The success of direct-acting antivirals (DAA) has transformed the management of hepatitis C virus (HCV) infection and has led to the expansion of the deceased donor organ pool for liver transplantation.
Material and methodsWe present a single center retrospective review of liver transplantations performed on HCV-seronegative recipients from HCV-seropositive organs from 11/2017 to 05/2020. HCV nucleic acid testing (NAT) was performed on HCV-seropositive donors to assess active HCV infection.
Results42 HCV-seronegative recipients underwent a liver transplant from a HCV-seropositive donor, including 21 NAT negative (20 liver, 1 simultaneous liver kidney transplant) and 21 NAT positive liver transplants. Two (9.5%) HCV antibody positive/NAT negative recipients developed HCV viremia and achieved sustained virologic response with DAA therapy. The remaining patients with available data (19 patients) remained polymerase chain reaction (PCR) negative at 6 months. 20 (95%) of HCV antibody positive/NAT positive recipients had a confirmed HCV viremia. 100% of patients with available data (15 patients) achieved SVR. Observed events include 1 mortality and graft loss and equivalent rates of post-transplant complications between NAT positive and NAT negative recipients.
ConclusionsHCV-seropositive organs can be safely transplanted into HCV-seronegative patients with minimal complications post-transplant.
Despite a record breaking 39,719 solid organ transplantations performed in the United States in 2019, there remains a discordance in the number of available organs for transplant compared to the number of patients requiring this life-saving intervention. Currently, there are 68,000 patients listed for solid organ transplant; however, 1 in 18 of these patients will expire on the waitlist prior to receiving a transplant [1]. This imbalanced demand versus availability of organs is expected to increase due to the rising prevalence of nonalcoholic steatohepatitis (NASH) along with the current opioid epidemic resulting in increased transmission of viral hepatitis [2–4].
There have been multiple initiatives to expand the donor pool, including the utilization of living donations and extended criteria donations [5–7]. Due to the current opioid epidemic there has been a significant increase in the number of high-risk donors due to infectious complications, specifically potential donors with chronic hepatitis C virus (HCV) and prior exposure to hepatitis B virus (HBV) [8]. The success of direct-acting antiviral (DAA) therapy has transformed the management of HCV infection and the utilization of HCV-seropositive donors for transplantation. Multiple centers have reported successful treatment of HCV post-transplantation in patients that developed a viremia after receiving a transplant from a HCV-seropositive donor [9,10].
The ethical and financial consideration of utilizing HCV-seropositive donors have been debated among the transplant community. Safety has been demonstrated in smaller single center studies in patients that received either a HCV antibody (Ab) positive/nucleic acid amplification (NAT) negative or a HCV Ab positive/NAT positive organ [11]. These studies demonstrate comparable rates of 1-year patient survival, graft loss, vascular and biliary complication and acute cellular rejection in HCV-seropositive recipients compared to HCV-seronegative recipients. In addition, recipients of HCV-seropositive organs with viremia post-transplantation have been successfully treated and cured with similar rates of a sustained virologic response (SVR) compared to patients with chronic HCV that have not undergone transplant [12–14].
We aim to study the outcomes after transplantation in recipients of a HCV-seropositive donation while emphasizing the safety of performing these higher risk transplants.
2Material and methods2.1DatabaseA retrospective analysis of an Institutional Review Board (IRB) approved study was performed on HCV negative patients that received a HCV-seropositive liver or simultaneous liver kidney transplantation at The Ohio State University Wexner Medical Center between November 2017 and May 2020.
2.2Study sampleTransplant candidates were deemed eligible to receive a HCV-seropositive organ if they did not have an active HCV viremia (defined as a detectable HCV RNA determined by NAT) at the time of evaluation. Candidates successfully treated for HCV with DAA therapy prior to transplant were excluded. Exemptions to the exclusion criteria were granted to patients deemed emergent for liver transplantation.
Informed consent was obtained from transplant candidates in the ambulatory setting prior to listing. If the liver transplantation was performed emergently, consent was obtained during the patient’s hospitalization from their medical power of attorney. Prior authorization for DAA therapy was not obtained prior to transplantation; however, if the transplant recipient’s insurance denied approval, The Ohio State Wexner Medical Center guaranteed to cover the cost of therapy. Patients who consented to receive a HCV-seropositive transplant were still eligible to receive a HCV-seronegative organ.
Patients included in this study received either a HCV Ab positive/NAT negative or a HCV Ab positive/NAT positive organ. HCV status of the donor was determined at the time of organ procurement from a blood draw analyzing the HCV Ab and NAT status of the donor. The transplant surgeon determined the organ to be adequate for transplant at the time of procurement.
2.3Outcomes of interestThe primary outcome of interest was to assess the safety of HCV-seropositive donations by evaluating rates of patient mortality, graft loss, acute cellular rejection, vascular and biliary complications, and cytomegalovirus (CMV) viremia. These outcomes were compared between HCV Ab positive/NAT negative recipients and HCV Ab positive/NAT positive recipients. Finally, we aimed to determine the incidence of HCV viremia in recipients of HCV-seropositive organs and determine the effectiveness of DAA therapy in patients that developed HCV viremia post-transplant.
2.4Variables and monitoringPatients who received a HCV-seropositive transplantation were monitored according to our institutions standard of care protocol.
If the recipient developed a post-transplantation HCV viremia, a genotype was checked and insurance approval for DAA therapy (preferably sofosbuvir/velpatasvir or glecaprevir/pibrentasvir) was sought. After approval was obtained, a pharmacist reviewed the patient’s medications and suggested appropriate modifications based on drug interactions. Patients were eligible to initiate DAA therapy after discharge from their index admission for transplantation.
Information collected on all patients included age, gender, race, blood group, type of organ received, date of transplantation, etiology of liver disease, the Model for End Stage Liver Disease sodium score (MELD-Na) at the time of transplant, CMV status based on serology, CMV viremia (one PCR elevated above 50 IU/mL), Epstein–Barr (EBV) status, acute cellular rejection (based on liver biopsy), vascular and biliary complications, graft loss, and mortality.
Age, sex, race, cause of death, type of donation, hepatitis B status, CMV and EBV status and allocation region and were collected on each donor. A liver biopsy from each donor was analyzed for fibrosis and steatosis, which was graded as mild (less than 10%), moderation (10 to 30%) and severe (greater than 30%) of the hepatocytes.
The following data was collected on all liver transplant recipients that developed a HCV viremia: date of first positive PCR, time to approval of DAA therapy and if an appeal was required, days from transplant to start of DAA therapy, HCV PCR 30 days after initiation of therapy, HCV PCR at end of treatment, SVR at 12 weeks after completion of therapy, HBV reactivation and HCV recurrence. If the patient did not develop a HCV viremia after transplantation in the first 3 days, a HCV PCR was obtained monthly for the first six months to monitor for HCV viremia.
The majority of patients received a standard protocol for immunosuppression including a rapid steroid induction tapered over 1 week, mycophenolate 720 mg BID and tacrolimus with a goal trough level of 8−12 ng/mL, 6−10 ng/mL, 5−8 ng/mL and 4−6 ng/mL for the first 90 days, 91–180 days, 181–365 and greater than 12 months post transplantation, respectively. If transplant recipients were unable to tolerate standard immunosuppression, they were switched to Cyclosporine, Everolimus or Sirolimus. Patients with renal dysfunction at the time of transplant that delayed initiation of Tacrolimus received Basiliximab. Patients were placed on appropriate antibacterial and antiviral prophylaxis after liver transplantation per protocol.
2.5Statistical analysisFisher exact tests were utilized to test for differences in categorical variables and student t-tests for differences in continuous variables between the groups of interest. A p-value of less than 0.05 was considered significant.
3ResultsSince 2017, a total of 42 HCV-seropositive livers have been transplanted in HCV-seronegative recipients at our medical center. This included 21 patients from HCV Ab positive/NAT positive donors and 21 organs from HCV Ab positive/NAT negative donors. The median time on the waitlist until receiving a HCV Ab positive/NAT negative liver transplantation was 49 days (IQR: 13-284) after listing compared to 58 days (IQR: 23-180) in patients that received a HCV Ab positive/NAT positive liver transplant. Time to transplant was not significantly different between the groups.
3.1Patient demographicsPatient demographics were similar between recipients of HCV Ab positive/NAT positive and HCV Ab positive/NAT negative organs. Alcoholic liver disease was the most common indication for liver transplant in both groups; however, patients were transplanted for a variety of indications including one patient that received a HCV Ab positive/NAT positive transplant who was previously achieved SVR after DAA therapy for HCV, but required status 1a listing after primary graft failure. The median MELD-Na score at time of transplant was 22 for HCV Ab positive/NAT positive recipients and 26 for HCV Ab positive/NAT negative recipients (p value = 0.02) (Table 1).
Information for HCV-seropositive recipients.
| Demographic information | HCV Ab Positive/NAT positive recipients (n = 21) | HCV Ab Positive/NAT negative recipients(n = 21)* | p value/t tests |
|---|---|---|---|
| Age (median, range) | 58 (43−70) | 56 (36−72) | 0.49 |
| Gender | 0.53 | ||
| Female | 7 | 10 | |
| Male | 14 | 11 | |
| Race | 1 | ||
| Caucasian | 20 | 21 | |
| African American | 0 | 0 | |
| Asian | 1 | 0 | |
| Blood type | 0.02 | ||
| A+ | 3 | 11 | |
| AB+ | 3 | 1 | |
| O+ | 13 | 6 | |
| O- | 2 | 2 | |
| B+ | 0 | 1 | |
| Etiology of liver disease | 0.59 | ||
| Alcohol | 9 | 10 | |
| NASH | 7 | 6 | |
| Primary biliary cholangitis | 1 | 1 | |
| Primary sclerosing cholangitis | 1 | 0 | |
| Autoimmune | 1 | 1 | |
| Hepatitis C virus | 1 | 0 | |
| Hemochromatosis | 1 | 0 | |
| Glycogen storage disease type 1 | 0 | 1 | |
| Cryptogenic | 0 | 2 | |
| Previous liver transplant | 1 | 1 | 1 |
| CVM status | 0.23 | ||
| Donor + / Recipient + | 8 | 8 | |
| Donor + / Recipient - | 2 | 7 | |
| Donor - / Recipient + | 9 | 3 | |
| Donor - / Recipient - | 2 | 3 | |
| MELD-Na score at transplant (median, range) | 22 (15−34) | 26 (15−40) | 0.02 |
| Time on waitlist (median, IQR) | 58 days (IQR: 23-180) | 49 days (IQR: 13-284) | 0.89 |
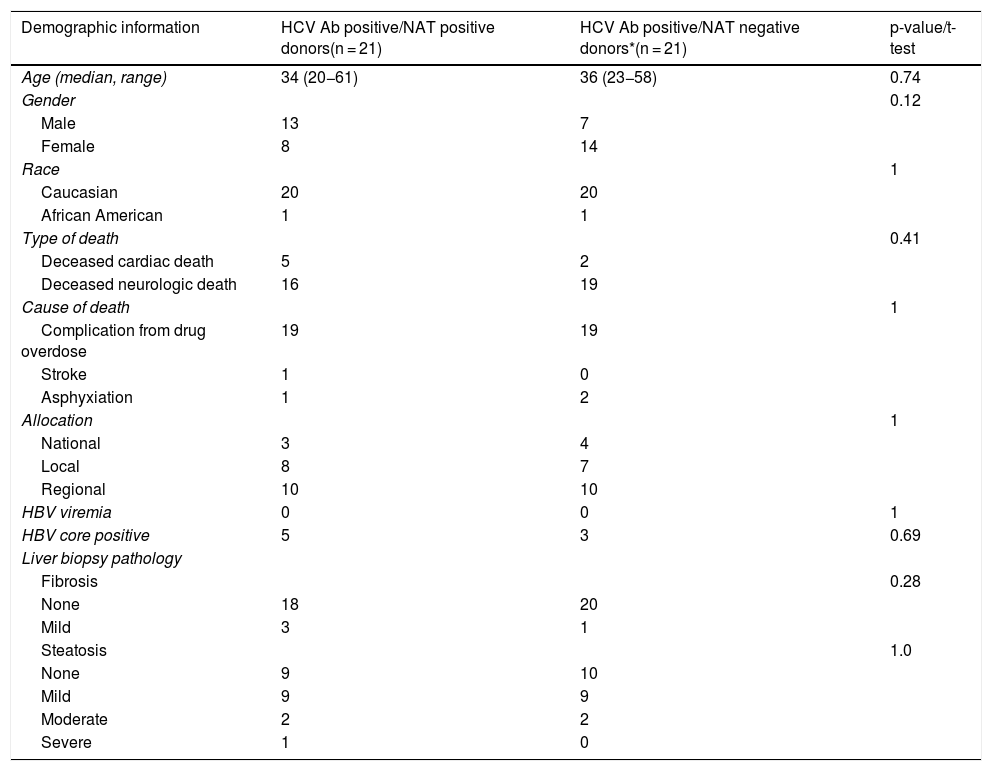
Donor demographics were comparable between HCV Ab positive/NAT positive and HCV Ab positive/NAT negative donors. Multiple patients received a deceased cardiac death donation including 5 HCV Ab positive/NAT positive recipients and 2 HCV Ab positive/NAT negative recipients. Five HCV Ab positive/NAT positive donors and 3 HCV Ab positive/NAT negative donors were also hepatitis B core antibody positive. The majority of donors were obtained from our region; however, 16.6% of the donors were obtained outside our region (Table 2).
Information for HCV-seropositive donors.
| Demographic information | HCV Ab positive/NAT positive donors(n = 21) | HCV Ab positive/NAT negative donors*(n = 21) | p-value/t-test |
|---|---|---|---|
| Age (median, range) | 34 (20−61) | 36 (23−58) | 0.74 |
| Gender | 0.12 | ||
| Male | 13 | 7 | |
| Female | 8 | 14 | |
| Race | 1 | ||
| Caucasian | 20 | 20 | |
| African American | 1 | 1 | |
| Type of death | 0.41 | ||
| Deceased cardiac death | 5 | 2 | |
| Deceased neurologic death | 16 | 19 | |
| Cause of death | 1 | ||
| Complication from drug overdose | 19 | 19 | |
| Stroke | 1 | 0 | |
| Asphyxiation | 1 | 2 | |
| Allocation | 1 | ||
| National | 3 | 4 | |
| Local | 8 | 7 | |
| Regional | 10 | 10 | |
| HBV viremia | 0 | 0 | 1 |
| HBV core positive | 5 | 3 | 0.69 |
| Liver biopsy pathology | |||
| Fibrosis | 0.28 | ||
| None | 18 | 20 | |
| Mild | 3 | 1 | |
| Steatosis | 1.0 | ||
| None | 9 | 10 | |
| Mild | 9 | 9 | |
| Moderate | 2 | 2 | |
| Severe | 1 | 0 |
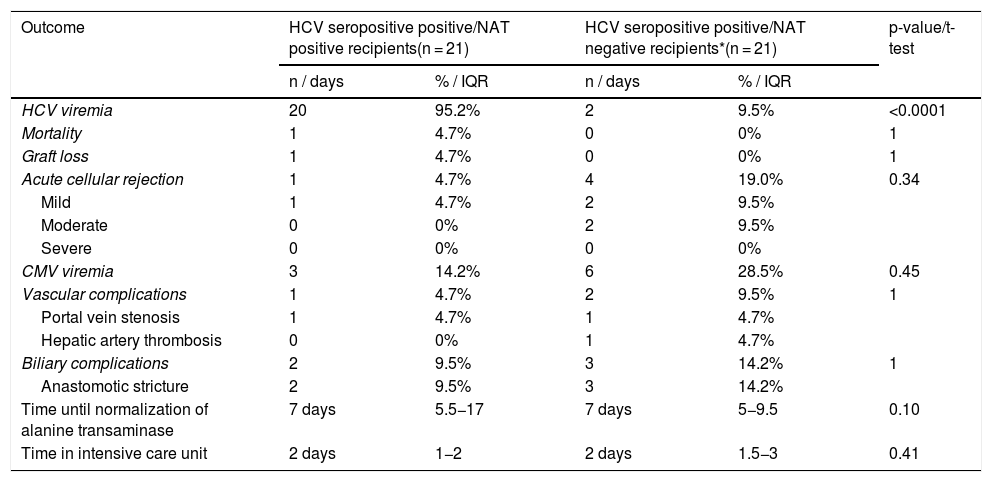
Patients that received a HCV Ab positive/NAT positive liver transplant had a 95% risk of developing a HCV viremia post transplantation compared to 9.5% of patients that received a HCV Ab positive/NAT negative liver transplant (Table 3).
Comparison of outcomes in HCV-seropositive recipients.
| Outcome | HCV seropositive positive/NAT positive recipients(n = 21) | HCV seropositive positive/NAT negative recipients*(n = 21) | p-value/t-test | ||
|---|---|---|---|---|---|
| n / days | % / IQR | n / days | % / IQR | ||
| HCV viremia | 20 | 95.2% | 2 | 9.5% | <0.0001 |
| Mortality | 1 | 4.7% | 0 | 0% | 1 |
| Graft loss | 1 | 4.7% | 0 | 0% | 1 |
| Acute cellular rejection | 1 | 4.7% | 4 | 19.0% | 0.34 |
| Mild | 1 | 4.7% | 2 | 9.5% | |
| Moderate | 0 | 0% | 2 | 9.5% | |
| Severe | 0 | 0% | 0 | 0% | |
| CMV viremia | 3 | 14.2% | 6 | 28.5% | 0.45 |
| Vascular complications | 1 | 4.7% | 2 | 9.5% | 1 |
| Portal vein stenosis | 1 | 4.7% | 1 | 4.7% | |
| Hepatic artery thrombosis | 0 | 0% | 1 | 4.7% | |
| Biliary complications | 2 | 9.5% | 3 | 14.2% | 1 |
| Anastomotic stricture | 2 | 9.5% | 3 | 14.2% | |
| Time until normalization of alanine transaminase | 7 days | 5.5−17 | 7 days | 5−9.5 | 0.10 |
| Time in intensive care unit | 2 days | 1−2 | 2 days | 1.5−3 | 0.41 |
One patient (4.7%) that received a HCV Ab positive/NAT positive liver transplant expired prior to initiation of DAA therapy. Mortality was due to post-surgical complications and sepsis. Rates of mortality and graft loss were not significantly different between HCV Ab positive/NAT positive recipients and HCV Ab positive/NAT negative recipients (Table 3).
3.5Post transplantation complicationsOne (4.7%) of the HCV Ab positive/NAT positive recipients experienced acute cellular rejection compared to 4 (19.0%) of the patients that received a HCV Ab positive/NAT negative transplant. The majority of patients experienced mild acute cellular rejection (Table 3). Three (14.2%) of the patients that received a HCV Ab positive/NAT positive liver transplant experienced CMV viremia compared to 6 (28.5%) of the HCV Ab positive/NAT negative recipients (Table 3). Vascular complications were experienced by 1 (4.7%) patient that received a HCV Ab positive/NAT positive transplant and 2 (9.5%) patients that received a HCV Ab positive/NAT negative transplant. Biliary complications were experienced by 2 (9.5%) patients that received a HCV Ab positive/NAT positive transplant compared to 3 (14.2%) patients that received a HCV Ab positive/NAT negative transplant (Table 3). Post-transplant complications were not significantly different between HCV Ab positive/NAT positive and HCV Ab positive/NAT negative recipients (Table 3).
3.6Time to normalization of alanine transaminase (ALT) and length of stay in the ICUThe median time to normalization of ALT in recipients of a HCV Ab positive/NAT positive transplant was 7 days (IQR 5.5-17) which was not significantly different from a median of 7 days (IQR 5-9.5) in recipients of a HCV Ab positive/NAT negative transplant (Table 3). There was also not a significant different in time admitted to the intensive care unit after transplant between recipients of a HCV Ab positive/NAT positive transplant (2 days, IQR1-2) and HCV Ab positive/NAT negative transplant (2 days IQR 1.5-3) (Table 3).
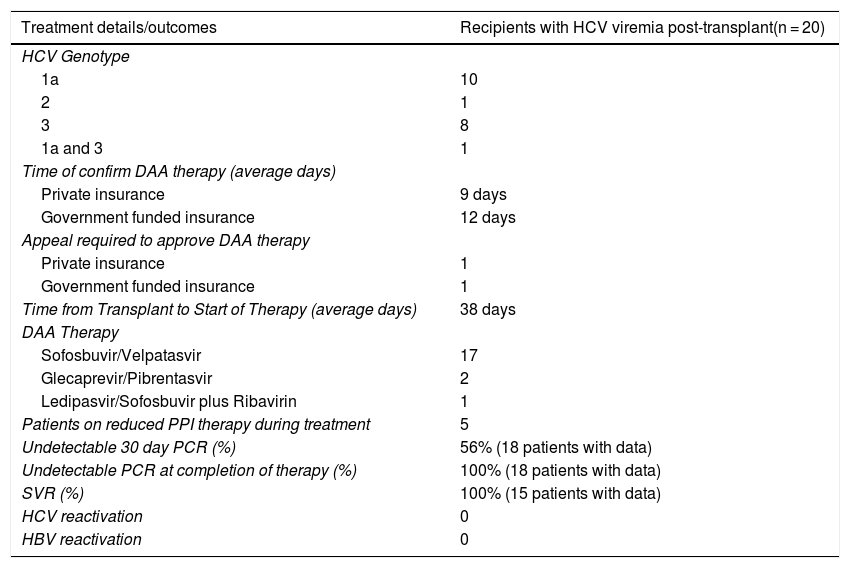
3.7Treatment of HCV post transplantationOf the 21 patients that received a HCV Ab positive/NAT positive transplant, 20 patients (95%) developed a HCV viremia with a detectable PCR within 3 days. HCV genotypes included 1a (50%), 2 (5%), 3 (40%) and one (5%) patient that had both 1a and 3. Insurance approval for DAA therapy varied based on provider and ranged from an average of 9 days in private insurance and 12 days in patients with government funded insurance. DAA therapy included sofosbuvir/velpatasvir (17 patients), glecaprevir/pibrentasvir (2 patients) and ledipasvir/sofosbuvir plus ribavirin (1 patient). On average, patients started DAA therapy 38 days after transplantation. Five patients remained on a reduced dose of proton pump inhibitors (40 mg or less) during treatment with DAA therapy. SVR was achieved in 100% of patients with available data (15 patients) (Table 4).
Details and outcomes of DAA treatment in recipients with HCV viremia after receiving a HCV Ab positive/NAT positive organ.
| Treatment details/outcomes | Recipients with HCV viremia post-transplant(n = 20) |
|---|---|
| HCV Genotype | |
| 1a | 10 |
| 2 | 1 |
| 3 | 8 |
| 1a and 3 | 1 |
| Time of confirm DAA therapy (average days) | |
| Private insurance | 9 days |
| Government funded insurance | 12 days |
| Appeal required to approve DAA therapy | |
| Private insurance | 1 |
| Government funded insurance | 1 |
| Time from Transplant to Start of Therapy (average days) | 38 days |
| DAA Therapy | |
| Sofosbuvir/Velpatasvir | 17 |
| Glecaprevir/Pibrentasvir | 2 |
| Ledipasvir/Sofosbuvir plus Ribavirin | 1 |
| Patients on reduced PPI therapy during treatment | 5 |
| Undetectable 30 day PCR (%) | 56% (18 patients with data) |
| Undetectable PCR at completion of therapy (%) | 100% (18 patients with data) |
| SVR (%) | 100% (15 patients with data) |
| HCV reactivation | 0 |
| HBV reactivation | 0 |
Two recipients (9.5%) of HCV Ab positive/NAT negative liver transplants experienced seroconversion with HCV viremia thus far and had a positive HCV PCR within 15 days after transplant. Both patients were treated with sofosbuvir/velpatasvir and have achieved SVR.
4DiscussionThis study validates the safety of liver transplantation from HCV-seropositive organ donors that were once considered inappropriate for transplantation. Our findings defend the utilization of HCV Ab positive/NAT positive and HCV Ab positive/NAT negative organs in a variety of clinical scenarios including liver and simultaneous liver kidney transplantation, different etiologies of liver disease and even in patients undergoing transplant after graft failure. We highlight a low risk of viremia in patients who have received a HCV Ab positive/NAT negative organ and a high rate of achieving SVR in patients that develop post-transplant HCV [11–14]. We emphasize the safety of HCV-seropositive transplants given the low risk of mortality, graft loss, vascular or biliary complications, acute cellular rejection and CMV. This study supports the utilization of HCV-seropositive donation in liver transplantation as a safe and effective way to expand the donor pool.
The risk of developing a HCV viremia after receiving a HCV-seropositive organ whether HCV Ab positive/NAT negative or HCV antibody positive/NAT positive is crucial information when counseling patients on the increased risk after transplant. Ten percent of the 21 HCV Ab positive/NAT negative recipients in our study experienced a HCV viremia thus far. Previous studies have also reported a similar rate of HCV viremia estimated around 10% [12,14,29]. We report the risk of HCV viremia in seronegative recipients after receiving a HCV Ab positive/NAT positive transplant of 95% which is similar to other studies [14,30].
One year survival post-transplantation is a key outcome that is monitored in the United States to determine the proficiency of a transplant center [15]. Due to advances in surgical techniques, immunosuppression and management of common post-operative complications, the one year survival rate has significantly increased with a national average around 92% after liver transplantation [15–17]. Prior to the advent of DAA therapy, patients with chronic HCV that underwent liver transplantation universally developed a viremia after transplant with higher rates of mortality and graft dysfunction compared to patients with other etiologies of liver disease [18]. Given the ability to now treat chronic HCV post liver transplantation, patients can expect equivalent, if not superior outcomes compared to patients that underwent a transplant for other indications [19]. This study highlights a 98% survival rate at 6 months post transplantation in patients that have undergone an HCV-seropositive organ. There was no significant difference in mortality rate between patients that received a HCV Ab positive/NAT positive organ compared to HCV Ab positive/NAT negative organ. While multiple factors are associated with an increased risk of one year mortality after transplant [20], previous studies suggest that a HCV-seropositive transplant does not independently increase the risk of one year mortality or graft dysfunction [12,14,21].
Twelve percent of the patients in our study had biopsy proven acute cellular rejection including 4 patients that received a HCV antibody positive/NAT negative transplant and 1 patient that received a HCV antibody positive/NAT positive organ. Rates of rejection were not significantly different between the two study groups and were lower than the average rate of acute cellular rejection after liver transplant reported to be 15–25% [22]. A previous case series had suggested a link between DAA therapy and acute cellular rejection with reported rates as high as 30% [23]. However, other subsequent analyses on patients that required DAA therapy after receiving a HCV-seropositive organ have highlighted a similar and acceptable acute cellular rejection rate [24]. We also highlight immunosuppression medications did not require adjustments while on DAA therapy.
Rates of CMV viremia after transplant are largely dependent on the donors and recipient’s status and the appropriate use of prophylactic antivirals with an incidence ranging from 1% to 65% [25,26]. We observed a rate of CMV viremia of 21% in liver recipients with no significant difference in the risk of CMV viremia between HCV antibody positive/NAT positive and HCV antibody positive/NAT negative organ recipients. Limited studies evaluating CMV viremia after HCV-seropositive transplant have confirmed a similar risk in patients receiving a HCV-seronegative organ [14,25,26].
The most common complications after liver transplantation include biliary and vascular complications which effects approximately 15%–30% of recipients [27,28]. Complications experienced in our transplant patients included anastomotic strictures, portal vein stenosis, and hepatic artery thrombosis. There was no significant difference in the rates of biliary and vascular complications between patients receiving a HCV Ab positive/NAT positive and HCV Ab positive/NAT negative organ. These findings confirm that HCV-seropositive organs are not at a higher risk of biliary or vascular complications after liver transplantation [14].
While multiple factors contribute to longer length of time in the intensive care unit of transplant and failure to achieve normalization of ALT, it should be noted that patients of a HCV antibody positive/NAT positive transplant did not experience longer time to achieve these goals. This suggests that the development of an active viremia in recipients after transplant has no effect on short term outcomes.
Successful treatment of HCV in patients that received a HCV Ab positive/NAT positive organ is vital as chronic HCV is associated with increased risks after transplant including fibrosing cholestatic hepatitis, graft loss and mortality [31–33]. In our report, 100% of the patients with available data have achieved SVR after DAA therapy. This is consistent with the expected SVR rates in patients who have been treated with DAA therapy after receiving a HCV-seropositive organ or treatment naïve patients with chronic hepatitis C treated prior to or after transplant [32,33]. Multiple donors were also HBV core antibody positive. Transplant recipients of these organs were placed on HBV prophylaxis and none of these patients experienced HBV reactivation while on DAA therapy.
The ability to successfully obtain HCV therapy is crucial to the success of transplanting HCV-seropositive organs into non-viremic patients. The average time to DAA therapy approval was 9 and 12 days in patients with private insurance and government funded patients, respectively. Two insurance companies denied therapy and required an appeal prior to securing therapy. DAA therapy was started in a median of 37 days after transplantation. As HCV-seropositive organ transplantation becomes a standard method to expand the donor pool, insurance approval should become less of a burden on the healthcare system and the multidisciplinary team.
This study has a few limitations that should be noted. Due to the ongoing nature of this study, data on follow up and response to DAA is incomplete. While our institution performed its first HCV-seropositive donor to HCV-seronegative recipient in 2017, the majority of the transplants analyzed in this study were performed in the past year limiting our ability to determine long term complications from this high risk organ donation. Limitations similar to this have been noted in other studies on this topic [13].
5ConclusionsIn conclusion, the utilization of HCV-seropositive organs, whether HCV Ab positive/NAT positive or HCV Ab positive/NAT negative is safe and should be considered across all liver transplant programs. This single center analysis evaluating liver and simultaneous liver and kidney transplants from HCV-seropositive donors revealed complications including graft loss, acute cellular rejection, biliary and vascular complications, CMV viremia and mortality were not significantly different between patients that received a NAT positive or NAT negative transplantation. Furthermore, recipients that develop a HCV viremia as a result of a HCV seropositive transplant had a 100% rate of SVR after DAA therapy. Ultimately, the use of these HCV seropositive organs is an effective means to expand the donor pool and provide more patients with timely, lifesaving transplants.
Conflict of interestHanje has disclosures of Salix and Intercept. Michael has disclosures of Gilead, Abbvie and Intercept. Remaining authors do not have disclosures.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to acknowledge Edward Wellner, MS as a statistical consultant for this project.