Renal dysfunction before liver transplantation (LT) is associated with higher post-LT mortality. We aimed to study if this association still persisted in the contemporary transplant era.
Materials and MethodsWe retrospectively reviewed data on 2871 primary LT performed at our center from 1998 to 2018. All patients were listed for LT alone and were not considered to be simultaneous liver–kidney (SLK) transplant candidates. SLK recipients and those with previous LT were excluded. Patients were grouped into 4 eras: era-1 (1998–2002, n = 488), era-2 (2003–2007, n = 889), era-3 (2008–2012, n = 703) and era-4 (2013–2018, n = 791). Pre-LT renal dysfunction was defined as creatinine (Cr) >1.5 mg/dl or on dialysis at LT. The effect of pre-LT renal dysfunction on post-LT patient survival in each era was examined using Kaplan Meier estimates and univariate and multivariate Cox proportional hazard analyses.
ResultsPre-LT renal dysfunction was present in 594 (20%) recipients. Compared to patients in era-1, patients in era-4 had higher Cr, lower eGFR and were more likely to be on dialysis at LT (P < 0.001). Pre-LT renal dysfunction was associated with worse 1, 3 and 5-year survival in era-1 and era-2 (P < 0.005) but not in era-3 or era-4 (P = 0.13 and P = 0.08, respectively). Multivariate analysis demonstrated the lack of independent effect of pre-LT renal dysfunction on post-LT mortality in era-3 and era-4. A separate analysis using eGFR <60 mL/min/1.73 m2 at LT to define renal dysfunction showed similar results.
ConclusionsPre-LT renal dysfunction had less impact on post-LT survival in the contemporary transplant era.
Renal dysfunction prior to liver transplantation (LT) is a common and complicated issue that is associated with higher post-LT mortality and is a risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD) after LT [1,2]. As serum creatinine (Cr) constitutes an important component of the MELD score, there was a surge in the number and proportion of patients being listed for LT with elevated serum Cr and/or on renal replacement therapy (RRT) with the introduction of the model for end-stage liver disease (MELD) score in 2002 [3]. Even within the last decade, pre-LT renal dysfunction continues to be a significant problem with an increase in the proportion of patients presenting for LT with renal dysfunction from 12% in 2002–2005 to 17% in 2014–2017 [4]. A similar increase in the proportion of LT recipients with pre-LT renal dysfunction was highlighted in a recent American Society of Transplant Surgeons (ASTS) report [5].
Pre-LT renal dysfunction has historically been shown to adversely affect post-LT outcomes even in patients with low MELD scores at LT [1,3,6]. Recent studies continue to document that patients with pre-LT renal dysfunction have a 15–20% increased risk of death compared to those with normal kidney function at LT [4,7]. However, these studies included patients who were transplanted from 2002 onward and did not study the effect of pre-LT renal dysfunction on mortality in recent transplant eras. Consequently, it remains unclear if pre-LT renal dysfunction impacts post-LT survival in the current transplant era, especially with the improvement in perioperative management of cirrhotic patients including those on RRT, and with the advancement in anesthesia and surgical techniques that occurred over the last 2 decades. In this retrospective study we sought to examine our center’s experience over the last 2 decades, comprising almost 3000 patients who underwent primary LT, to determine if there has been any change in the association between pre-LT renal dysfunction and post-LT survival over time.
2Materials and methods2.1Patient populationAfter obtaining the Institution Review Board (IRB) approval, data on 2871 primary LT performed at our center from 1998 to 2018 were obtained from a prospectively maintained transplant database. Simultaneous liver-kidney (SLK) transplant recipients and other multi-organ transplant recipients, recipients of previous LT and those who died or lost their allograft within the first week post-LT were excluded. Patients were grouped into 4 time eras of 5-year increments: era-1 (1998–2002, n = 488), era-2 (2003–2007, n = 889), era-3 (2008–2012, n = 703) and era-4 (2013–2018, n = 791). Kidney function was assessed using serum Cr obtained at LT and patients were grouped into normal kidney function [Cr ≤ 1.5 mg/dl (n = 2272)] and renal dysfunction [Cr > 1.5 or on RRT (n = 594)] groups. This Cr cut off was chosen as previous studies demonstrated that it correlates with glomerular filtration rate (GFR) of 30–35 mL/min in cirrhotic patients [8]. Patients in the renal dysfunction group were all listed for LT alone and were not considered to be SLK candidates. In a separate analysis, kidney function at LT was calculated using the 4 points Modification of Diet in Renal Diseases (MDRD) equation [9]. In this analysis, a Cr of 4 mg/dl was assigned to patients on RRT and patients with estimated GFR (eGFR) >60 mL/min/1.73 m2 (n = 1920) were considered to have normal kidney function while those with eGFR ≤ 59 mL/min/1.73 m2 (n = 951) were considered to have renal dysfunction. Early allograft dysfunction (EAD) was defined according to criteria which have previously been validated as the presence of one or more of the following: 1) total bilirubin ≥10 mg/dl on post-operative day (POD) 7, 2) international normalized ratio (INR) ≥1.6 on POD 7, and 3) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≥2000 IU/mL in the first post-operative week [10,11].
2.2Data collection, surgical technique and post-LT immunosuppression managementBaseline recipient demographics, donor and transplant related characteristics were recorded. Surgical techniques for organ procurement and the recipient operation have been described previously [12]. The majority of operations were performed via the piggyback technique without porto-caval shunt, caval clamping or veno-venous bypass. Post-transplant immunosuppression (IS) management has previously been described [13]. The renal dysfunction group (Cr > 1.5 or on RRT) received induction treatment with interleukin-2 receptor blocker with delayed calcineurin-inhibitor (CNI) introduction while patients without renal dysfunction received no induction treatment. Intravenous methylprednisolone starting from the day of the surgery was given for 4–5 doses followed by oral prednisone which was tapered to 5 mg/day and later discontinued by the 4th month post-LT. CNIs with either tacrolimus or cyclosporine were the primary post-LT IS medications with a target trough level between 7–10 ng/mL in the 1st month post-LT, and 5–8 ng/mL thereafter for tacrolimus treated patients, and 155–200 ng/mL in the 1st month and 150 ng/mL thereafter in those who received cyclosporine. Mycophenolate mofetil (MMF) 1000 mg twice daily was started at LT with subsequent dose adjustment based on development of leukopenia, thrombocytopenia or other MMF related side effects. MMF was subsequently discontinued by the 4th month post-LT. Azathioprine was used in MMF intolerant patients. Mammalian target of rapamycin (mTOR) inhibitors with either sirolimus or everolimus was substituted for CNI in selected cases based on the clinicians’ recommendations. Data on IS medications 90-days at LT are provided in Supplemental Table 2.
2.3Primary aimTo determine the relationship between pre-LT renal dysfunction and post-LT survival in different transplant eras.
2.4Statistical analysisOverall comparisons of categorical variables between eras were completed using Chi-square tests. Continuous factors were compared using Kruskal-Wallis tests. Overall mortality was estimated using the Kaplan-Meier method. A comparison of these curves among the ERAs was completed with a log-rank test. Univariate analysis was first conducted to identify factors associated with post-LT mortality in each era. Factors with a P-value <0.05 in era-1 were included in a multivariate Cox proportional hazard model to identify independent predictors of post-LT mortality in each transplant era. The analysis was completed using SAS version 9.4.
3Results3.1Patients’ characteristics by transplant eraRecipient, donor, and transplant-related characteristics by transplant era are presented in Table 1. Compared to patients in era-1, patients in era-4 were older [median (IQR) 60 years (53–65), versus 53 years (47–61), P < 0.001], had higher Cr at LT [median (IQR) 1.0 mg/dl (0.8–1.5) versus 0.8 mg/dl (0.7–1.0), P < 0.001], had lower eGFR [median (IQR) 70 (42.7–97.6) mL/min/173 m2 versus 88.6 (66.3–113.9) mL/min/173 m2, P < 0.001) and were more likely to be on RRT [number (%) 14 (2.9) versus 67 (8.5), P < 0.001]. INR, and recipient bilirubin levels were also higher in era-4 compared to era-1 (P < 0.001 for all). Calculated MELD score progressively increased from a median (IQR) of 14 (11–18) in era-1 to 19 (12–27) in era-4 (P < 0.001). The number (%) of patients in the ICU at time of LT decreased from 69 (14%) in era-1 to 76 (9.6%) in era-4 while more patients were hospitalized at time of LT in era-4 than in era-1, P < 0.001 for both. The number (%) of SLK transplants performed in our center who were not included in this analysis has also increased from 6 (1.2%) in era-1 to 83 (10.5%) in era-4, P < 0.001. The etiology of end-stage liver disease (ESLD) also changed over the years with an increase of LT due to non-alcoholic steatohepatitis (NASH) from 0% in era-1 to 17% in era-4 and a decline of LT due to hepatitis C (HCV) from 41% in era-1 to 28% in era-4, (P < 0.001 for both) while LT for alcoholic cirrhosis remained constant over time. Concomitantly, there was a progressive increase in hepatocellular carcinoma (HCC) as the primary indication for LT from 8.6% in era 1–29% in era 4 (P < 0.001). Days on the waiting list almost doubled from a median of 51 days in era 1 to a median of 98 days in era 4 (P < 0.001). Cold and warm ischemia times progressively declined over the 4 different transplant eras and post-LT EAD declined from 36% in era-1 to 24% in era-4 despite the increase in DCD LT from 3.7% in era-1 to almost 16% in era-4.
Recipient, donor and transplant characteristics by 4 different eras.
| Era-1 1998–2002 (N = 488) | Era-2 2003–2007 (N = 889) | Era-3 2008–2012 (N = 703) | Era-4 2013–2018 (N = 791) | p value | |
|---|---|---|---|---|---|
| Recipient Characteristics | |||||
| Age at transplant (years) median (IQR) | 53 (47, 61) | 56 (50, 63) | 58 (52, 64) | 60 (53, 65) | <0.001a |
| Male gender number (%) | 318 (65.2) | 591 (66.5) | 477 (67.9) | 523 (66.1) | 0.797b |
| Recipient race number (%) | 0.004b | ||||
| Caucasian | 389 (79.7) | 748 (84.1) | 572 (81.4) | 661 (83.6) | |
| African American | 18 (3.7) | 38 (4.3) | 51 (7.3) | 39 (4.9) | |
| Other | 81 (16.6) | 103 (11.6) | 80 (11.4) | 91 (11.5) | |
| BMI (kg/m2) median (IQR) | 27.6 (24.4, 31.5) | 27.3 (23.8, 31.7) | 27.8 (24.9, 32.0) | 27.7 (24.4, 32.2) | 0.213a |
| Diabetes Mellitus number (%) | 105 (22.2) | 247 (27.8) | 191 (27.2) | 224 (28.4) | 0.086b |
| Alcoholic cirrhosis number (%) | 62 (12.7) | 106 (11.9) | 84 (11.9) | 89 (11.3) | 0.892b |
| Cholestatic Liver Disease number (%) | 50 (10.2) | 109 (12.3) | 70 (10.0) | 61 (7.7) | 0.023b |
| NASH number (%) | 0 (0.0) | 38 (4.3) | 66 (9.4) | 136 (17.2) | <0.001b |
| HCV number (%) | 202 (41.5) | 336 (37.8) | 290 (41.3) | 219 (27.7) | <0.001b |
| HCC as secondary diagnosis number (%) | 42 (8.6) | 88 (9.9) | 32 (4.6) | 229 (29.0) | <0.001b |
| Days on LT waiting list median (IQR) | 51 (19, 123) | 23 (8, 56) | 54 (18, 115) | 98 (27, 203) | <0.001b |
| Calculated MELD at LT median (IQR) | 14 (11, 18) | 16 (12, 21) | 18 (13, 25) | 19 (12, 27) | <0.001a |
| INR at LT median (IQR) | 1.4 (1.2, 1.7) | 1.5 (1.3, 1.9) | 1.6 (1.3, 2.1) | 1.6 (1.3, 2.2) | <0.001a |
| Recipient Bilirubin at LT (mg/dl) median (IQR) | 2.2 (1.3, 4.0) | 2.8 (1.5, 5.6) | 3.1 (1.7, 6.8) | 2.8 (1.3, 7.1) | <0.001a |
| Creatinine at LT (mg/dl) median (IQR) | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.2) | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.5) | <0.001a |
| eGFR at LT (mg/dl) median (IQR) | 88.6 (66.3, 113.9) | 77.9 (54.1, 103.6) | 71.8 (46.6, 100.4) | 70.0 (42.7, 97.6) | <0.001a |
| Pre-LT renal dysfunction number (%) | <0.001b | ||||
| No (Creatinine ≦ 1.5 mg/dl) | 431 (89.2) | 722 (81.2) | 546 (77.7) | 573 (72.4) | |
| Yes (Creatinine > 1.5 mg/dl) | 38 (7.9) | 123 (13.8) | 117 (16.6) | 151 (19.1) | |
| Yes (On RRT) | 14 (2.9) | 44 (4.9) | 40 (5.7) | 67 (8.5) | |
| Medical condition at LT number (%) | <0.001b | ||||
| in ICU | 69 (14.2) | 52 (5.8) | 49 (7.0) | 76 (9.6) | |
| in hospital bed | 29 (6.0) | 70 (7.9) | 65 (9.2) | 90 (11.4) | |
| at home | 389 (79.9) | 767 (86.3) | 589 (83.8) | 625 (79.0) | |
| Transplant and Donor Characteristics | |||||
| CIT (hours) median (IQR) | 7.0 (5.7, 8.8) | 6.4 (5.4, 7.8) | 6.1 (5.3, 7.2) | 5.8 (5.1, 6.7) | <0.001a |
| Recipient WIT median (IQR) | 33.0 (27, 41) | 32.0 (26, 39) | 30.0 (25, 36) | 29.0 (25, 32) | <0.001a |
| DCD donor number (%) | 18 (3.7) | 103 (11.6) | 83 (11.8) | 125 (15.8) | <0.001b |
| DCD WIT (minutes) median (IQR) | 13 (6, 19) | 16 (8, 22) | 19 (14, 24) | 18 (14, 21) | 0.008a |
| OR Time (minutes) median (IQR) | 245 (200, 300) | 244 (187, 325) | 227 (188, 277) | 246 (207, 291) | <0.001a |
| EAD number (%) | 175 (36.2) | 256 (28.9) | 165 (23.5) | 188 (23.9) | <0.001b |
| DRI median (IQR) | 1.6 (1.2, 2.0) | 1.7 (1.3, 2.0) | 1.5 (1.2, 1.9) | 1.6 (1.3, 1.9) | 0.001a |
| Donor Age (years) median (IQR) | 51 (33, 67) | 49 (35, 62) | 47 (29, 59) | 48 (34, 59) | <0.001a |
| Donor Race number (%) | <0.001b | ||||
| Caucasian | 367 (75.4) | 614 (69.5) | 463 (65.9) | 507 (64.1) | |
| African American | 63 (12.9) | 128 (14.5) | 131 (18.6) | 163 (20.6) | |
| Other | 57 (11.7) | 142 (16.1) | 109 (15.5) | 121 (15.3) | |
| Donor Terminal Total Bilirubin (mg/dl) median (IQR) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.1) | 0.7 (0.5, 1.1) | 0.6 (0.4, 1.0) | <0.001a |
BMI: Body mass index; CIT: Cold ischemia time; Cr: creatinine; DCD: Donation after cardiac death; DRI: Donor risk index; EAD: Early allograft dysfunction; HCC: hepatocellular carcinoma; HCV: Hepatitis C virus; ICU: Intensive care Unit; INR: International normalization ratio; LT: Liver transplant; NASH: Non-alcoholic steatohepatitis; OR: Operation room; RRT: Renal replacement therapy; WIT: Warm ischemia time.
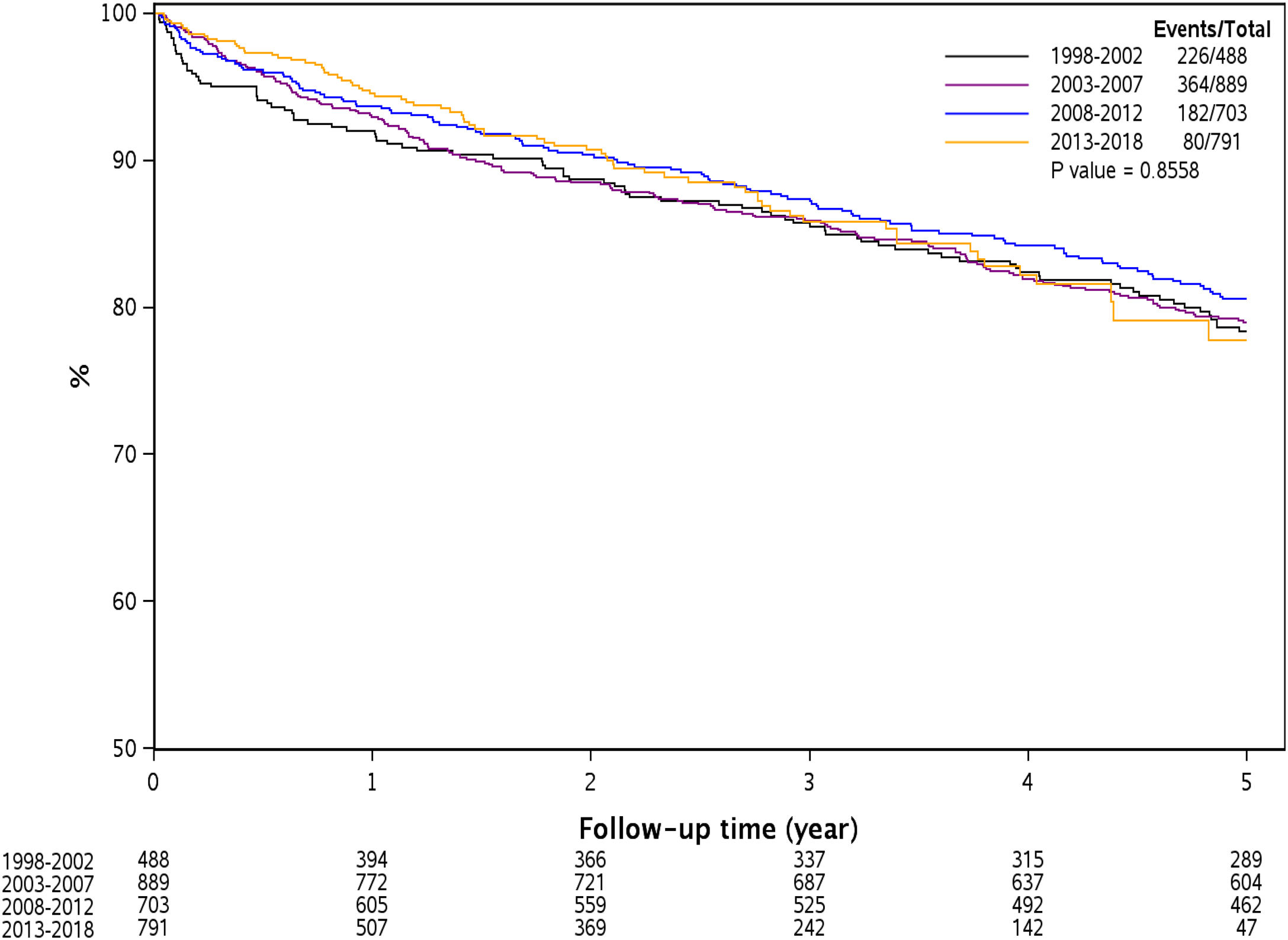
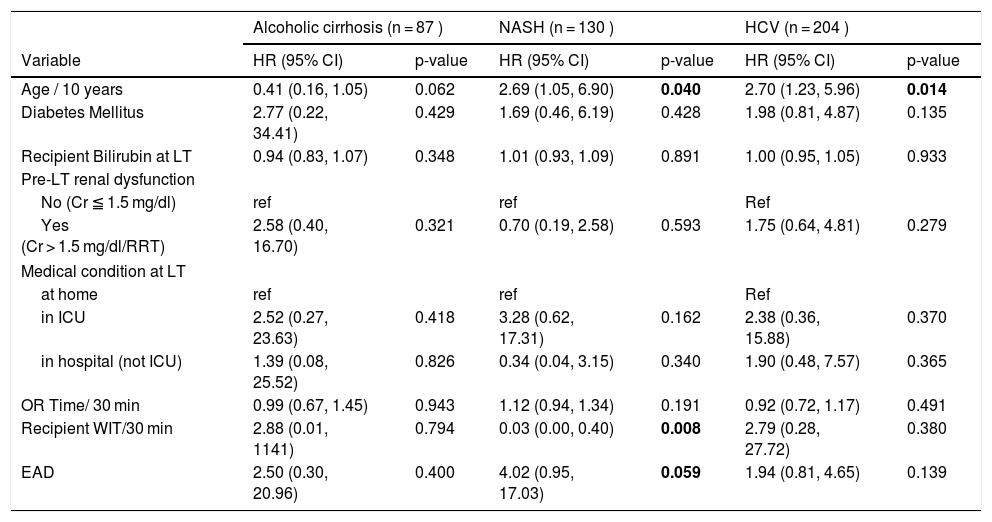
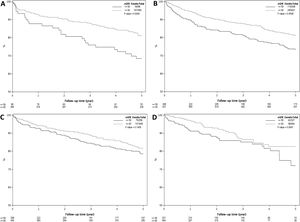
Fig. 1 represents Kaplan Meier estimate of patient survival by transplant era. As demonstrated, there was no difference in patient survival among the 4 different transplant eras (Log-rank P = 0.85).
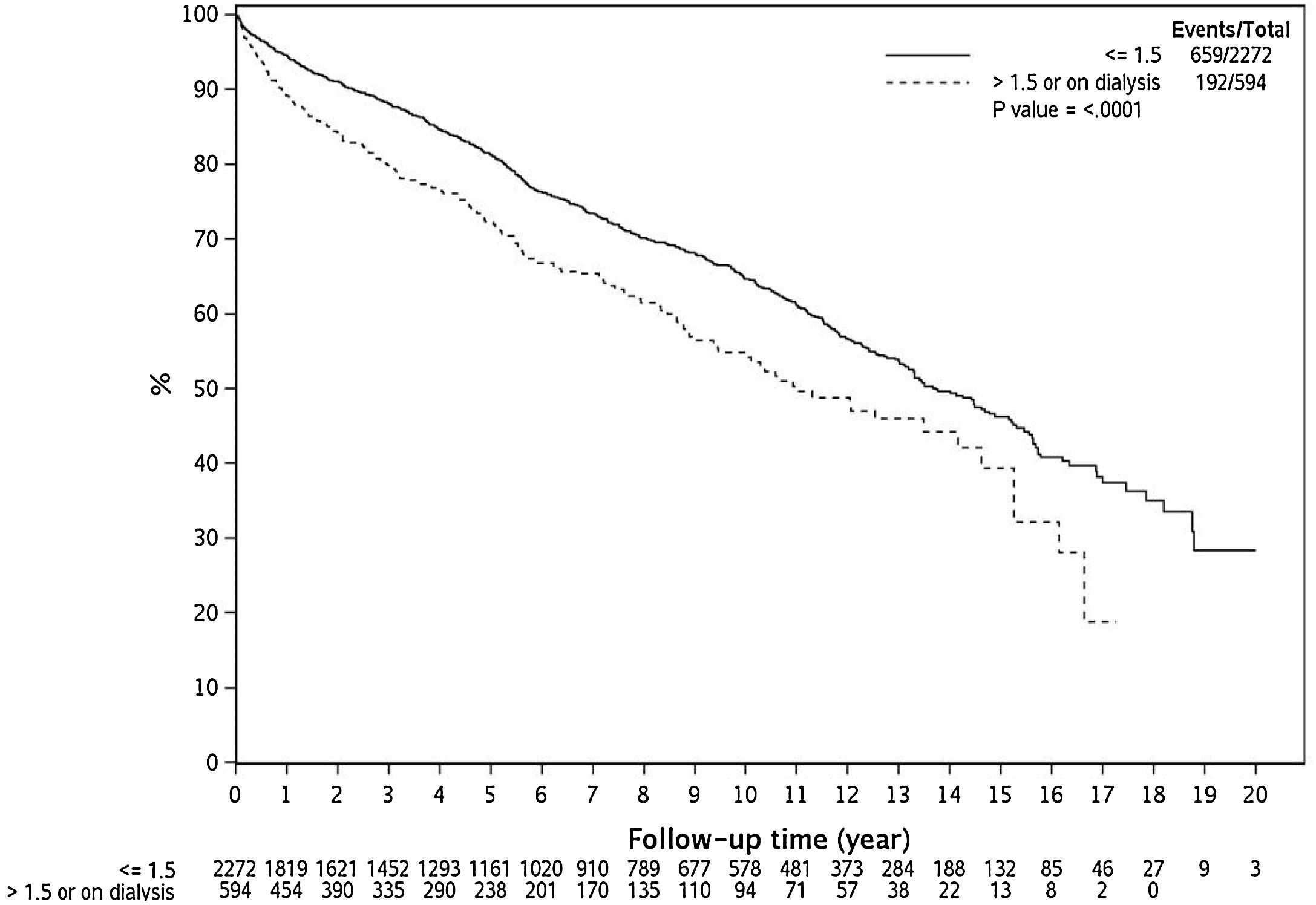
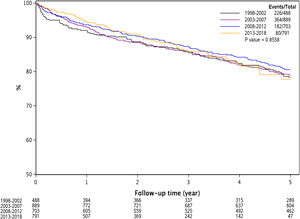
3.3Effect of renal dysfunction on post-LT survivalAs demonstrated in Fig. 2, over the 20 year time period, renal dysfunction at LT was associated with lower survival compared to those with Cr ≤ 1.5 mg/dl at LT (Log Rank P < 0.0001).
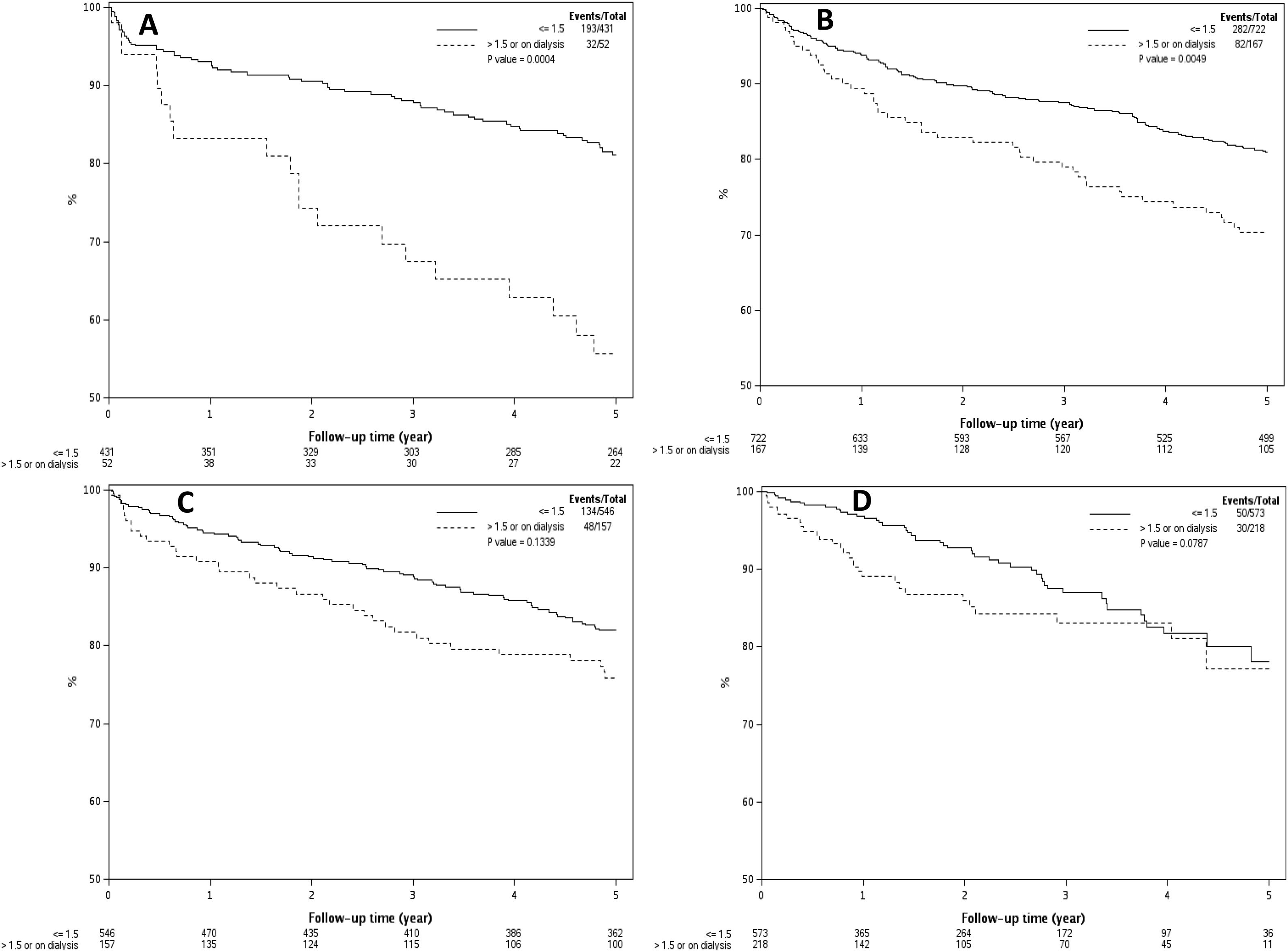
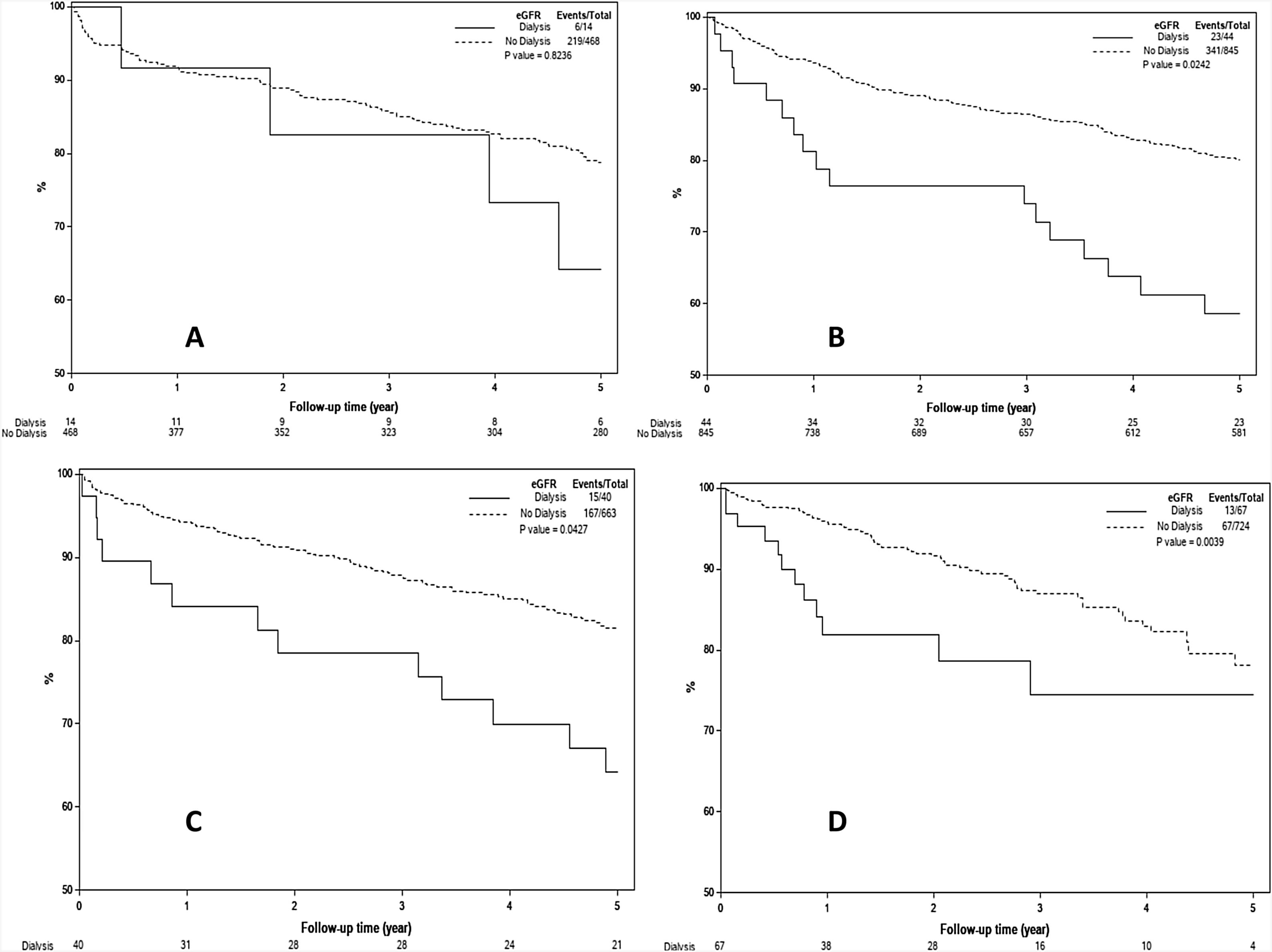
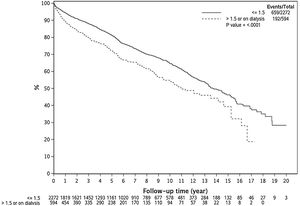
3.4Effect of pre-LT renal dysfunction on post-LT survival by transplant eraFig. 3A–D demonstrates the effect of renal dysfunction in each transplant era on patient survival. As shown in Fig. 3A, the 1, 3 and 5 year survival of patients with pre-LT renal dysfunction in era 1 was 83%, 67% and 56%, respectively, compared to 93%, 88% and 81% for those without renal dysfunction (P < 0.001). In era 2, the 1,3 and 5 year survival was 89%, 79% and 70% for the pre-LT renal dysfunction group and 94%, 87% and 81% in those without renal dysfunction, P = 0.005 (Fig. 3B). In era 3 and era 4, there was no significant difference in survival between groups with 1, 3 and 5-year survival of 91%, 82% and 76% for the pre-LT renal dysfunction group and 94%, 89% and 82% in those without renal dysfunction in era 3 (P = 0.13) and 1, 3 and 5 year survival were 89%, 83% and 77% for the renal dysfunction group and 97%, 87% and 78% in those without renal dysfunction in era 4 (P = 0.08) (Fig. 3 C and D).
A–D) Kaplan Meier plots representing post-LT patient survival in those with and without pre-LT renal dysfunction in 4 different transplant eras. As demonstrated, pre-LT renal dysfunction was associated with higher mortality in era 1 (Fig. 3A) and era 2 (Fig. 3B), P < 0.001 for both. There was statistically increased risk of death in the pre-LT renal dysfunction patients transplanted in era 3 and era 4, P > 0.05 for both (Fig. 3C and D).
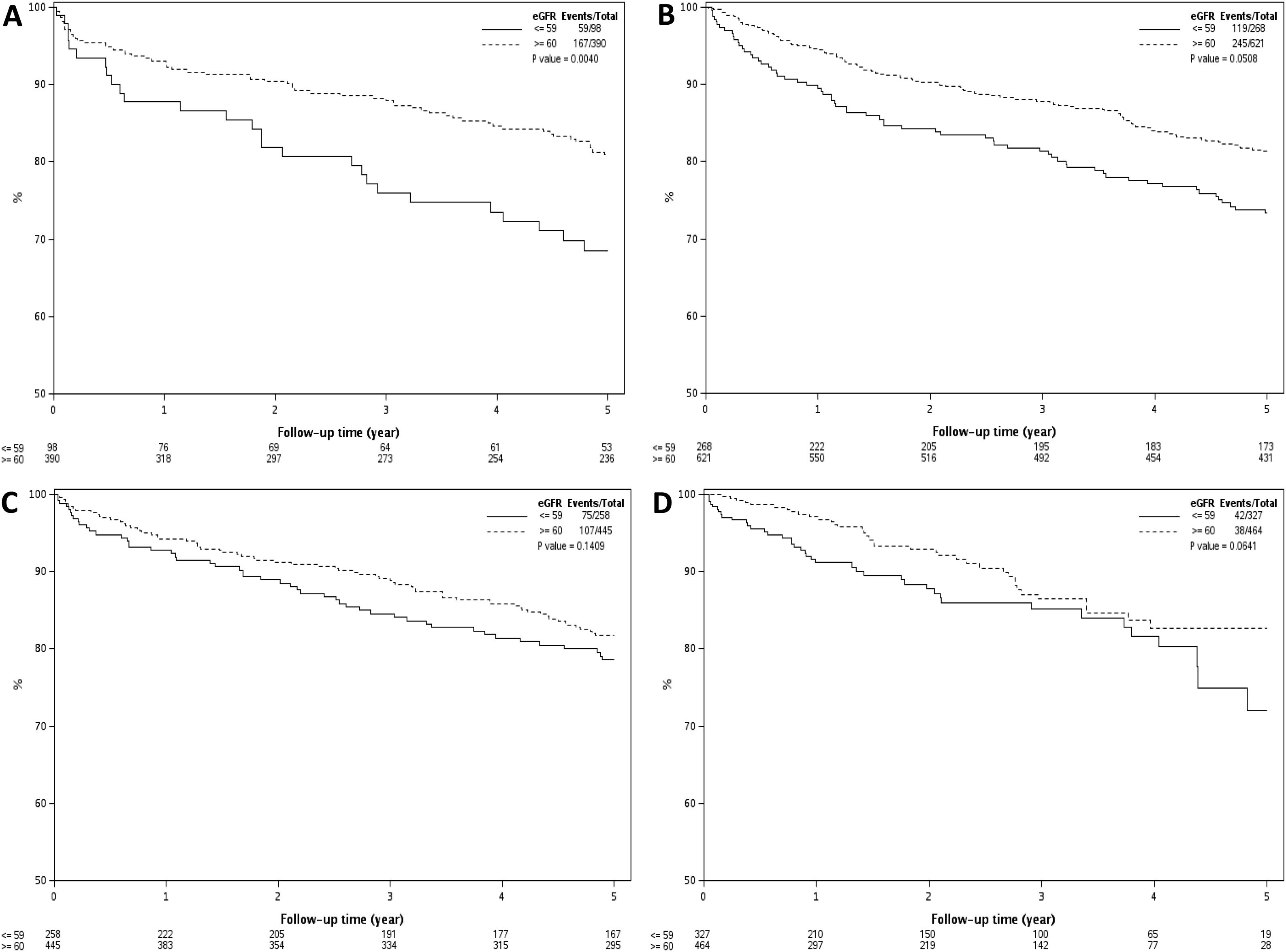
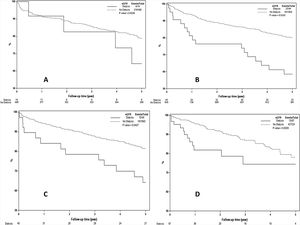
Results remained unchanged when a cutoff eGFR of 60 mL/min/1.73 m2 was used to define renal dysfunction. In era 1, patients with eGFR ≤ 59 mL/min/1.73 m2 had 1, 3 and 5 year survival of 88%, 76% and 69%, respectively, compared to 93%, 88% and 81% for those with eGFR >60 mL/min/1.73 m2 (P = 0.004). In era 2, the 1, 3 and 5 year patient survival for those with eGFR ≤59 mL/min/1.73 m2 were 86%, 77% and 68% compared to 93%, 85% and 78% for those with eGFR >60 mL/min/1.73 m2 (P = 0.05). There was no significant difference in 1, 3 and 5 year survival in those with eGFR ≤59 mL/min/1.73 m2 compared those with eGFR > 60 mL/min/1.73 m2 in era 3 (90%, 80%, and 73% vs 92%, 92% and 78%, P = 0.14) and in era 4 (88%, 80% and 61% vs 95%, 82% and 74%, P = 0.06). Results of this analysis are presented in Fig. 4A–D. The results remain unchanged when the analysis was repeated using a eGFR cut-off of 45 mL/min/1.73 m2 (data not shown). However, when the analysis was repeated using patients on RRT versus those not on RRT at time of LT, patients on RRT at time of LT had worse survival in all eras 2 to era 4 (P < 0.05 for all), as shown in Fig. 5A–D. Post-LT survival for patients on RRT at LT was also comparable between the 4 transplant eras (Log Rank P = 0.96, Supplemental Fig. 1).
A–D) Kaplan Meier estimates of post-LT survival by eGFR at LT in 4 different transplant eras. As demonstrated, patients with eGFR ≤ 59 mL/min/1.73 m2 at time of LT had worse post-LT survival in era 1 (Fig. 4A, P = 0.004) and era 2 (Fig. 4B, P = 0.05) but not in era 3 (Fig. 4C, P = 0.14) and era 4 (Fig. 4D, P = 0.06). Using eGFR of 45 mL/min/1.73 m2 as a cut-off for renal dysfunction showed similar results (data not shown).
A–D) Kaplan Meier estimates of post-LT survival by RRT requirement at time of LT in 4 different transplant eras. As demonstrated, patients on RRT at time of LT had inferior post-LT survival era-2, -3 and -4 (P < 0.05 for all) but not in era-1 (P = 0.82) which included only 14 patients on RRT at time of LT.
On univariate analysis, factors associated with post-LT death in era-1 included recipient’s age (HR 1.26 for each 10 years increase in age, CI: 1.09–1.45, P = 0.002), diabetes mellitus (HR 1.58, CI:1.17–2.13, P = 0.003), pre-LT renal dysfunction (HR 1.94, CI: 1.33–2.83, P < 0.001), higher recipient bilirubin at LT (HR 1.02, CI: 1.00–1.03, P = 0.04), intensive care unit (ICU) stay at LT (HR 1.55, CI: 1.1–2.19, P = 0.013), longer operative (OR) time (HR 1.09 for each 30 min increase, CI: 1.04–1.13, P < 0.001) and longer recipient warm ischemia time (WIT) (HR 1.46 for each 30 min increase, CI: 1.1–1.94, P = 0.008) and post-LT EAD (HR 1.39, CI: 1.05–1.82, P = 0.02) (Table 2).
Univariate COX Proportional Hazards model for Death Following Transplant by Transplant Era.
| Era-1 1998–2002 (N = 488) | Era-2 2003–2007 (N = 889) | Era-3 2008–2012 (N = 703) | Era-4 2013–2018 (N = 791) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age / 10 years | 1.26 (1.09, 1.45) | 0.002 | 1.30 (1.17, 1.45) | <0.001 | 1.23 (1.05, 1.45) | 0.010 | 1.22 (0.95, 1.57) | 0.120 |
| Female gender | 1.00 (0.76, 1.31) | 0.972 | 0.76 (0.61, 0.96) | 0.019 | 1.08 (0.79, 1.47) | 0.619 | 0.84 (0.52, 1.34) | 0.461 |
| BMI | 1.01 (0.99, 1.03) | 0.551 | 1.00 (0.98, 1.02) | 0.810 | 1.00 (0.98, 1.03) | 0.733 | 0.97 (0.93, 1.01) | 0.123 |
| Diabetes Mellitus | 1.58 (1.17, 2.13) | 0.003 | 1.40 (1.13, 1.75) | 0.002 | 1.50 (1.11, 2.04) | 0.009 | 1.49 (0.95, 2.35) | 0.084 |
| Alcoholic cirrhosis | 1.05 (0.73, 1.51) | 0.806 | 0.99 (0.73, 1.36) | 0.970 | 0.96 (0.62, 1.48) | 0.839 | 0.98 (0.49, 1.97) | 0.963 |
| Cholestatic Liver disease | 0.66 (0.41, 1.05) | 0.080 | 0.83 (0.60, 1.15) | 0.260 | 0.66 (0.38, 1.14) | 0.138 | 0.12 (0.02, 0.88) | 0.037 |
| NASH | NA | NA | 0.67 (0.38, 1.19) | 0.170 | 1.22 (0.77, 1.92) | 0.395 | 1.30 (0.75, 2.25) | 0.356 |
| HCV | 1.23 (0.97, 1.57) | 0.093 | 1.02 (0.84, 1.25) | 0.833 | 0.81 (0.60, 1.10) | 0.173 | 1.45 (0.93, 2.27) | 0.103 |
| HCC as secondary diagnosis | 1.07 (0.67, 1.72) | 0.769 | 1.60 (1.18, 2.18) | 0.003 | 1.00 (0.49, 2.03) | 1.000 | 1.50 (0.95, 2.37) | 0.082 |
| Days on LT waiting list / 365 | 1.26 (0.85, 1.88) | 0.249 | 0.54 (0.28, 1.02) | 0.056 | 0.74 (0.46, 1.19) | 0.212 | 0.75 (0.41, 1.34) | 0.325 |
| Calculated MELD at LT | 1.02 (0.98, 1.06) | 0.363 | 1.00 (0.99, 1.02) | 0.660 | 1.00 (0.98, 1.01) | 0.662 | 1.01 (0.99, 1.03) | 0.595 |
| INR at LT | 1.04 (0.82, 1.31) | 0.775 | 1.00 (0.83, 1.20) | 0.989 | 0.85 (0.67, 1.08) | 0.188 | 0.85 (0.65, 1.12) | 0.243 |
| Recipient Bilirubin at LT | 1.02 (1.00, 1.03) | 0.040 | 1.00 (0.99, 1.02) | 0.690 | 0.99 (0.97, 1.01) | 0.335 | 1.00 (0.98, 1.02) | 0.858 |
| Pre-LT renal dysfunction | ||||||||
| No (Cr ≦ 1.5 mg/dl) | ref | ref | ref | ref | ||||
| Yes (Cr > 1.5 mg/dl or RRT) | 1.94 (1.33, 2.83) | <0.001 | 1.42 (1.11, 1.82) | 0.005 | 1.29 (0.93, 1.79) | 0.135 | 1.50 (0.95, 2.36) | 0.081 |
| Medical condition at LT | ||||||||
| at home | ref | ref | ref | ref | ||||
| in ICU | 1.55 (1.10, 2.19) | 0.013 | 1.31 (0.85, 2.03) | 0.217 | 1.26 (0.74, 2.14) | 0.400 | 1.89 (1.03, 3.45) | 0.040 |
| in hospital (not ICU) | 0.76 (0.40, 1.43) | 0.393 | 1.22 (0.85, 1.75) | 0.289 | 1.04 (0.63, 1.72) | 0.871 | 1.15 (0.60, 2.20) | 0.671 |
| CIT/hour | 1.01 (0.95, 1.07) | 0.806 | 1.03 (1.00, 1.07) | 0.072 | 1.06 (1.00, 1.12) | 0.056 | 1.04 (0.91, 1.20) | 0.545 |
| Recipient WIT/30 min | 1.46 (1.10, 1.94) | 0.008 | 1.14 (0.87, 1.47) | 0.342 | 1.06 (0.67, 1.66) | 0.811 | 0.44 (0.15, 1.23) | 0.116 |
| DCD WIT per minute | 1.14 (0.97, 1.35) | 0.118 | 1.00 (0.97, 1.02) | 0.930 | 1.06 (1.00, 1.12) | 0.052 | 1.00 (0.91, 1.11) | 0.950 |
| DCD | 0.93 (0.44, 1.98) | 0.858 | 1.03 (0.75, 1.43) | 0.837 | 1.06 (0.68, 1.66) | 0.800 | 1.10 (0.61, 2.00) | 0.750 |
| DRI per unit | 1.28 (0.94, 1.75) | 0.119 | 1.07 (0.84, 1.37) | 0.586 | 1.47 (1.05, 2.06) | 0.026 | 1.41 (0.84, 2.38) | 0.196 |
| OR Time / 30 min | 1.09 (1.04, 1.13) | <0.001 | 1.04 (1.01, 1.08) | 0.011 | 1.00 (0.95, 1.06) | 0.910 | 1.00 (0.96, 1.04) | 0.883 |
| EAD | 1.39 (1.05, 1.82) | 0.020 | 1.27 (1.01, 1.59) | 0.038 | 1.11 (0.79, 1.57) | 0.545 | 1.25 (0.76, 2.04) | 0.380 |
| Age Donor / 10 years | 1.06 (0.99, 1.12) | 0.090 | 1.02 (0.96, 1.08) | 0.503 | 1.15 (1.06, 1.25) | <0.001 | 1.05 (0.92, 1.19) | 0.482 |
| Bilirubin Donor /mg/dl | 0.99 (0.89, 1.09) | 0.777 | 0.94 (0.82, 1.08) | 0.375 | 0.91 (0.76, 1.09) | 0.319 | 1.00 (0.71, 1.43) | 0.983 |
BMI: Body mass index; CIT: Cold ischemia time; Cr: creatinine; DCD: Donation after cardiac death; DRI: Donor risk index; EAD: Early allograft dysfunction; HCC: hepatocellular carcinoma; HCV: Hepatitis C virus; ICU: Intensive care Unit; INR: International normalization ratio; LT: Liver transplant; NASH: Non-alcoholic steatohepatitis; OR: Operation room; RRT: Renal replacement therapy; WIT: Warm ischemia time.
Bold values signifies the values are statistically significant.
Results of a multivariate cox proportional hazards analysis that included recipient age, diabetes mellitus, pre-LT renal dysfunction, recipient bilirubin at LT, ICU stay at LT, OR time, WIT and EAD demonstrated that factors that pre-LT renal dysfunction independently affected post-LT death in era-1 (aHR 1.74, CI: 1.14–2.66, P = 0.01) In contrast, pre-LT renal dysfunction had no independent impact on post-LT mortality in era 4 (aHR 1.26, CI: 0.74–2.15, P = 0.39) (Table 3). A separate multivariate analysis using eGFR cut-off of 60 mL/min/1.73 m2 at LT to define pre-LT renal dysfunction demonstrated that although eGFR ≤ 59 mL/min/1.73 m2 was associated with 40% increased risk of death in era-1 (aHR 1.4, CI:1.02–1.93, P0.037), eGFR ≤ 59 mL/min/1.73 m2 had no statistically significant impact on post LT mortality in era 3 and era-4 (aHR 1.12, CI: 0.81–1.53, P = 0.5 and aHR 1.24, CI: 0.76–2.02, P = 0.39, respectively) (Supplemental Table 1). Results remained unchanged when the analysis was repeated using a eGFR cut-off of 45 mL/min/1.73 m2 (data not shown).
Results of a Multivariate COX Proportional Hazards model for Death Following Transplant by Transplant Era.
| Era-1 1998–2002 (n = 456) | Era-2 2003–2007 (n = 883) | Era-3 2008–2012 (n = 701) | Era-4 2013–2018 (n = 756) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age / 10 years | 1.18 (1.16, 1.38) | 0.030 | 1.30 (1.15, 1.45) | <0.001 | 1.19 (1.01, 1.40) | 0.043 | 1.24 (0.95, 1.62) | 0.111 |
| Recipient Bilirubin at LT | 1.01 (0.99, 1.03) | 0.506 | 1.00 (0.99, 1.02) | 0.980 | 0.99 (0.97, 1.01) | 0.208 | 1.00 (0.97, 1.02) | 0.766 |
| Diabetes Mellitus | 1.46 (1.07, 1.99) | 0.018 | 1.26 (1.00, 1.57) | 0.048 | 1.43 (1.05, 1.95) | 0.025 | 1.30 (0.81, 2.08) | 0.272 |
| Pre-LT renal dysfunction | ||||||||
| No (Cr ≦ 1.5 mg/dl) | ref | ref | ref | ref | ||||
| Yes (Cr > 1.5 mg/dl/RRT) | 1.74 (1.14, 2.66) | 0.010 | 1.25 (0.95, 1.65) | 0.108 | 1.24 (0.86, 1.77) | 0.251 | 1.26 (0.74, 2.15) | 0.392 |
| Medical condition | ||||||||
| at home | ref | ref | ref | ref | ||||
| in ICU | 1.21 (0.82, 1.80) | 0.338 | 1.17 (0.70, 1.96) | 0.545 | 1.59 (0.85, 2.99) | 0.149 | 1.93 (0.89, 4.17) | 0.096 |
| in hospital (not ICU) | 0.74 (0.38, 1.44) | 0.376 | 1.12 (0.75, 1.66) | 0.583 | 1.18 (0.69, 2.03) | 0.540 | 1.26 (0.61, 2.60) | 0.525 |
| OR Time/ 30 min | 1.06 (1.01, 1.11) | 0.021 | 1.05 (1.01, 1.10) | 0.012 | 1.00 (0.92, 1.09) | 0.960 | 1.00 (0.99, 1.01) | 0.882 |
| Recipient WIT/30 min | 1.22 (0.88, 1.69) | 0.242 | 0.81 (0.57, 1.17) | 0.261 | 0.96 (0.48, 1.93) | 0.909 | 0.39 (0.13, 1.12) | 0.079 |
| EAD | 1.39 (1.04, 1.86) | 0.026 | 1.29 (1.03, 1.62) | 0.029 | 1.15 (0.81, 1.63) | 0.442 | 1.44 (0.87, 2.38) | 0.153 |
EAD: Early allograft dysfunction; ICU: Intensive care Unit; OR: Operation room; RRT: Renal replacement therapy; WIT: Warm ischemia time.
Bold values signifies the values are statistically significant.
We subsequently explored the possibility that the effect of pre-LT renal dysfunction on post-LT death could have varied by the cause of ESLD. A separate multivariate Cox proportional hazards model was constructed to identify predictors of post-LT death in era-4 patients by the 3 most common causes of ESLD. Included in this analysis were 87 patients with alcoholic cirrhosis, 130 patients with NASH, and 204 patients with HCV. The same factors were included in the multivariate model as in the previous analysis. As shown in Table 4, the effect of pre-LT renal dysfunction on post-LT death did not differ by the cause of ESLD (P > 0.3 for all 3 groups).
Multivariate COX Proportional Hazards model for Death Following Transplant by Cause of Liver Disease in Era-4 (2013–2018).
| Alcoholic cirrhosis (n = 87 ) | NASH (n = 130 ) | HCV (n = 204 ) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age / 10 years | 0.41 (0.16, 1.05) | 0.062 | 2.69 (1.05, 6.90) | 0.040 | 2.70 (1.23, 5.96) | 0.014 |
| Diabetes Mellitus | 2.77 (0.22, 34.41) | 0.429 | 1.69 (0.46, 6.19) | 0.428 | 1.98 (0.81, 4.87) | 0.135 |
| Recipient Bilirubin at LT | 0.94 (0.83, 1.07) | 0.348 | 1.01 (0.93, 1.09) | 0.891 | 1.00 (0.95, 1.05) | 0.933 |
| Pre-LT renal dysfunction | ||||||
| No (Cr ≦ 1.5 mg/dl) | ref | ref | Ref | |||
| Yes (Cr > 1.5 mg/dl/RRT) | 2.58 (0.40, 16.70) | 0.321 | 0.70 (0.19, 2.58) | 0.593 | 1.75 (0.64, 4.81) | 0.279 |
| Medical condition at LT | ||||||
| at home | ref | ref | Ref | |||
| in ICU | 2.52 (0.27, 23.63) | 0.418 | 3.28 (0.62, 17.31) | 0.162 | 2.38 (0.36, 15.88) | 0.370 |
| in hospital (not ICU) | 1.39 (0.08, 25.52) | 0.826 | 0.34 (0.04, 3.15) | 0.340 | 1.90 (0.48, 7.57) | 0.365 |
| OR Time/ 30 min | 0.99 (0.67, 1.45) | 0.943 | 1.12 (0.94, 1.34) | 0.191 | 0.92 (0.72, 1.17) | 0.491 |
| Recipient WIT/30 min | 2.88 (0.01, 1141) | 0.794 | 0.03 (0.00, 0.40) | 0.008 | 2.79 (0.28, 27.72) | 0.380 |
| EAD | 2.50 (0.30, 20.96) | 0.400 | 4.02 (0.95, 17.03) | 0.059 | 1.94 (0.81, 4.65) | 0.139 |
Cr: creatinine; EAD: Early allograft dysfunction; ICU: Intensive care Unit; OR: Operation room; RRT: WIT: Warm ischemia time.
Bold values signifies the values are statistically significant.
We also analyzed the rates of RRT at 90 days from LT and KALT at any time point after LT. Results of this analysis are provided in Supplemental Table 2. There was slight but statistically significant increase in RRT requirement at 90 days from LT from era-1 (n = 0, 0%) to era 4 (n = 11, 1.4%), P = 0.002. Although there was no observed difference in KALT among the 4 transplant eras (P = 0.70), days to KALT decreased from a median (IQR)
4DiscussionIn this large single-center study that included 2871 primary LT recipients transplanted over a 20 year period, we demonstrated that LT recipients with pre-LT renal dysfunction had an increased overall risk of death compared to their LT counterparts with normal renal function. However, when analyzed separately by 5-year eras, the effect of pre-LT renal dysfunction on post-LT mortality has declined over time, with no statistically observed difference in survival between LT recipients with or without renal dysfunction in the most recent time period. Multivariate analysis also demonstrated that, while pre-LT renal dysfunction was independently associated with mortality in the pre-MELD era, the impact of pre-LT renal dysfunction on post-LT survival has decreased over time and was no longer evident in the latest transplant era (2013–2018). These results persisted when pre-LT renal dysfunction was redefined using different eGFR cut-offs. Despite these promising results, LT recipients on RRT at time of LT demonstrated worse post-LT survival in irrespective of the transplant era.
Previous reports that spanned both the pre-MELD and post-MELD eras established the negative impact of pre-LT renal dysfunction on post-LT survival [3,4,7]. A recent report by Cullaro et al. analyzed the UNOS data on 78,640LT and SLK transplants performed in the US between 2002 and 2017. The authors demonstrated that different forms of pre-LT renal dysfunction including acute kidney injury (AKI) or chronic kidney disease (CKD) were associated with comparable increased risk of post-LT mortality even after adjusting for multiple other factors that affect post-LT survival indicating that pre-LT renal dysfunction itself rather than the mechanism of renal dysfunction that impacts mortality [4]. In this report, the authors did not analyze the data by transplant era and did not exclude SLK transplant recipients. Other recent studies that confirmed the continuous negative impact of pre-LT renal dysfunction on post-LT survival also did not assess the effect the era of transplant on outcome [6,7]. Overall, the literature assessing the effect of pre-LT renal dysfunction on post-LT mortality in the last 5–10 years is limited. In the current study, we demonstrated that when LT recipients transplanted over the last 2 decades were pooled together, there was a clear negative impact of pre-LT renal dysfunction on post-LT survival, consistent with these prior reports. However, the negative impact of pre-LT renal dysfunction on post-LT outcomes that was clearly evident prior to MELD implementation (era 1) and immediately after the introduction of MELD in organ allocation (era 2), had declined over time with no statistically significant impact observed in the last 10 years of transplant activity, as demonstrated on univariate or multivariate analysis (Tables 3 and 4). Our results did not change when we repeated the analysis using different eGFR cut-offs including eGFR < 60 mL/min and eGFR < 45 mL/min. One exception to this observation was the lower post-LT survival in patients on RRT at time of LT compared with their non-RRT counterparts in the last 3 transplant eras.
What may explain the lower postoperative mortality rate in the last decade in non RRT patients with pre-LT renal dysfunction? One possible explanation is that the lower proportion of recipients with HCV transplanted in recent years could have favorably affected post-LT mortality especially in those with pre-LT renal dysfunction. However, the improved survival in the renal dysfunction patients still remained even after controlling for the cause of ESLD (Table 4). Another explanation is more restrictive approach to listing critically ill patients in the recent eras. We observed that the proportion of patients in the ICU at time of LT decreased from 14% in era 1–7% and 9.6% in eras 3 and 4, respectively, reflecting a trend noted in national data [5,14]. This decline underscores the overall reluctance of listing these patients due to their higher post-LT mortality [15]. However, the demographics of listed patients have changed over time as our recent LT recipients were older, had higher calculated MELD score and there was a higher proportion of patients with creatinine >1.5 mg/dl (19.1% in era 4 vs 7.9% in era 1) or on RRT (8.5% in era 4 vs 2.9% in era 1) at time of LT, again comparable to national data and recently published reports [4,5,16]. Therefore, patient selection alone does not explain the lower post-LT mortality rate in patients with pre-LT renal dysfunction. A third possible explanation for the lower post-operative mortality rate in recently transplanted patients with pre-LT renal dysfunction could be better organ selection as demonstrated by the lower donor risk index (DRI), lower donor age, and lower terminal donor bilirubin in eras 3 and 4, despite the increased number of DCD liver transplants, whose outcomes have improved over the last decade [17]. The shorter CIT, shorter recipient WIT, and the lower EAD rates observed in the recent transplant eras also indicate that improvements in organ handling and surgical expertise had beneficial effects on post-LT survival. However, despite adjusting for some of these factors, we still observed a progressive decline over the years of the negative impact of pre-LT renal function on post-LT survival. We, therefore, cannot exclude that other factors not captured in this report, such as reduced waiting times in patients with high MELD scores after the implementation of the share 35 policy in 2013, better anesthetic management, improvement in the peri-operative care of LT recipients and better selection of SLK transplant candidates could have contributed to the improved outcome in LT recipients with pre-LT renal dysfunction. Of note, although we observed similar rates of KALT among the 4 different transplant eras, the waiting time for KALT was much shorter in era-3 and -4 which could have also contributed to the improved post-LT outcome over the years. Irrespective of the explanation, our results are encouraging and should be confirmed in other large volume transplant centers. It also remains to be determined if there is comparable improvement in the incidence of post-LT CKD and ESRD rates in LT recipients with pre-LT renal dysfunction. Our results also call for caution when transplanting cirrhotic patients on RRT at time of LT as our findings showed that these patients continue to experience inferior post-LT outcomes without any observed improvement in overall post-LT survival in these patients over the last 2 decades. Factors such as older age, longer time on the waiting list may “set the stage” for worse outcomes after transplant in the presence of RRT.
Our findings have important clinical implications for patients with renal dysfunction at time of LT. One important practical application is that with the improvement in 1-year survival in the recent era, patients with renal dysfunction, in whom the possibility of post-LT renal recovery is unknown, consideration should be made for LT alone hoping for either renal recovery or KALT within “the safety net” period adopted by UNOS, especially since recent studies demonstrated that early KALT performed between 60 and 365 days after LT alone have comparable outcomes to SLK transplantation [18].
With the obesity epidemic, NASH is becoming a leading cause of LT in the US [19]. LT recipients with NASH tend to have more diabetes, hypertension and features of the metabolic syndrome. Consequently, CKD is more prevalent in cirrhotic patients with NASH than those with other causes of liver disease [20,21]. A recent publication that included almost 4000 LT recipients with NASH who were transplanted between 2002 and 2013 demonstrated that pre-LT renal function was an independent predictor of post-LT survival [7]. Here we studied if the effect of pre-LT renal dysfunction on post-LT survival varied by etiology of liver disease, with special emphasis on patients with NASH who were transplanted between 2013 and 2018 (era 4). Multivariate Cox proportion analysis demonstrated that after adjusting for age, EAD and other factors, NASH patients with pre-LT renal dysfunction had no increased risk of death compared to those without renal dysfunction (Table 4). The analysis however could have been limited by the relatively small number of NASH patients (n = 130) transplanted in this time frame. Further studies are required to assess the relationship between the underlying cause of liver disease, pre-LT renal dysfunction and post-transplant survival.
Our study is limited by its retrospective nature and lack of important information such as dialysis duration, etiology of renal dysfunction (AKI vs CKD) and information on hypertension as we did not capture this information. We suspect that these missing data were likely evenly distributed among the 4 defined eras, especially giving the comparable number of cases in each group. We also strived to limit selection bias by excluding patients who died in the first week post-LT in whom a definition of EAD could have not been applied and by excluding SLK and re-transplant patients. We used an arbitrary Cr cut off to define pre-LT renal dysfunction. However, this Cr cut-off has been utilized to define pre-LT renal dysfunction in previous reports and roughly corresponds to an eGFR of 30 mL/min/1.73 m2 [3,8,22]. We also used eGFR calculated at time of LT to further validate our results. Results showed that eGFR cut-offs of ≤59 mL/min/1.73 m2 and ≤44 mL/min/1.73 m2 were not associated with post-LT survival on univariate or multivariate analysis in the recent transplant eras. Although the 6 point MDRD equation, that also includes serum albumin and blood urea nitrogen levels, has been shown to be the most accurate equation to estimate GFR in cirrhotic patients [23,24], we adopted the 4 point MDRD equation due to its widespread and ease of use, given that serum albumin level was not always available at time of LT. We could only speculate why pre-LT renal dysfunction had lower impact on post-LT survival in the recent transplant eras. Therefore, future studies are needed to confirm our findings using different eGFR equations and to identify reasons for the improvement in post-LT survival in those with pre-LT renal dysfunction at LT. As expected, smaller numbers of patients in era 4 were available for the 5-year survival analysis. However, there were almost 500 LT recipients transplanted in era 3 who completed a 5-year follow-up, and the results obtained for era 3 and era 4 were comparable. We still cannot exclude a type B error especially when analyzing the effect of pre-LT renal dysfunction on mortality in NASH patients.
5ConclusionIn conclusion, our study demonstrated that pre-LT renal dysfunction had less impact on post-LT survival in the last decade compared to the time prior to MELD and early post MELD implementation in prioritizing patients for LT. One exception to this observation was the lower post-LT survival in patients on RRT at time of LT compared with their non-RRT counterparts, irrespective of the transplant era. Future studies are required to determine if our findings represent a center effect or reflect more widespread changes in practice.
FundingNone.
DisclosuresNone of the authors have any conflict of interest regarding the content of this manuscript.
The following are Supplementary data to this article: