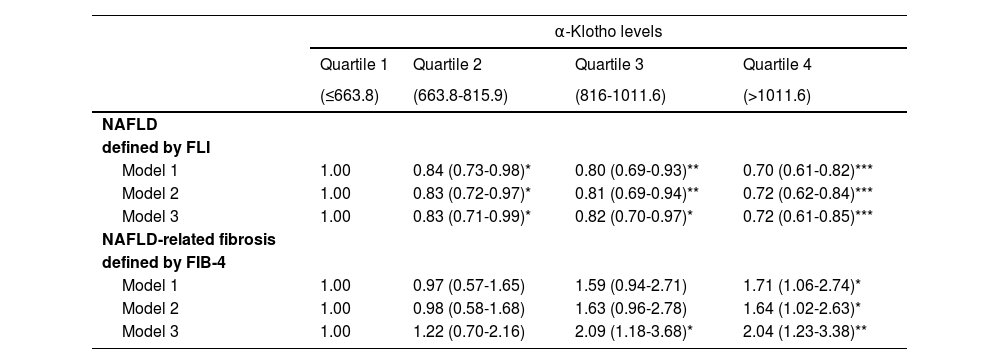
Three multivariable logistic regression models were constructed (Table 2): model 1, no covariates were adjusted; model 2, gender and race/ethnicity were adjusted; model 3, gender, race/ethnicity, PIR, education level, cotinine, hypertension and diabetes were adjusted. We did not put age, BMI as well as other factors into the model because they were related to the dependent variable. We calculated the OR and 95% CIs using logistic regression analyses to assess the association between klotho and NAFLD. The results, shown in Table 2, indicated a negative association between klotho and NAFLD in all klotho levels across all models. The negative associations were strengthened in the all multivariable-adjusted model, and the results were OR=0.83 (95%CI: 0.71-0.97), OR=0.82 (95%CI: 0.70-0.96), OR=0.74 (95%CI: 0.63-0.87), respectively. Regarding NAFLD-related fibrosis, although the associations between VAI and liver fibrosis were not statistically significant in the first three quartiles of klotho, they were statistically significant in the highest level of klotho.
The multivariable OR (95%CI) for NAFLD and fibrosis according to alpha-Klotho levels.
| α-Klotho levels | ||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| (≤663.8) | (663.8-815.9) | (816-1011.6) | (>1011.6) | |
| NAFLD | ||||
| defined by FLI | ||||
| Model 1 | 1.00 | 0.84 (0.73-0.98)* | 0.80 (0.69-0.93)** | 0.70 (0.61-0.82)*** |
| Model 2 | 1.00 | 0.83 (0.72-0.97)* | 0.81 (0.69-0.94)** | 0.72 (0.62-0.84)*** |
| Model 3 | 1.00 | 0.83 (0.71-0.99)* | 0.82 (0.70-0.97)* | 0.72 (0.61-0.85)*** |
| NAFLD-related fibrosis | ||||
| defined by FIB-4 | ||||
| Model 1 | 1.00 | 0.97 (0.57-1.65) | 1.59 (0.94-2.71) | 1.71 (1.06-2.74)* |
| Model 2 | 1.00 | 0.98 (0.58-1.68) | 1.63 (0.96-2.78) | 1.64 (1.02-2.63)* |
| Model 3 | 1.00 | 1.22 (0.70-2.16) | 2.09 (1.18-3.68)* | 2.04 (1.23-3.38)** |
OR, odds ratio; 95% CI, 95% confidence interval. Model 1: no covariates were adjusted. Model 2: gender and race/ethnicity were adjusted. Model 3: gender, race/ethnicity, PIR, education level, cotinine, hypertension and diabetes were adjusted. p-value in comparison to the lowest category is shown as *** p < 0.001; ** p < 0.01; * p < 0.05.
We conducted subgroup analysis to reduce the effects of confounding factors, with the lowest category as the reference. As depicted in Supplementary Table 1 when stratified by age, we found that the quartile 4 and 3 of klotho had negative associations with NAFLD in participants aged ≤51 years and 51–63 years, respectively. When stratified by gender, we found a negative correlation between klotho and NAFLD in females only in the highest level of klotho. When stratified by race, there were negative associations between klotho and NAFLD in all level among Non-Hispanic Whites. When stratified by education level, we found that the quartile 3 and 4 of klotho had negative associations with NAFLD in participants who completed education beyond high school. When stratified by smoking status, the results were statistically significant in non-smoking participants. When stratified by diabetes, the results were statistically significant among the participants without the aforementioned diseases. As for NAFLD- related fibrosis, we just grouped by age, gender, education level, smoking status, and diabetes, because there were not enough people to stratify in other groups. Notably, a positive association between klotho and NAFLD-related fibrosis persisted in the highest level of klotho in most subgroups as shown in Supplementary Table 1.
4DiscussionIn this study, we investigated the relationship between klotho and NAFLD in 10,949 participants from NHANES 2007–2016 wave to explore the association between klotho and NAFLD. We discovered a negative correlation between klotho and NAFLD as defined by FLI, while a positive association between klotho and NAFLD-related fibrosis as defined by FIB-4 was only observed in the highest level of klotho. To the best of our knowledge, this is the first study to assess the associations between klotho and NAFLD based on a large population.
As far as we are aware, although many studies have examined klotho in relation to chronic kidney disease, cardiovascular disease, metabolism and so on, there has yet to be a study that has investigated klotho in subjects with NAFLD. Klotho has many functions such as maintaining mineral metabolism, suppressing Wnt signal transduction, inhibiting the activation of the IGF/insulin signaling pathway, resisting to oxidative stress, modifying TRPV5 and ROMK and so on [25]. Klotho governs multiple metabolic processes in mammals [26], as the Klotho proteins are essential components of endocrine fibroblast growth factor (FGF) receptor complexes. Additionally, some studies have demonstrated that α-Klotho functions as a tumor suppressor in human hepatocellular carcinoma via Wnt signaling [27]. Moreover, klotho may act as an inhibitor in the insulin/IGF-1 signaling pathway to promote lipid oxidation and reduce lipogenesis [28]. It also plays a role in multiple reactive oxygen species (ROS) buffering systems, and attenuates oxidative stress [5,29]. Furthermore, klotho has been reported to inhibit tissue fibrosis [30,31] and mitigate some pathological changes [32] caused by hyperglycemia.
At the same time, the mechanisms leading to NAFLD are still unclear. Currently, steatosis, lipotoxicity, and inflammation are referred to as the ‘three hit’ of NAFLD in the pathological progression [33,34]. Overfeeding, gut microbiota [35,36], genetic factors [37], and de novo lipogenesis [38] are thought to be the triggers for steatosis, which is the excessive fat accumulation in the liver. Steatosis increases the signaling of the transcription factor NF-κβ [33], which can induce pro-inflammatory mediators such as IL-6, IL-1β, and others, causing oxidant stress and persistent inflammation, and evidence has suggested [39–41] that increased oxidative stress had a lipid-elevating effect in hepatocytes, while lipid decreased as oxidative stress decreased in the liver. The last hit, lipotoxicity, is caused by the excess fat accumulation in the liver and leads to organelle failure due to mitochondrial dysfunction and endoplasmic reticulum stress [42,43]. This can lead to inflammation, oxidative stress, and ultimately cell death, resulting in liver damage and cirrhosis.Therefore, Klotho may affect NAFLD through the above mechanisms. This could explain why we found NAFLD to be robustly and negatively correlated with klotho. As for the positive association between the highest level of klotho and NAFLD- related fibrosis, the current evidence strongly suggests [44] that klotho has a cytoprotective function, possibly as an expression of a compensatory rise of Klotho in an attempt to protect the cell from aggression.
Among the majority of subgroups, differences were statistically significant in the highest level of klotho, with the lowest category as the reference. As can be seen in the Supplementary Table 1, results were statistically significant in Quartile 3/4 with age ≤51 and Quartile 3 with age 51–63, indicating that klotho is not very effective in the older age group. Similarly, results were statistically significant in Quartile 4 with female, suggesting that the effect of klotho might be due to the differences between males and females, such as sex hormone levels. When subgroup analysis was conducted by race, we found a negative association between klotho and NAFLD in the non-Hispanic white population, indicating that the effect of klotho might be different among the races. Klotho showed a strong negative correlation with NAFLD in the participants who had completed education beyond high school, suggesting that the life status, cognitive level, living habits and other factors associated with low-level education might account for the discrepancy. Participants were divided into two groups according to serum cotinine level, so we further performed a subgroup analysis based on different smoking group. Klotho showed a strong negative correlation with NAFLD in the non-smoking group. It is known that smoking causes oxidative stress, inflammation, and elevates the levels of inflammatory factors, and klotho also affects NAFLD via inflammatory pathways [45,46]. In the subgroup analysis of disease, hypertension and diabetes affect the protective effect of klotho. The mechanisms are not clear, but some studies have reported [47] that the liver is crucial to the regulation of metabolism and insulin sensitivity, and T2DM increases the risk of liver diseases such as liver cirrhosis and hepatocellular carcinoma (HCC). There is a study which reported that hypertension is a plausible risk factor for liver damage and hepatic fibrosis, primarily induced by glucose intolerance and decreased levels of IL-10-mediated or HO-1-induced anti-inflammatory pathways [48,49]. Therefore, we should pay more attention to patients with hypertension or diabetes during the treatment of NAFLD.
Previous research has indicated that Klotho gene variants are related to liver injury in children with NAFLD [48,49], and the mechanism may be that Klotho can prevent hepatocyte lipotoxicity, inflammation, and liver fibrosis [50]. Studies have found that klotho can reduce lipid accumulation in the liver and adipose tissue of obese mice and regulate lipid homeostasis, leading to an oxidation-preferred metabolism over storage, revealing the tissue-specific mechanism of α-Klotho in energy metabolism [50]. Furthermore, overexpression of Klotho can inhibit liver cancer progression and induce cell apoptosis by negatively regulating the Wnt/β-catenin signaling pathway [12]. Additionally, research has shown that a lack of β-Klotho can protect against obesity through interactions between the liver, microbiota, and brown adipose tissue [51]. All of the above suggest that Klotho may have a therapeutic effect on NAFLD and liver fibrosis, and more research is needed to explore potential mechanisms.
According to the EPV (Events Per Variable) principle, a sample size of at least 10 times the number of variables is required for multivariate regression analysis. For 17 variables and a NAFLD incidence rate of 30% [3], at least 567 samples are necessary. Using a formula-based method, with 17 variables, an expected risk ratio of 0.7, 90% statistical power, and 95% confidence level, at least 2344 samples are required [52]. Our study included 10,949 samples, which possessed higher statistical power. The study may have simultaneously considered multiple potential confounders and performed sub-group analysis, making the results more robust and enabling a more accurate evaluation of the impact of klotho on NAFLD. The survey utilized in this study was conducted and regulated by NHANES, a powerful resource that allows for the exploration of the intricate correlations between population health and disease. Researchers were able to verify the reliability of the data through the use of standardized testing, questionnaires, medical evaluations, and other methods, thus lending high levels of validity and reliability to the findings [53–55]. The findings of this study must be interpreted with caution due to several limitations. Our study found associations between klotho and NAFLD, but NAFLD is defined by FLI and FIB-4 since it is an indirect measurement. Furthermore, it is important to note that the association between klotho and NAFLD cannot be established as causal since NHANES is a cross-sectional study. Further studies are required to verify the exact mechanism for the association between klotho and NAFLD.
5ConclusionsThe results of our study indicate a possible link between levels of α-Klotho in the bloodstream and the presence of non-alcoholic fatty liver disease (NAFLD) among adult patients. This association seems to be stronger among younger individuals, females, and those of Non-Hispanic White ethnicity. Additionally, elevated levels of α-Klotho may have potential therapeutic applications for NAFLD. Our study sheds new light on the management of this condition, although further research is necessary to validate these findings.
FundingThis work was supported by the International Qihuang Scholar Program under project number [2020]7. The corresponding author was funded by the program during the course of this research. The aim of the program is to support outstanding scholars in their academic research and foster international cooperation.
Author contributionsZhenfei Chi: Conceptualization, Data curation, Formal analysis, Writing – original draft, Resources, Investigation. Yun Teng: Conceptualization, Data curation, Formal analysis, Writing – original draft, Resources, Investigation. Yuting Liu: Conceptualization, Data curation, Formal analysis, Resources, Investigation. Lu Gao: Conceptualization, Data curation, Formal analysis, Resources, Investigation. Junhan Yang: Conceptualization, Data curation, Formal analysis, Resources, Investigation. Zhe Zhang: Conceptualization, Validation, Investigation.
We gratefully acknowledge the financial support by the State Administration of Traditional Chinese Medicine under grant numbers [2020] NO.7.