Chronic hepatitis B virus (HBV) infection exerts an impact on lipid metabolism, but its interaction with dysmetabolism-based non-alcoholic fatty liver disease (NAFLD) remains uncertain. The purpose of the study was to investigate the effects of HBV infection on lipid metabolism, hepatic steatosis and related impairments of NAFLD patients.
MethodsBiopsy-proven Chinese NAFLD patients with (NAFLD-HBV group, n = 21) or without chronic HBV infection (NAFLD group, n = 41) were enrolled in the case-control study. Their serum lipidomics was subjected to individual investigation by ultra-performance liquid chromatography–tandem mass spectrometry. Steatosis, activity, and fibrosis (SAF) scoring revealed the NAFLD-specific pathological characteristics.
ResultsChronic HBV infection was associated with global alteration of serum lipidomics in NAFLD patients. Upregulation of phosphatidylcholine (PCs), choline plasmalogen (PC-Os) and downregulation of free fatty acids (FFAs), lysophosphatidylcholine (LPCs) dominated the HBV-related lipidomic characteristics. Compared to those of NAFLD group, the levels of serum hepatoxic lipids (FFA16:0, FFA16: 1, FFA18:1, FFA18:2) were significantly lowered in the NAFLD-HBV group. These low-level FFAs demonstrated correlation to statistical improvements in aspartate aminotransferase activity (FFA16:0, r = 0.33; FFA16:1, r = 0.37; FFA18:1, r = 0.32; FFA18:2, r = 0.42), hepatocyte steatosis (FFA16: 1, r = 0.39; FFA18:1, r = 0.39; FFA18:2, r = 0.32), and ballooning (FFA16:0, r = 0.30; FFA16:1, r = 0.45; FFA18:1, r = 0.36; FFA18:2, r = 0.30) (all P < 0.05).
ConclusionChronic HBV infection may impact on the serum lipidomics and steatosis-related pathological characteristics of NAFLD.
Chronic hepatitis B virus (HBV) appears a worldwide chronic liver disease that affects over 250 million people, especially in the Chinese population [1,2]. By the studies published to date, there is an inverse association of viral indices (e.g., HBsAg, HBV-DNA) and incidence of hyperlipidemia (e.g., hypertriglyceridemia, hypercholesterolemia), fatty liver and metabolic syndrome (MetS) [3–5]. Thus, chronic HBV infection is proposed to exert a beneficial impact on lipid metabolism, probably on the basis of HBV-host interaction [6–9].
Recent decades have witnessed the rapid growing incidence of non-alcoholic fatty liver disease (NAFLD), a metabolic stress-induced chronic liver disease, in China by the prevalence of overweight/obesity and/or sedentary lifestyle [10,11]. In result, concurrent NAFLD is now identified in 13.5% (12/ 91) and 14% (260/1915) of chronic hepatitis B infection patients from Hong Kong (China) and Hang Zhou (Zhe Jiang, China), respectively [8,12].
Serving as one of the components of MetS, NAFLD has been well described to demonstrate an intimate association with abnormalities in systemic lipid metabolism. The individuals with NAFLD exhibit a strong positive association with hyperlipidemia [13]. While patients with hypertriglyceridemia are independently predisposed to the risk of NAFLD [14]. Given the reciprocal causation of NAFLD and hyperlipidemia, they are supposed to be affected by the concurrent HBV infection. However, the role of chronic HBV infection in NAFLD and related lipometabolic disorders remains uncertain until now.
Therefore, we conducted a case-control study of the biopsy-proven Chinese NAFLD patients, with or without chronic HBV infection, to investigate their difference in serum lipidomics. Then integrated analysis of demographic and clinical manifestations, serum lipidomics, and hepatic pathological characteristics was performed so as to uncover the effect of chronic HBV infection on lipid metabolism, hepatic steatosis and related impairments of NAFLD patients.
2Materials and methods2.1PatientsA total of 62 NAFLD patients with (NAFLD-HBV group, n = 21) or without chronic HBV infection (NAFLD group, n = 41) were enrolled from the inpatients of Xinhua Hospital, Shanghai during May 2012 to May 2014. Subjects with ongoing or recent alcohol abuse (alcohol intake >20 g/day for male, >10 g/day for female), anti-HCV IgG/IgM positive, autoimmune hepatitis, drug-induced liver injury, primary biliary cholangitis, Wilson’s disease and other causes of liver steatosis were excluded. Each participant of the study was exposed to pathological evaluation by liver biopsy. Patients with hepatocyte steatosis (>5%) were diagnosed to be NAFLD, and those with seropositivity for hepatitis B surface antigen (HBsAg) for at least six months were defined as chronic HBV infection (Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection, 2015) [15]. This study was approved by the Research Ethics Committee of Xinhua Hospital, and informed consent was obtained from each patient.
2.2Clinical assessment and laboratory analysisDemographical characteristics including age, gender, height, weight, waist-to-hip ratio and body mass index (BMI) were obtained from medical record. Blood samples were collected from each patient after 12-h fasting. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γ-GT) and alkaline phosphatase (ALP) test were performed by a multichannel automatic analyzer (Bayer ADVIA 1650, Moss, Norway). Fasting plasma glucose (FPG), triacylglycerol (TG) and total cholesterol (TC) were measured using Wako Bioproducts (Wako Pure Chemical Industries, Richmond, VA, USA). Hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), hepatitis B e antigen (HBeAg) and antibody against HBeAg (anti-HBe)) were tested by Enzyme-linked immunosorbent assay (Abbott Laboratories, North Chicago, IL, USA) and HBV DNA by real-time polymerase chain reaction assay (COBAS® TaqMan HBV Test, Roche Diagnostics, Basel, Switzerland). The mean HBV DNA level of NAFLD-HBV patients were 2.35*108 IU/mL.
2.3Hepatic histopathological examinationEach liver biopsy sample was reviewed by three pathologists who were blinded to the present study. The steatosis, activity, and fibrosis (SAF) scoring system was employed for the evaluation of NAFLD [16,17]. In detail, the SAF score was assessed on the basis of Steatosis (S0, <5%; S1, 5–33%; S2, 34–66%; S3, >66%), Activity (sum of lobular inflammation: 0, no foci per 200× field; 1, <2 foci per 200× field; 2, 2‐4 foci per 200× field; 3, >4 foci per 200× field), ballooning (0, none; 1, few balloon cells; 2, many cells/prominent ballooning), and Fibrosis (F0, none; F1, perisinusoidal or portal fibrosis; F2, perisinusoidal and periportal fibrosis without bridging; F3, bridging fibrosis; F4, cirrhosis). Chronic hepatitis B infection was defined by the typical periportal/portal hepatitis with piecemeal necrosis of hepatocytes.
2.4UPLC-MS/MSThe serum lipidomics were analyzed as described previously [18]. In brief, lipids were extracted from the collected serum samples, and analyzed by UPLC (Waters, Milford, USA) combined with a triple TOF 5600 mass spectrometer (AB SCIEX, USA) platform together with thirteen quality control (QC) samples. Lipids separation was performed using a UPLC ACQUITY C8 BEN column (2.1 × 100 mm; 1.7 μm; Waters, Milford, USA). The mobile phases consisted of (A) 60% acetonitrile in water, 10 mmol/L ammonium acetate, and (B) 90% isopropanol in acetonitrile, 10 mmol/L ammonium acetate. Gradient elution was carried out at a flow rate of 0.26 mL/min with the gradient conditions as follows: 0–1.5 min, 32% B; 1.5–14 min, 32–85% B; 15.5–15.6 min, 85–97% B; 15.6–18 min, 97% B; 18–20 min, 97−32% B. Mass spectrometry was performed in positive and negative electrospray ion modes. Data acquisitions were applied using Analyst TF 1.6 software (AB SCIEX, Framingham, MA). LipidView/PeakView and MultiQuant 2.0 (AB SCIEX, Framingham, MA) were used for lipid identification and quantification, respectively. After being normalized with corresponding internal standards, the detected lipids data in QCs were evaluated based on their relative standard deviation (RSD), and only those with RSD below 30% were subjected to further analysis.
2.5Statistical analysisStatistical analyses were performed using SPSS Statistics software version 23.0 and R software version 4.0.2. Demographic, clinical and pathological characteristics were presented as mean ± SD, median (interquartile range) or percentage, where appropriate. Continuous variables were compared between groups using unpaired Student’s t test or Mann-Whitney U test. Categorical data were analyzed by Chi-square test or Fisher’s exact test. Multivariate analysis including principal component analysis (PCA) was performed using R 4.0.2, and orthogonal partial least squares-discriminant analysis (OPLS-DA) was performed, using SIMCA 14.1 (MKS Umetrics, Malmö, Sweden). The differential serum lipids with both multivariate and univariate significance (OPLS-DA VIP > 1.0 and P < 0.05) were filtered on the basis of variable importance in the projection (VIP), S-plot and P value (unpaired Student’s t-test). Spearman’s correlation was used to exploring the correlativity between serum lipidomics and hepatic pathological parameters. Statistical significance was defined as a two-side P value <0.05.
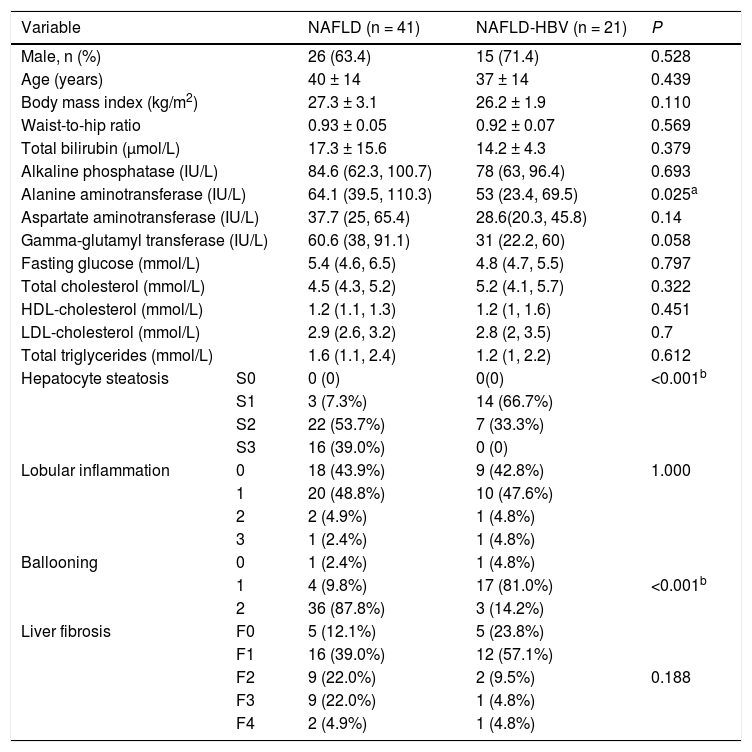
3Results3.1Demographic characteristics of NAFLD patients with and without HBVIn the present study, demographic, clinical and pathological indices were compared between NAFLD patients with (NAFLD-HBV group) or without chronic HBV infection (NAFLD group). The two groups displayed similar gender distribution, BMI, waist-to-hip ratio and fasting glucose level. Interestingly, most patents (14, 66.7%) in NAFLD-HBV were at S1 while most patients (22, 53.7%) in NAFLD were at S2 based on the SAF score. Consistently, most patients (17, 81.0%) in NAFLD-HBV had lessened ballooning (Table 1). This indicated that an amelioration of NAFLD-specific pathological characteristics, including hepatocyte steatosis and ballooning, was documented in the NAFLD-HBV instead of NAFLD group. In contrast to the comparability in most biochemical indices, there was a statistically decreased ALT activity in the NAFLD-HBV group in comparison to that of the NAFLD group (NAFLD group vs NAFLD-HBV group: 64.1 IU/L (39.5 IU/L, 110.3 IU/L) vs 53 IU/L (23.4 IU/L, 69.5 IU/L), P = 0.025) (Table 1).
Demographic, clinical and pathological data of all patients.
| Variable | NAFLD (n = 41) | NAFLD-HBV (n = 21) | P | |
|---|---|---|---|---|
| Male, n (%) | 26 (63.4) | 15 (71.4) | 0.528 | |
| Age (years) | 40 ± 14 | 37 ± 14 | 0.439 | |
| Body mass index (kg/m2) | 27.3 ± 3.1 | 26.2 ± 1.9 | 0.110 | |
| Waist-to-hip ratio | 0.93 ± 0.05 | 0.92 ± 0.07 | 0.569 | |
| Total bilirubin (μmol/L) | 17.3 ± 15.6 | 14.2 ± 4.3 | 0.379 | |
| Alkaline phosphatase (IU/L) | 84.6 (62.3, 100.7) | 78 (63, 96.4) | 0.693 | |
| Alanine aminotransferase (IU/L) | 64.1 (39.5, 110.3) | 53 (23.4, 69.5) | 0.025a | |
| Aspartate aminotransferase (IU/L) | 37.7 (25, 65.4) | 28.6(20.3, 45.8) | 0.14 | |
| Gamma-glutamyl transferase (IU/L) | 60.6 (38, 91.1) | 31 (22.2, 60) | 0.058 | |
| Fasting glucose (mmol/L) | 5.4 (4.6, 6.5) | 4.8 (4.7, 5.5) | 0.797 | |
| Total cholesterol (mmol/L) | 4.5 (4.3, 5.2) | 5.2 (4.1, 5.7) | 0.322 | |
| HDL-cholesterol (mmol/L) | 1.2 (1.1, 1.3) | 1.2 (1, 1.6) | 0.451 | |
| LDL-cholesterol (mmol/L) | 2.9 (2.6, 3.2) | 2.8 (2, 3.5) | 0.7 | |
| Total triglycerides (mmol/L) | 1.6 (1.1, 2.4) | 1.2 (1, 2.2) | 0.612 | |
| Hepatocyte steatosis | S0 | 0 (0) | 0(0) | <0.001b |
| S1 | 3 (7.3%) | 14 (66.7%) | ||
| S2 | 22 (53.7%) | 7 (33.3%) | ||
| S3 | 16 (39.0%) | 0 (0) | ||
| Lobular inflammation | 0 | 18 (43.9%) | 9 (42.8%) | 1.000 |
| 1 | 20 (48.8%) | 10 (47.6%) | ||
| 2 | 2 (4.9%) | 1 (4.8%) | ||
| 3 | 1 (2.4%) | 1 (4.8%) | ||
| Ballooning | 0 | 1 (2.4%) | 1 (4.8%) | |
| 1 | 4 (9.8%) | 17 (81.0%) | <0.001b | |
| 2 | 36 (87.8%) | 3 (14.2%) | ||
| Liver fibrosis | F0 | 5 (12.1%) | 5 (23.8%) | |
| F1 | 16 (39.0%) | 12 (57.1%) | ||
| F2 | 9 (22.0%) | 2 (9.5%) | 0.188 | |
| F3 | 9 (22.0%) | 1 (4.8%) | ||
| F4 | 2 (4.9%) | 1 (4.8%) | ||
Continuous variables were presented as mean ± SD or median (interquartile range). Categorical variables were presented as percentage. HDL, high density lipoprotein; LDL, low density lipoprotein.
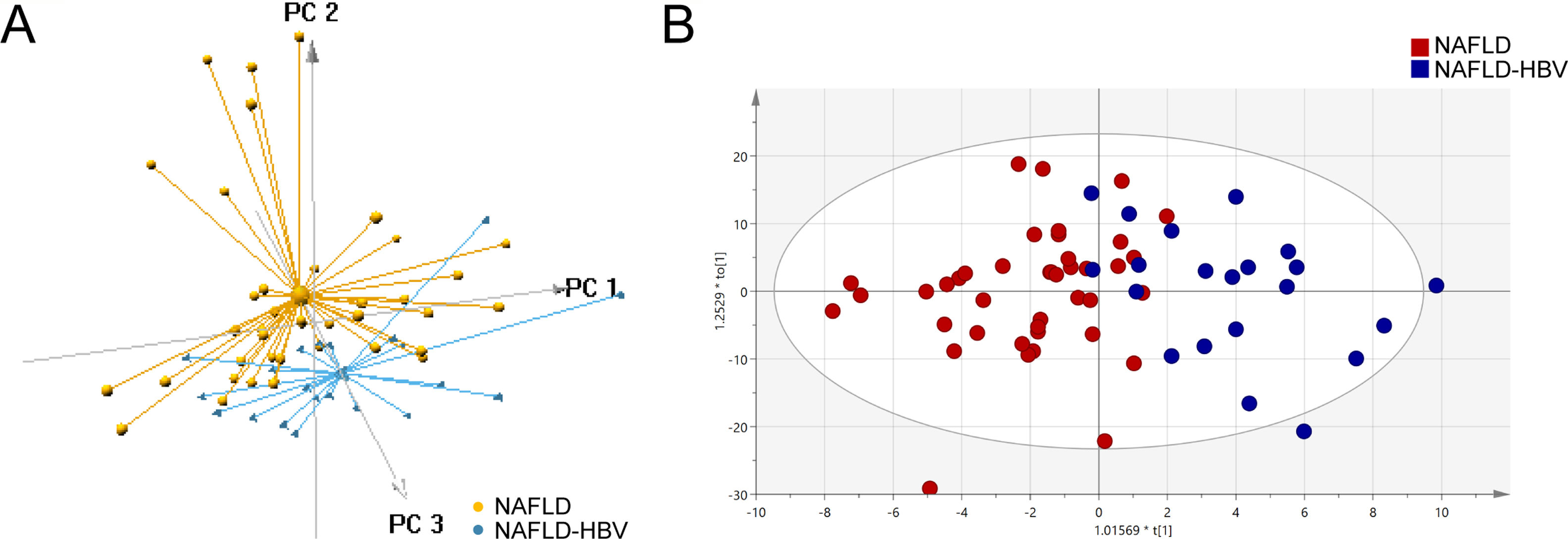
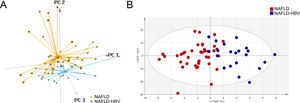
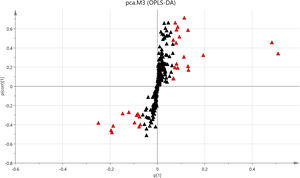
Multivariate analyses were employed in our study to take an overview of the serum lipidomics between NAFLD and NAFLD-HBV groups. Dramatically, 3D PCA score plot of serum lipidomics distinctly differentiated the NAFLD patients with or without chronic HBV infection (Fig. 1A). Similar group discrimination was also obtained by the OPLS-DA score plot (Fig. 1B).
Lipidomics differentiates patients with or without chronic HBV infection. (A) Score plot of 3D principal component analysis (PCA) for the patients with (NAFLD-HBV group) or without chronic HBV infection (NAFLD group). (B) Score plot of orthogonal partial least squares-discriminant analysis (OPLS-DA) for the NAFLD and NAFLD-HBV groups.
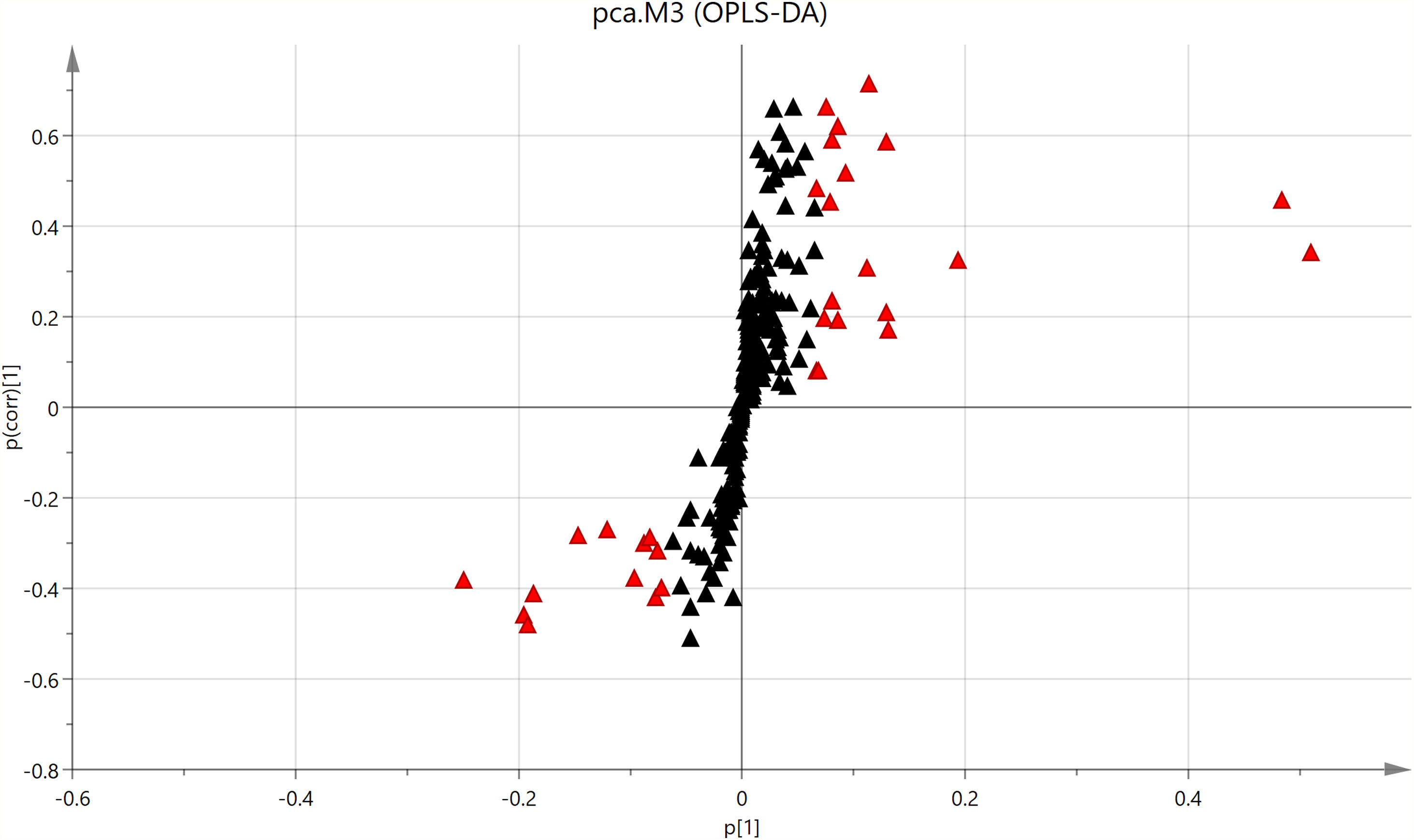
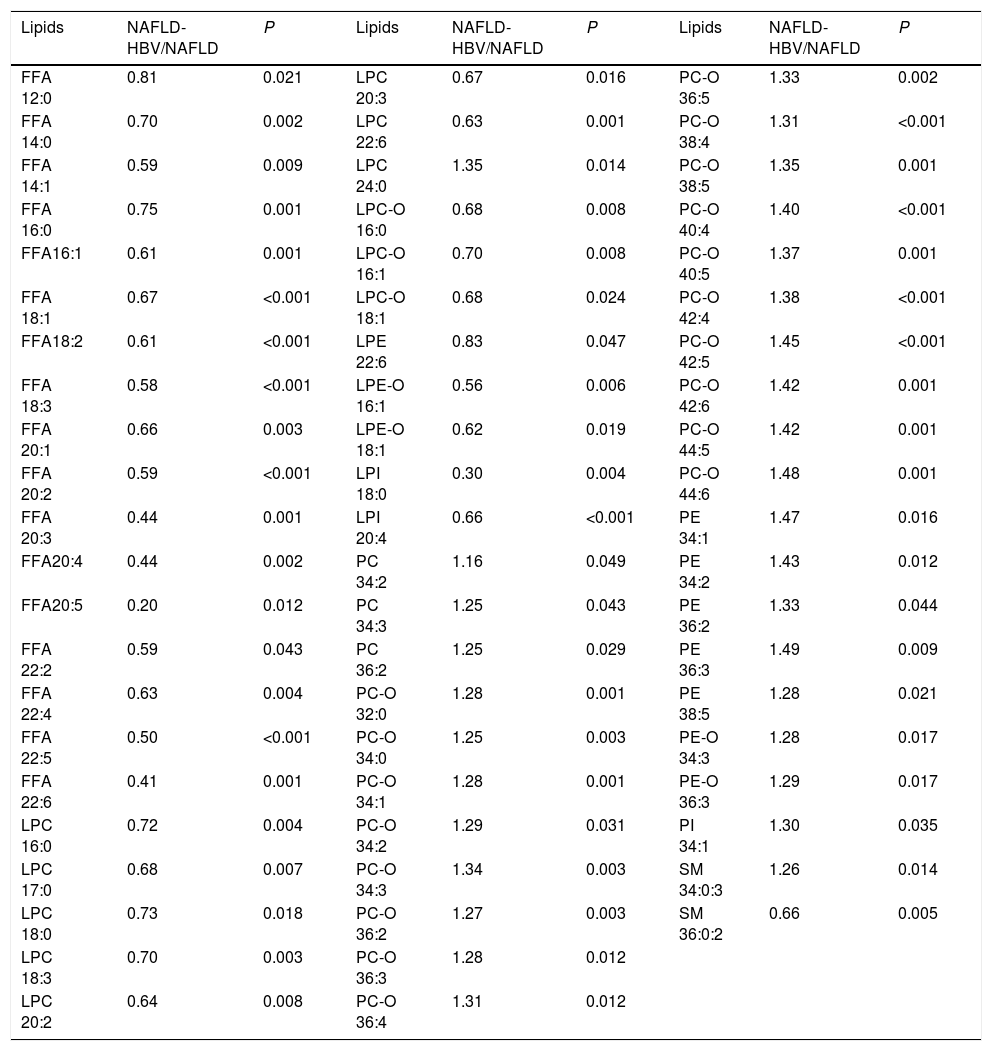
To reveal the role of chronic HBV infection in lipid metabolism, a total of 239 serum lipids was exposed to UPLC-MS/MS in both NAFLD and NAFLD-HBV groups. In result, 64 lipids among these ones (26.78%) were filtered to be statistically different by unpaired Student’s t-test. Detailedly, the profile of differential serum lipids comprised 17 free fatty acid (FFAs), 8 lysophosphatidylcholine (LPCs), 3 lysophosphatidylcholine plasmalogen (LPC-Os), 1 lysophosphatidylethanolamine (LPE), 2 lysophosphatidylethanolamine plasmalogen (LPE-Os), 2 lysophosphatidylinositol (LPIs), 3 phosphatidylcholine (PCs), 18 cholineplasmalogen (PC-Os), 5 phosphatidylethanolamine (PEs), 2 ethanolamine plasmalogen (PE-Os), 1 phosphatidylinositol (PI) and 2 sphingomyelin (SMs) (Table 2). On the other hand, S-plot put forward 31 differential serum lipids with VIP > 1.0, including 7 free fatty acid (FFAs), 3 lysophosphatidylcholine (LPCs), 10 phosphatidylcholine (PCs), 8 cholineplasmalogen (PC-Os), 1 sphingomyelin (SM) and 2 triacylglycerol (TGs) (Fig. 2).
Differential serum lipids between NAFLD patients with or without chronic HBV infection (unpaired Student’s t test).
| Lipids | NAFLD-HBV/NAFLD | P | Lipids | NAFLD-HBV/NAFLD | P | Lipids | NAFLD-HBV/NAFLD | P |
|---|---|---|---|---|---|---|---|---|
| FFA 12:0 | 0.81 | 0.021 | LPC 20:3 | 0.67 | 0.016 | PC-O 36:5 | 1.33 | 0.002 |
| FFA 14:0 | 0.70 | 0.002 | LPC 22:6 | 0.63 | 0.001 | PC-O 38:4 | 1.31 | <0.001 |
| FFA 14:1 | 0.59 | 0.009 | LPC 24:0 | 1.35 | 0.014 | PC-O 38:5 | 1.35 | 0.001 |
| FFA 16:0 | 0.75 | 0.001 | LPC-O 16:0 | 0.68 | 0.008 | PC-O 40:4 | 1.40 | <0.001 |
| FFA16:1 | 0.61 | 0.001 | LPC-O 16:1 | 0.70 | 0.008 | PC-O 40:5 | 1.37 | 0.001 |
| FFA 18:1 | 0.67 | <0.001 | LPC-O 18:1 | 0.68 | 0.024 | PC-O 42:4 | 1.38 | <0.001 |
| FFA18:2 | 0.61 | <0.001 | LPE 22:6 | 0.83 | 0.047 | PC-O 42:5 | 1.45 | <0.001 |
| FFA 18:3 | 0.58 | <0.001 | LPE-O 16:1 | 0.56 | 0.006 | PC-O 42:6 | 1.42 | 0.001 |
| FFA 20:1 | 0.66 | 0.003 | LPE-O 18:1 | 0.62 | 0.019 | PC-O 44:5 | 1.42 | 0.001 |
| FFA 20:2 | 0.59 | <0.001 | LPI 18:0 | 0.30 | 0.004 | PC-O 44:6 | 1.48 | 0.001 |
| FFA 20:3 | 0.44 | 0.001 | LPI 20:4 | 0.66 | <0.001 | PE 34:1 | 1.47 | 0.016 |
| FFA20:4 | 0.44 | 0.002 | PC 34:2 | 1.16 | 0.049 | PE 34:2 | 1.43 | 0.012 |
| FFA20:5 | 0.20 | 0.012 | PC 34:3 | 1.25 | 0.043 | PE 36:2 | 1.33 | 0.044 |
| FFA 22:2 | 0.59 | 0.043 | PC 36:2 | 1.25 | 0.029 | PE 36:3 | 1.49 | 0.009 |
| FFA 22:4 | 0.63 | 0.004 | PC-O 32:0 | 1.28 | 0.001 | PE 38:5 | 1.28 | 0.021 |
| FFA 22:5 | 0.50 | <0.001 | PC-O 34:0 | 1.25 | 0.003 | PE-O 34:3 | 1.28 | 0.017 |
| FFA 22:6 | 0.41 | 0.001 | PC-O 34:1 | 1.28 | 0.001 | PE-O 36:3 | 1.29 | 0.017 |
| LPC 16:0 | 0.72 | 0.004 | PC-O 34:2 | 1.29 | 0.031 | PI 34:1 | 1.30 | 0.035 |
| LPC 17:0 | 0.68 | 0.007 | PC-O 34:3 | 1.34 | 0.003 | SM 34:0:3 | 1.26 | 0.014 |
| LPC 18:0 | 0.73 | 0.018 | PC-O 36:2 | 1.27 | 0.003 | SM 36:0:2 | 0.66 | 0.005 |
| LPC 18:3 | 0.70 | 0.003 | PC-O 36:3 | 1.28 | 0.012 | |||
| LPC 20:2 | 0.64 | 0.008 | PC-O 36:4 | 1.31 | 0.012 |
The serum lipids are expressed in a pattern of name carbon numbers: double bond numbers. FFA, free fatty acids; LPC, lysophosphatidylcholine; LPC-O, lysophosphatidylcholine plasmalogen; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PC-O, choline plasmalogen; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SM, sphingomyelin.
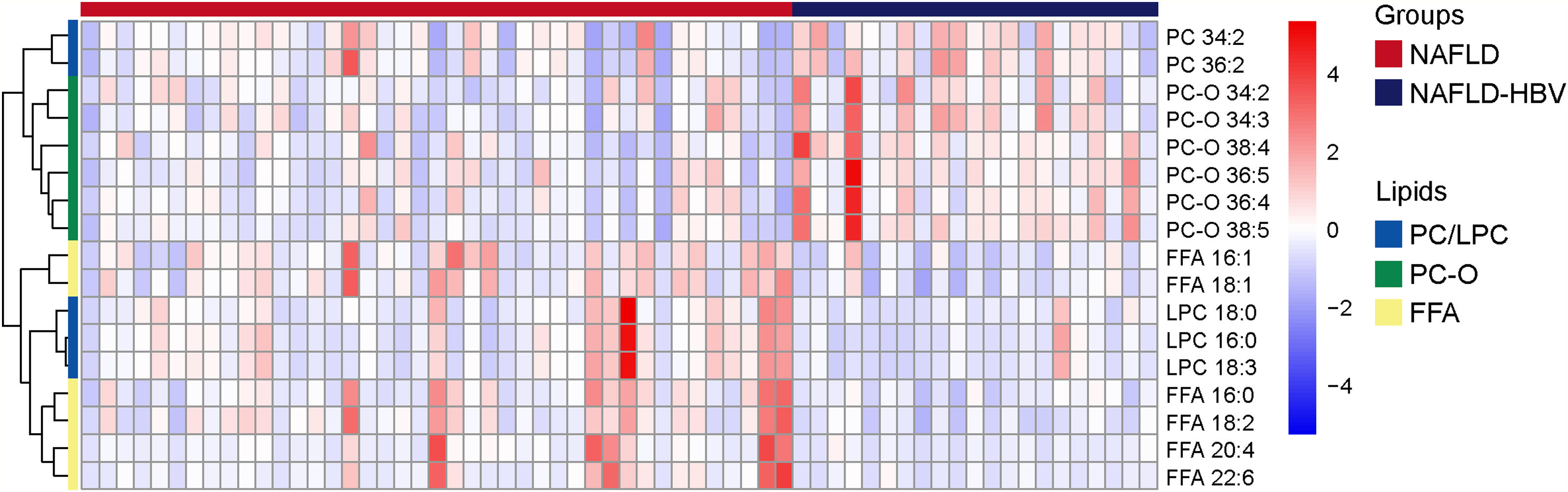
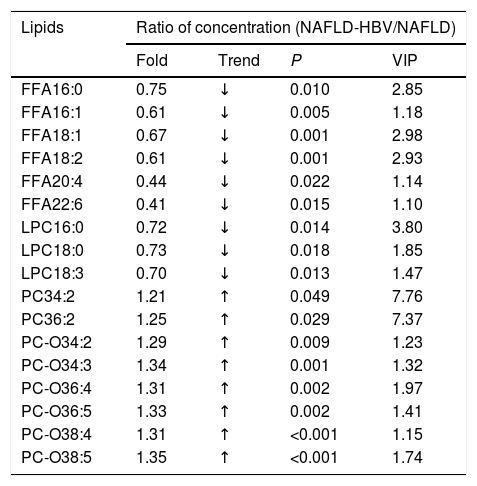
Integrating unpaired Student’s t-test and S-plot, 17 differential serum lipids with P < 0.05 and VIP > 1.0 were identified to characterize the lipidomics of NAFLD patients upon chronic HBV infection. They were classified into FFAs (FFA 16:0, FFA 16:1, FFA 18:1, FFA 18:2, FFA 20:4, FFA 22:6), LPCs (LPC 16:0, LPC 18:0, LPC 18:3), PCs (PC 34:2, PC 36:2), and PC-Os (PC-O 34:2, PC-O 34:3, PC-O 36:4, PC-O 36:5, PC-O 38:4, PC-O 38:5), respectively (Table 3). When compared to those of the NAFLD group, serum PCs (NAFLD-HBV/NAFLD: 1.21–1.25) and PC-Os levels (NAFLD-HBV/NAFLD: 1.29–1.35) in the NAFLD-HBV group exhibited significant upregulation (Table 3, Fig. 3). Contrastively, serum levels of FFAs (NAFLD-HBV/NAFLD: 0.41–0.75) and LPCs (NAFLD-HBV/NAFLD: 0.70–0.73) experienced statistical downregulation in the NAFLD patients with concurrent chronic HBV infection (Table 3, Fig. 3). These characteristics convinced the dominating role of FFAs, LPCs, PCs and PC-Os in differential serum lipidomics.
Concentration ratio of serum lipids between NAFLD and NAFLD-HBV groups (P < 0.05, VIP > 1).
| Lipids | Ratio of concentration (NAFLD-HBV/NAFLD) | |||
|---|---|---|---|---|
| Fold | Trend | P | VIP | |
| FFA16:0 | 0.75 | ↓ | 0.010 | 2.85 |
| FFA16:1 | 0.61 | ↓ | 0.005 | 1.18 |
| FFA18:1 | 0.67 | ↓ | 0.001 | 2.98 |
| FFA18:2 | 0.61 | ↓ | 0.001 | 2.93 |
| FFA20:4 | 0.44 | ↓ | 0.022 | 1.14 |
| FFA22:6 | 0.41 | ↓ | 0.015 | 1.10 |
| LPC16:0 | 0.72 | ↓ | 0.014 | 3.80 |
| LPC18:0 | 0.73 | ↓ | 0.018 | 1.85 |
| LPC18:3 | 0.70 | ↓ | 0.013 | 1.47 |
| PC34:2 | 1.21 | ↑ | 0.049 | 7.76 |
| PC36:2 | 1.25 | ↑ | 0.029 | 7.37 |
| PC-O34:2 | 1.29 | ↑ | 0.009 | 1.23 |
| PC-O34:3 | 1.34 | ↑ | 0.001 | 1.32 |
| PC-O36:4 | 1.31 | ↑ | 0.002 | 1.97 |
| PC-O36:5 | 1.33 | ↑ | 0.002 | 1.41 |
| PC-O38:4 | 1.31 | ↑ | <0.001 | 1.15 |
| PC-O38:5 | 1.35 | ↑ | <0.001 | 1.74 |
The serum lipids are expressed in a pattern of name carbon numbers: double bond numbers. FFA, free fatty acids; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PC-O, choline plasmalogen. VIP, variable importance in the projection.
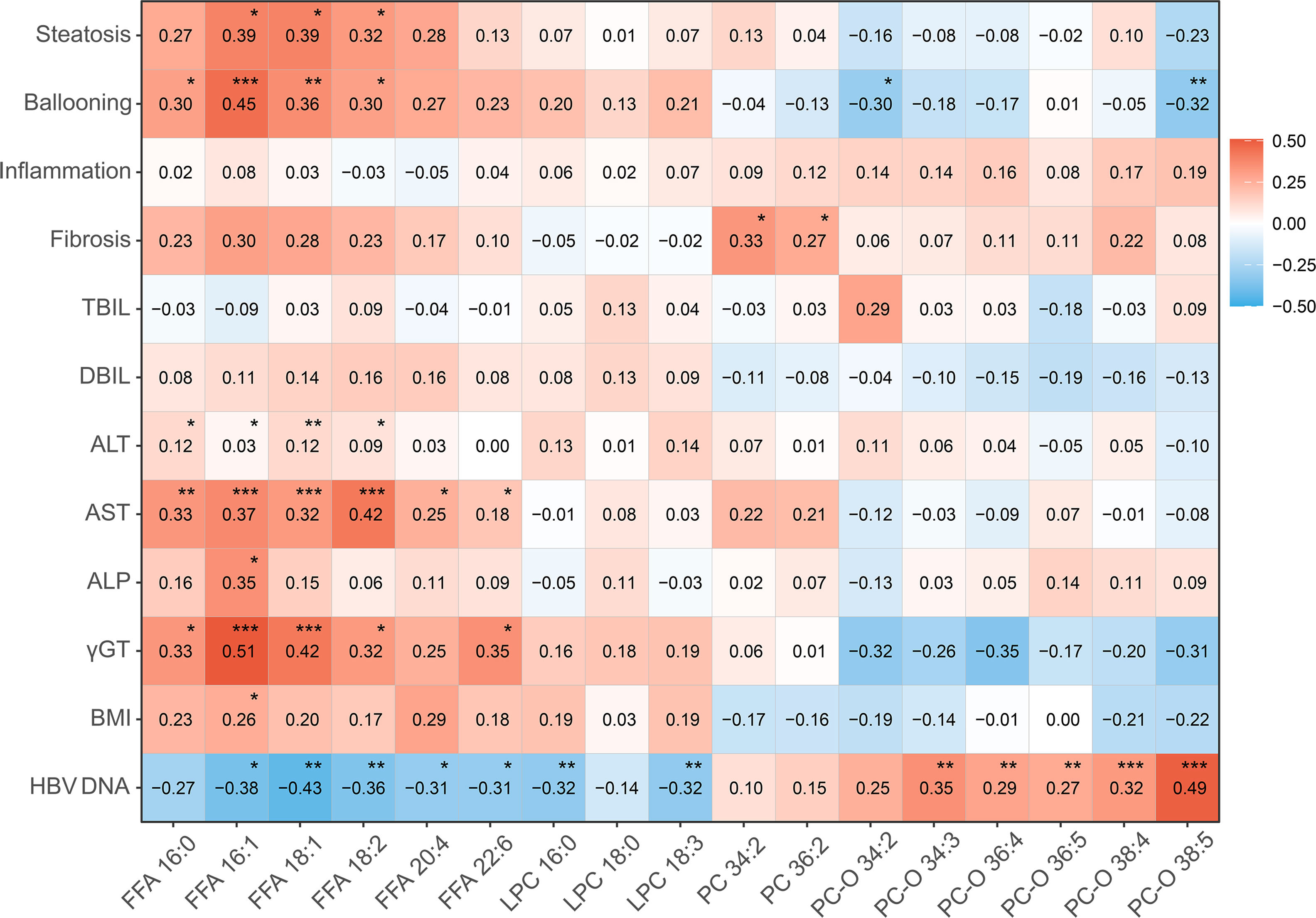
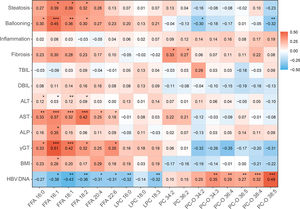
Correlation between differential serum lipids (FFAs, LPCs, PCs and PC-Os), biochemical indices (TBIL, DBIL, ALP, γ-GT, ALT and AST), and HBV DNA level, together with NAFLD-specific pathological characteristics (hepatocyte steatosis, ballooning, lobular inflammation, fibrosis), was subjected to assessment in this study. Noticeably, HBV DNA level was negatively associated with the hepatoxic lipids (FFA16: 1, r = −0.38, P = 0.015; FFA18:1, r = −0.43, P = 0.006; FFA18:2, r = −0.36, P = 0.006; LPC 16:0, r = −0.32, P = 0.004; LPC 18:3, r = −0.32, P = 0.003) and was positively associated with the lipids with antioxidant property (PC-O 34:3, r = 0.35, P = 0.009; PC-O 36:4, r = 0.29, P = 0.004; PC-O 36:5, r = 0.27, P = 0.006; PC-O 38:4, r = 0.32, P < 0.001; PC-O 38:5, r = 0.49, P < 0.001). Furthermore, the low-level FFAs among these lipids showed close association with lessened hepatocyte steatosis (FFA16: 1, r = 0.39, P = 0.010; FFA18:1, r = 0.39, P = 0.015; FFA18:2, r = 0.32, P = 0.036) and ballooning (FFA16:0, r = 0.30, P = 0.037; FFA16:1, r = 0.45, P < 0.001; FFA18:1, r = 0.36, P = 0.005; FFA18:2, r = 0.30, P = 0.013) (Fig. 4). Additionally, the reduction of ALT (FFA16: 0, r = 0.12, P = 0.043; FFA16: 1, r = 0.03, P = 0.020; FFA18:1, r = 0.12, P = 0.004; FFA18:2, r = 0.09, P = 0.017) and AST activities (FFA16: 0, r = 0.33, P = 0.003; FFA16: 1, r = 0.37, P < 0.001; FFA18:1, r = 0.32, P < 0.001; FFA18:2, r = 0.42, P < 0.001) in patients with low-level FFAs reflected an attenuation of steatosis-related hepatic impairments (Fig. 4). The down-regulatory effect of chronic HBV infection on FFAs, and the association of low-level FFAs and steatosis improvements, resultantly convinced a beneficial role of chronic HBV infection in the serum lipidomics and related NAFLD.
Correlations between differential serum lipids and pathological and clinical data in HBV-NAFLD patients. TBIL, total bilirubin; DBIL, direct bilirubin; ALP, Alkaline phosphatase; γGT, Gamma-glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase. * P < 0.05, ** P < 0.01, *** P < 0.001.
In contrast to previous concept of the less connection of HBV and glycolipid metabolism, accumulating proofs shed light on the fact that chronic HBV infection deeply involves in the metabolic profiles and, subsequently, affects multiple components of MetS [3,4,6–9,19,20]. When compared to the uninfected control subjects, those with chronic HBV infection demonstrate a decreased prevalence of hypertriglyceridemia and lowered level of serum TG [3,7]. Whereas hypertriglyceridemia inversely associates with the viral load in HBeAg seropositives [20]. There is also a negative correlation between HBV viral load (HBV-DNA) and serum TG level [7]. After adjusting for demographic and metabolic factors, HBV infection is now recognized to be the independent factor associated with lower risk of NAFLD, mainly attributed to the HBV-related reduction of serum and intrahepatic TG concentration [8,9,20]. Some other MetS components, including hypercholesterolemia and high blood pressure, are likely to be improved in the patients with HBV seropositivity [3,9,21,22]. Furthermore, both Third Korean National Health and Nutrition Examination Survey (KNHANES III) and cross-sectional population study in Hong Kong Chinese uncovered an association of HBsAg positivity and low prevalence of MetS [4,6,8]. In similar, HBV infection was associated with lower risk of dyslipidaemia after adjusting for BMI and exercise [23].
Clinical trials have recently shown that NAFLD takes place on the basis of chronic HBV infection with an increasing annual prevalence [24], yet the viral impact on lipid metabolism, hepatic steatosis and related impairments remains to be explored. In the present study that integrated ready-made clinical, lipidomic, and pathological data, we identified 26.78% (64/239) differential lipids in the serum lipidomics of NAFLD-HBV group in comparison to those of NAFLD group. The wide-range alterations verified by P < 0.05 and/or VIP > 1.0, including differential FFAs, LPCs, LPEs, LPIs, PCs, PC-Os, PEs, PE-Os, and SMs, confirmed the changes of serum lipidomics upon chronic HBV infection in the NAFLD patients. Moreover, both 3D PCA and OPLS-DA score plots for serum lipidomics distinctly differentiated NAFLD patients with or without chronic HBV infection. Thus chronic HBV infection is suggested to shape, to some extent, the feature of serum lipidomics.
To take further insight into the HBV-specific serum lipodomics, we assessed the differential lipids obtained from Student’s t - test and S-plot. When compared to those of the NAFLD group, upregulation of PCs, PC-Os and downregulation of FFAs, LPCs resultantly characterized the lipidomics of NAFLD-HBV group with statistical significance. Serving as inhibitors of hepatic lipogenesis, PCs induce the alleviation of orotic acid-induced rodent hepatocyte steatosis [25]. On the other hand, PCs take an essential place in the membrane integrity [26,27]. They prevent the membrane leakage to abolish hepatocyte injury and, subsequently, lobular inflammation and liver fibrosis [28,29]. PC-Os are a class of phospholipids that contain a vinyl ether linkage at the sn-1 position and highly arachidonic acid at the sn-2 position. They have been reported to act as potential protector against oxidative stress [30,31]. Contrastively, NAFLD patients demonstrate high serum level of FFAs, which are described to be cytotoxic and potential in the early diagnosis [32,33]. LPCs, a kind of lipid intermediate elevated in rodent and human nonalcoholic steatohepatitis (NASH), mediate the interaction of saturated fatty acid and insulin resistance [34,35]. Given their hepatic activities, these differential lipids are conferred to interact with lipid metabolism and related pathological alterations in the liver.
We further investigate the interaction between serum lipidomics of PCs, PC-Os, FFAs, LPCs and both biochemical and pathological indices in patients with concurrent chronic HBV infection and NAFLD. In the multivariate model of our study, HBV DNA positivity was significantly associated with lowered serum lipotoxic lipids such as FFA16: 1, FFA18:1 and FFA18:2. These low-level FFAs of 16:1, 18:1, and 18:2 showed close association with alleviated hepatocyte steatosis. Consistently, FFAs reduction was accompanied by the improvements in steatosis-based hepatic injury (ballooning, down-regulated aminotransferase activities). With their reflux from adipose tissue to the liver, FFAs lead to lipotoxicity that contributes to hepatic steatosis and related impairments [36]. On the contrary, FFAs reduction upon chronic HBV infection abrogates these lipotoxicity-induced abnormalities. Taken together, chronic HBV infection may be presented to have beneficial impact on the lipid metabolism and steatosis-related liver impairments, likely on the basis of lipidomic improvements.
Despite these metabolic benefits observed in patients with chronic HBV infection, there are still some controversial results that HBx facilitates hepatic steatosis in both high fat-stimulated hepatocellular model and HBx-transgenic mice, perhaps by the activation of liver X receptor (LXR)/sterol regulatory element-binging protein (SREBP)-1c signaling [37]. Peng et al. also present a higher prevalence of hepatic steatosis in patients with chronic HBV infection rather than healthy controls with correlation to increased BMI [38]. The difference in clinical manifestation of chronic HBV infection and HBV component (e.g., HBx) overexpression may be attributed, to some extent, to the acquisition of health consciousness and healthy dietary habits (e.g, less low-nutrient foods, less high-fat sources of meat/protein, less high-sodium foods) in patients [39]. Age- and obesity-related metabolic disorder may serve as another explanation for these disputes.
There are some limitations in this study. First, our study was performed on a limited-number, case-control basis. In spite of an intimate association between chronic HBV infection and serum lipidomics had been uncovered, factors underlying such effects require further investigation in a multi-center, large-scale population study. Second, the effects of HBV infection on lipid metabolism may differ in phases of immune tolerance, immune clearance, immune reactivation and inactive carrier [40]. In addition, the non-target method of lipidomic analysis kept serum lipids from absolute quantification. Moreover, the position of double bonds has not been identified in various kinds of multiple unsaturated serum lipids. Finally, some potential metabolic confounding factors (e.g., total calorie intake, physical activity) could not be fully excluded in the present study. These limitations should be taken into consideration for an interpretation of our findings.
5ConclusionsIn summary, chronic HBV infection may exert global effect on serum lipidomics of NAFLD patients. Alteration of FFAs, LPCs, PCs and PC-Os dominates the HBV-related lipidomic characteristics. Low-level FFAs (FFA16: 0, FFA16:1, FFA18:1, FFA18:2) upon chronic HBV infection demonstrate association with significant improvements in hepatocyte steatosis, ballooning, and decreased aminotransferase activities. The effects of chronic HBV infection on lipid metabolism deserve our further concerns of its potential role in multiple dyslipidemia-related disorders, such as coronary artery disease, metabolic syndrome and type 2 diabetes.
FundingThis work is supported by the National Key Research and Development Plan ‘Precision Medicine Research’ (2017YFC0908903), and the National Natural Science Foundation of China (81270492, 81470859).
Authors’ contributionsHL and QYX contributed to study concept, study design, data interpretation and original draft writing. YX and JJL contributed to data collection, data analysis and data interpretation. HXC and QP contributed to resources, conceptualization, funding acquisition, supervision, writing-review and editing.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank Guo-Wang Xu and Chen-Xiu Hu of Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences for technical assistance.