Cardiovascular disease (CVD) is the major cause of death in non-alcoholic fatty liver disease (NAFLD), a clinical condition without any approved pharmacological therapy. Probiotics are often indicated for the disease, but their results are controversial in part due to the poor quality of studies. Thus, we investigated the impact of 24-week probiotics supplementation on cardiovascular risk (CVR) in biopsy-proven non-alcoholic steatohepatitis (NASH) patients.
Patients and MethodsDouble-blind, placebo-controlled, single-center study (NCT03467282), adult NASH, randomized for 24 weeks daily sachets of probiotic mix (109CFU of Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei and Bifidobacterium lactis) or placebo. Clinical scores (atherogenic indexes, atherosclerotic cardiovascular disease-ASCVD and systematic coronary risk evaluation-SCORE), biochemistry, miR-122, miR-33a, plasminogen activator inhibitor-1 (PAI-1), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), were determined before and after the intervention.
ResultsForty-six patients were enrolled (23 received probiotics and 23 placebo), with a mean age of 51.7 years, most of them females and whites. Clinical and demographic features were similar between the groups at the baseline. The Median NAFLD activity score was 4.13 in both groups. Fibrosis was mild in most patients (15.2% and 65.2% F0 and F1, respectively). Treatment did not promote any clinically significant changes in body mass index or laboratory, including lipid and glucose profile. High CVR patients through atherogenic indexes decreased from baseline in both groups, as well as PAI-1 and miR-122 levels, although there was no difference between probiotics and placebo.
ConclusionsA 24-week probiotic mix administration was not superior to placebo in reducing CVR markers in patients with NASH.
Non-alcoholic fatty liver disease (NAFLD), recently renamed metabolic-associated fatty liver disease (MAFLD) [1], is the leading cause of chronic liver disease worldwide [2], with an estimated prevalence between 30-40% in the general population [3, 4]. The increase in the prevalence of this disease has caused a great impact on the clinical and economic burden on society, such that nearly 1 billion people globally are affected [5]. The term NAFLD comprises liver conditions varying in the severity of the injury as hepatic steatosis and non-alcoholic steatohepatitis (NASH). The more advanced the disease (NASH, especially with fibrosis), the greater the cardiovascular risk (CVR) [6].
Cardiovascular disease (CVD) is the major cause of death in NAFLD patients [7], independently of other traditional CVR factors or metabolic syndrome (MS) [4, 8]. Abnormalities in microRNAs, inflammatory and endothelial injury markers, as well as gut dysbiosis can alter NAFLD and CVR [9–12]. Inflammation has been linked to coronary syndrome [13]. Soluble forms of vascular adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) are over-regulated in patients with higher hepatic fat content, suggesting greater endothelial dysfunction and vascular injury [14], as well as endothelial markers such as plasminogen activator inhibitor-1 (PAI-1) [15] and pro-coagulation factors such as fibrinogen [15]. NAFLD is also associated with the dysregulation of many microRNAs, responsible for the regulation of gene expression at the post-transcriptional level and also the synthesis of pro-inflammatory cytokines, culminating in the influx of inflammatory cells into the liver and activation of stellate cells, mechanisms linked to the progression of the clinical picture [16–18]. miR-122 is a liver-characteristic miRNA that composes about 70% of the total miRNAs found in normal hepatocytes, most probably due to the fact that it positively regulates the accumulation of cholesterol and triglycerides and the metabolism of fatty acids [19]. Additionally, miR-33a inhibits genes involved in HDL synthesis and reverse cholesterol transport, which may contribute to the development of NAFLD-related metabolic disorders and CVD [16].
Probiotics are living microorganisms that provide benefits to the host [20]. They can modulate the pathways of liver inflammation and improve lipid and glycemic profiles [21]. Despite their importance, CVD and CVR are outcomes rarely evaluated in NASH studies [22–25]. The effect of probiotics on human CVR is still conflicting [10, 26-28] and studies focusing CVR effects of probiotics in NAFLD patients are lacking. This study aims to evaluate the impact of probiotics supplementation on CVR markers in patients with biopsy-proven NASH.
2Patients and methodsTriple-blind, randomized, placebo-controlled, single-center study carried out in a university hospital in southern Brazil - NCT03467282 with the aim of evaluating CVR as the primary outcome through clinical scores, microRNAs, inflammatory and adherence molecules. Adult patients (>18 years) presenting with NASH (liver biopsy less than one year before inclusion) were enrolled from January to June 2018. Patients with cirrhosis and those infected with human immunodeficiency, hepatitis B or hepatitis C virus, with significant alcohol intake (> 15g ethanol/day) were excluded, as well as pregnant women, transplant recipients, patients using immunosuppressant, corticosteroids, valproic acid, tetracycline and amiodarone, and those carriers of other chronic inflammatory diseases and history of diarrhea. Patients using antibiotics were also excluded or included after three months of withdrawal. Potentially eligible patients were identified at the NAFLD outpatient clinic. The patient's eligibility was confirmed by the responsible researcher. As there are no previous studies considering CVR in NASH patients receiving probiotics, a convenience sample was used. This study is registered at ClinicalTrials.gov as NCT0346782.
2.1Randomization and interventionPatients were allocated through a randomization list made by an online program (randomization.com website) being divided into two groups: intervention (probiotics) and control (placebo). Patients allocated to the intervention group received mixed probiotics supplementation, which consists of a 1g sachet containing Lactobacillus acidophilus NCFM (1 × 109 CFU) + Lactobacillus rhamnosus HN001 (1 × 109 CFU) + Lactobacillus paracasei LPC-37 (1 × 109 CFU) + Bifidobacterium lactis HN019 (1 × 109 CFU) while those allocated to the placebo group received a 1g sachet with an identical appearance (physical and organoleptic) containing polydextrose/maltodextrin as the placebo. Patients were instructed to ingest two sachets daily with water at room temperature for a period of 24 weeks. All patients received diet and physical activity general guidelines. Every 45 days, patients were instructed to return for a follow-up appointment, carrying notes on a standard spreadsheet to check adherence to the supplement, possible side effects, or even unusual medications (the participants were instructed to advise the research team about the need to use any other non-routine medications and to inform the team when they use a product that contains probiotics).
Patients and the researchers administering the study did not know the composition of each sachet of supplements and the participant's allocation treatment. An external researcher was unblinded. Researchers will know which supplements each participant received only at the end of the study. The external researcher was informed about the composition of each supplement if needed.
2.2Diagnosis and liver histologyLiver biopsy was done in the Hospital setting and reviewed for histological examination by a blinded pathologist, graded through NASH-Clinical Research Network, NAFLD activity score (NAS) and steatosis-activity-fibrosis (SAF) [29, 30].
2.3Clinical and physical evaluationA medical doctor carried out the evaluations. Clinical evaluation included data on age, sex and ethnicity. The presence of T2DM, hypertension, hypothyroidism, dyslipidemia with or without treatment and current or previous smoking was seen before the interventions started and after the 24-week follow-up. Hypertension, dyslipidemia and T2DM diagnosis followed the 2018, 2016 and 2013 European Society of Cardiology guidelines, respectively [31–33]. All medications taken by the patient were analyzed. Family history of coronary artery disease in a first-degree relative [34] was asked. The presence of acute myocardial infarction, coronary syndrome, previous arterial revascularization and stroke were analyzed before the study. During the same, the presence of coronary syndrome or the presence of cardiovascular events was seen.
Anthropometric variables (body mass index [BMI] and waist circumference [WC]) were evaluated before and after the follow-up period by experienced nutritionists. Blood pressure was always checked by the same researcher. For the diagnosis of MS, the presence of ≥ 3 of the criteria presented in the study conducted by Alberti et al. [35], and for the purpose of WC the database of the ELSA study (Longitudinal Study of Adult Health) was used (men ≥ 92 cm, women ≥ 86 cm) [36].
2.4Physical-activity assessmentThe International Physical Activity Questionnaire – Short Form (IPAQ) was applied to evaluate the weekly time spent in physical exercise before the interventions start and after de 24-week follow-up period [37].
2.5Laboratory assessmentWe conducted blood laboratory analysis before and after the intervention, consisting of complete blood count, liver enzymes, factor VII, fibrinogen, C-reactive protein (CRP) lipid and glucose profile, and these evaluations were performed using the Labmax 560 equipment. Insulin resistance was determined by the homeostasis model assessment for insulin resistance (HOMA-IR) [38].
2.6Cardiovascular risk scoresThe CVR scores were assessed before and after the intervention. The American College of Cardiology Atherosclerotic Cardiovascular Disease (ASCVD) score was used. Patients were classified as low CVR (<5%), borderline (5-7.4%), intermediate (7.5 - 19.9%) and high CVR (≥ 20%) [39]. Framingham score was also calculated for all patients, classifying them in low CVR (≤ 10%), intermediate (10-20%), or high CVR (≥ 20%) [40]. Additionally, the European Society of Cardiology (SCORE) was performed, categorizing patients into different grades: very high ≥ 10%, high ≥ 5% and <10%, moderate ≥ 1 and < 5%, and low CVR < 1% [41]. The presence of altered high-density lipoprotein (HDL) and elevated WC was also investigated; the latter was seen using the usual patterns [35] and the ELSA study [36].
Atherogenic ratios were calculated using the results of the lipid profile to predict CVR. Lipid profile included low-density lipoprotein (LDL), HDL and total cholesterol (TC). Such ratios were calculated in the following ways: Castelli's Risk Index (CRI)-I = TC/HDL, CRI-II= LDL/HDL and Atherogenic Coefficient (AC) = (TC–HDL)/HDL [42]. The cut-off values for atherogenic indices were obtained from previous studies and stratified by sex [43, 44]. Low risk was considered if CRI-I > 3.5 for men and > 3.0 for women; CRI-II and AC values were considered low risk when less than 3.0 and 2.0, respectively, for both sexes [45].
2.7Analysis of markers of endothelial dysfunctionTo detect serum changes before and after the intervention in the endothelial dysfunction markers, we analyzed ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) using the multiplex assay of the Luminex platform (Millipore, Germany). The results were expressed in ng/mL. Serum evaluation of the PAI-1 was performed using the ELISA kit (Invitrogen, USA). The absorbance was measured in a spectrophotometer at a wavelength of 450 nm (Zenyth 200 rt) and the results were expressed in pg/mL. All processes were performed according to the manufacturer's instructions and analyzes were performed in duplicate.
2.8Analysis of the circulating microRNAsTotal RNA was extracted from serum using miRNeasy serum/plasma kit (Qiagen, USA) to analyze the circulating microRNAs before and after the intervention. Then, cel-miR-39 (1.6 × 108 copies) spike in control (Qiagen, USA) was added to provide an internal reference. cDNA conversion was performed from 10ng of total RNA using the TaqMan microRNA Reverse Transcription kit (Applied Biosystems, USA). Analysis of the gene expression of miR-122 and miR-33a, together with the cell-miR-39 normalizer, was performed by RT-qPCR using TaqMan assay (Applied Biosystems, USA). The sequences and codes of the assessed microRNAs are described in Supplementary Table 1. Values were calculated by formula 2 −(ΔΔCt)[46].
2.9Sample size and statistical analysisSample size estimation was performed using the WINPEPI 11.20 program (Brixton Health, Israel), based on a published study that found a mean reduction in fibrosis score of 9.36 ± 1.9 to 6.38 ± 1.5 in NAFLD patients taking symbiotic supplementation (p < 0.001, compared to placebo) [47]. Thus, considering a power of 90% and a significance of 5%, adding 10% to compensate for eventual losses, it will be necessary to include 46 patients with NAFLD in the present study.
Normality was verified for all variables using the Shapiro-Wilk test and histograms. Quantitative variables were described as mean and standard deviation / standard error and categorical variables as absolute and relative frequencies. Comparisons over time were evaluated using the Generalized Estimation Equations model complemented by the Least Significant Difference test. The comparison of means was performed by the t-student test and the comparison of proportions by the chi-square test or Fisher's exact test. The level of significance adopted was 5% (p <0.05) and the analyzes were performed using the SPSS version 21.0 program.
2.10Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Hospital de Clínicas de Porto Alegre Ethics Committee (CAAE 86120718.6.0000.5327 and CAAE 97777318.2.0000.5327).
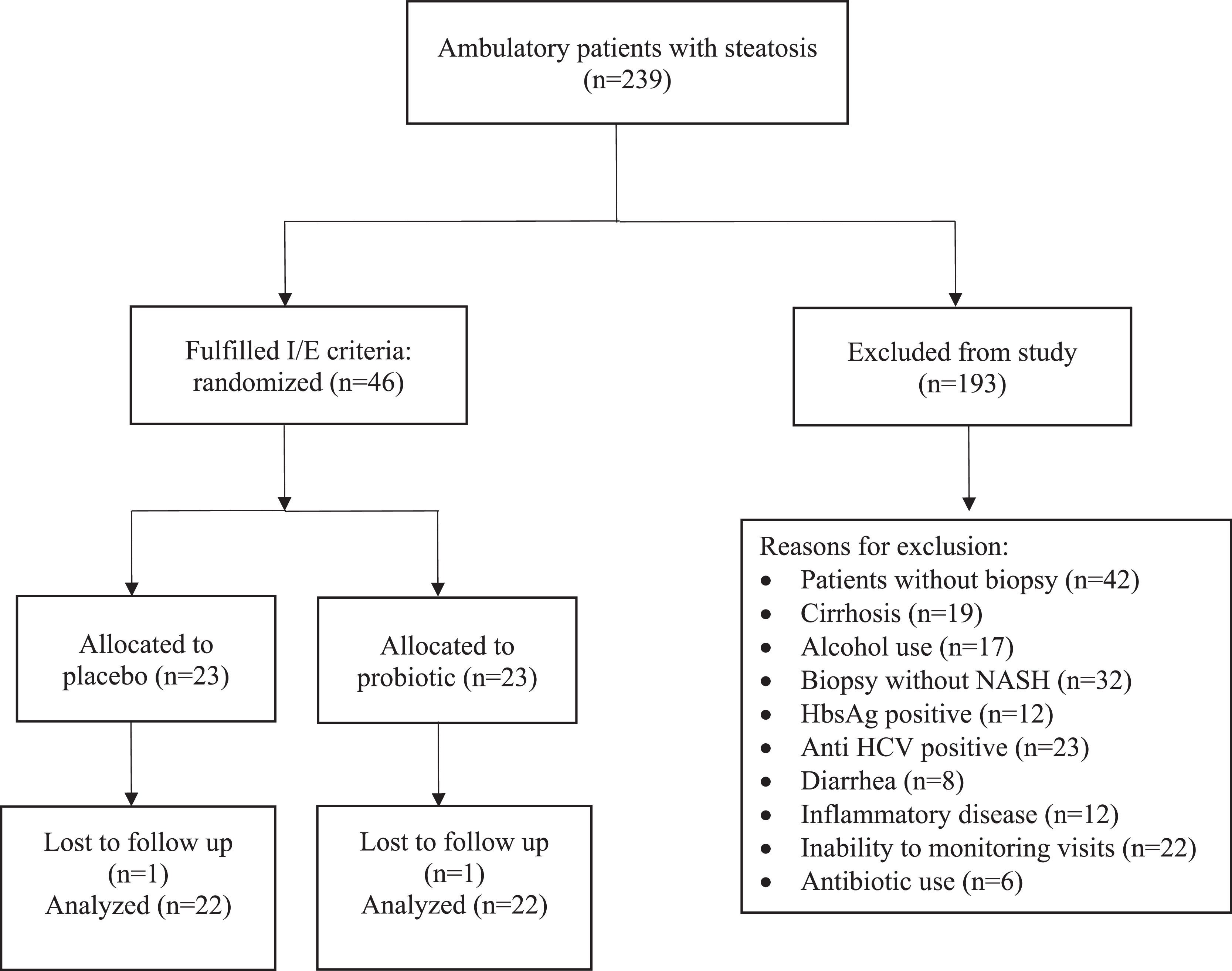
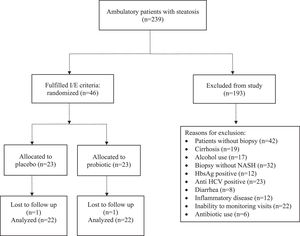
3ResultsDuring the study period, 239 patients were evaluated, of which 46 met the inclusion criteria, were randomized and effectively underwent the intervention (Fig. 1). During the study, one patient was lost to follow-up in each group.
The clinical and demographic baseline data of the patients under study are shown in Table 1. The study sample had a mean age of 51.7 years and a predominance of females and white ethnicity in both groups. As shown, there was no statistically significant difference between the placebo and the probiotics group regarding BMI, WC, smoking, cardiovascular disease, MS features and exercise, although patients in the placebo group presented a higher percentage of T2DM and MS than those in the probiotics group. There was also a statistical difference concerning the presence of previous cardiovascular disease, with predominance in the placebo group, and although the randomization could not separate those patients as equal, we adjusted the cardiovascular variables for these features.
Clinical and demographic data of patients under study
| Variables# | Placebo (n = 23) | Probiotic (n = 23) | p |
|---|---|---|---|
| Age (years) | 51.7 ± 11.9 | 51.7 ± 11.4 | 0.990 |
| Gender | |||
| Female | 12 (52.2) | 15 (65.2) | 0.549 |
| Male | 11 (47.8) | 8 (34.8) | |
| Ethnicity | |||
| White | 19 (82.6) | 20 (87.0) | 1.000 |
| Non-white | 4 (17.4) | 3 (13.0) | |
| Menopause | 9 (64.3) | 9 (60.0) | 1.000 |
| Mean WC | 104.2 ± 11 | 105.143 ± 13.1 | 0.801 |
| Mean BMI | 32.3 ± 5.6 | 32.852 ± 7.1 | 0.767 |
| Smoking | |||
| Current | 1 (4.3) | 1 (4.3) | 1.000 |
| Previous | 4 (17.4) | 9 (39.1) | 0.190 |
| Previous cardiovascular disease | 6 (26.1) | 1 (4.3) | 0.029 |
| Atherosclerosis* | 3 (13) | 4 (17.4) | 1.000 |
| Family history⁎⁎ | 6 (26.1) | 7 (30.4) | 1.000 |
| Hypothyroidism | 3 (13) | 6 (26.1) | 0.457 |
| Dyslipidemia | 11 (47.8) | 11 (47.8) | 1.000 |
| Hypertension | 14 (60.9) | 16 (69.6) | 0.757 |
| Diabetes | 13 (56.5) | 9 (39.1) | 0.376 |
| Metabolic syndrome | 19 (82.6) | 16 (69.6) | 0.489 |
| IPAQ | |||
| Low | 9 (39.1) | 9 (39.1) | 0.909 |
| Moderate | 10 (43.5) | 11 (47.8) | |
| High | 4 (17.4) | 3 (13.0) |
Baseline liver biopsy findings are shown in Table 2. There was no significant difference between the two groups. All included patients had NASH (NAS 4.13 ± 0.87 - placebo group; and 4.13 ± 0.87 - probiotics group; p = 1.000). Most cases were of absent (F0) or mild (F1) fibrosis (19 - placebo group; 18 - probiotics group; p = 0.730). Only five patients taking placebo and four receiving probiotics had intermediate (F2) or advanced (F3) fibrosis (p = 0.730). Twenty-three patients received placebo and twenty-three patients received probiotics. At the end of 24 weeks, 44 patients completed the study. Antibiotics were used by six patients in the placebo group (26.1%) and four (17.4%) in the probiotics group (p = 0.721). No patient has any adverse event.
Histological findings assessed according to NAS and SAF
| Variables# | Placebo (n = 23) | Probiotic (n = 23) | p |
|---|---|---|---|
| NAS | 4.13 ± 0.87 | 4.13 ± 0.87 | 1.000 |
| Steatosis | |||
| Mild | 9 (39.1) | 9 (39.1) | 0.654 |
| Moderate | 10 (43.5) | 12 (52.2) | |
| Severe | 4 (17.4) | 2 (8.7) | |
| Lobular inflammation | |||
| Mild | 17 (73.9) | 20 (87.0) | 0.459 |
| Moderate | 6 (26.1) | 3 (13.0) | |
| Ballooning | |||
| Mild | 21 (91.3) | 16 (69.6) | 0.135 |
| Moderate | 2 (8.7) | 7 (30.4) | |
| SAF score | |||
| S - Steatosis | |||
| 1 | 9 (39.1) | 11 (47.8) | 0.799 |
| 2 | 11 (47.8) | 10 (43.5) | |
| 3 | 3 (13.0) | 2 (8.7) | |
| A - Inflammation activity | |||
| 1 | 4 (17.4) | 3 (13) | 0.841 |
| 2 | 11 (47.8) | 14 (60.9) | |
| 3 | 7 (30.4) | 5 (21.7) | |
| 4 | 1 (4.3) | 1 (4.3) | |
| F - Fibrosis | |||
| 0 | 4 (17.4) | 3 (13.0) | 0.730 |
| 1 | 14 (60.9) | 16 (69.6) | |
| 2 | 1 (4.3) | 2 (8.7) | |
| 3 | 4 (17.4) | 2 (8.7) |
The mean weight, BMI and WC were not statistically different between placebo and probiotics before and after the intervention, as well as mean systolic and diastolic blood pressure and IPAQ (Supplementary Table 2 and Supplementary Fig. 1). The baseline and final laboratory are shown in Table 3 and Supplementary Table 3. As for the findings referring to CRP, creatine kinase, fibrinogen and factor VII, there was no difference after intervention in both groups (Supplementary Table 4). Combining high WC and low HDL (according to the International Diabetes Federation or ELSA Study recommendations), there were no differences between the two groups either in the baseline or after intervention (Supplementary Table 5).
Comparison of laboratory tests before and after intervention
| Variables# | Placebo (n = 23) | Probiotic (n = 23) | p |
|---|---|---|---|
| AST (U/L) | |||
| Before | 39.3 ± 4.80 | 38.2 ± 5.34 | 0.880 |
| After | 37.6 ± 5.65 | 36.6 ± 4.18 | 0.887 |
| Difference % (CI 95%) | -1.66 (-7.41 to 4.07) | -1.57 (-10.2 to 7.12) | 0.994 |
| p | 0.570 | 0.722 | |
| ALT (U/L) | |||
| Before | 50.8 ± 5,24 | 49.6 ± 8.13 | 0.896 |
| After | 50.0 ± 6,99 | 49.4 ± 7.91 | 0.959 |
| Difference % (CI 95%) | -0.87 (-12.0 to 10.3) | -0.15 (-12.1 to 11.8) | 0.933 |
| P | 0.879 | 0.980 | |
| GGT (U/L) | |||
| Before | 101.1 ± 46.7 | 81.8 ± 19.6 | 0.704 |
| After | 114.8 ± 61.0 | 98.7 ± 32.1 | 0.816 |
| Difference % (CI 95%) | 13.6 (-16.1 to 43.4) | 16.9 (-17.3 to 51.1) | 0.735 |
| p | 0.369 | 0.333 | |
| Fasting glucose (mg/dL) | |||
| Before | 124.8 ± 9.32 | 103.8 ± 6.34 | 0.063 |
| After | 120.8 ± 6.45 | 110.7 ± 7.50 | 0.310 |
| Difference % (CI 95%) | -4.00 (-17.1 to 9.09) | 6.90 (-3.45 to 17.2) | 0.200 |
| p | 0.549 | 0.191 | |
| Insulin (lU/L) | |||
| Before | 17.1 ± 1.94 | 22.6 ± 3.99 | 0.213 |
| After | 17.6 ± 2.19 | 19.46 ± 2.96 | 0.619 |
| Difference % (CI 95%) | 0.464 (-2.42 to 3.35) | -3.22 (-7.61 to 1.16) | 0.162 |
| p | 0.753 | 0.150 | |
| HbA1C (%) | |||
| Before | 6.38 ± 0.29 | 6.10 ± 0.24 | 0.473 |
| After | 6.44 ± 0.26 | 6.44 ± 0.32 | 0.991 |
| Difference % (CI 95%) | 0.05 (-0.36 to 0.47) | 0.33 (0.01 to 0.65) | 0.292 |
| p | 0.801 | 0.037 | |
| HOMA IR | |||
| Before | 5.52 ± 0.94 | 5.65 ± 0.83 | 0.919 |
| After | 5.30 ± 0.80 | 5.70 ± 1.24 | 0.791 |
| Difference % (CI 95%) | -0.22 (-1.87 to 1.43) | 0.04 (-1.43 to 1.52) | 0.811 |
| p | 0.792 | 0.954 | |
| Total cholesterol (mg/dL) | |||
| Before | 173.0 ± 8.38 | 178.6 ± 6.46 | 0.593 |
| After | 171.7 ± 9.17 | 184.7 ± 8.65 | 0.304 |
| Difference% (CI 95%) | -1.22 (-11.4 to 8.96) | 6.07 (-3.09 to 15.2) | 0.297 |
| p | 0.813 | 0.194 | |
| HDL (mg/dL) | |||
| Before | 44.1 ± 2.60 | 46.7 ± 2.67 | 0.492 |
| After | 45.5 ± 2.98 | 46.9 ± 2.38 | 0.722 |
| Difference % (CI 95%) | 1.37 (-1.98 to 4.72) | 0.17 (-2.26 to 2.60) | 0.570 |
| p | 0.423 | 0.891 | |
| LDL (mg/dL) | |||
| Before | 97.9 ± 7.50 | 98.9 ± 6.26 | 0.920 |
| After | 95.5 ± 7.72 | 103.1 ± 7.71 | 0.483 |
| Difference % (CI 95%) | -2.48 (-10.5 to 5.60) | 4.20 (-4.73 to 13.1) | 0.277 |
| Triglycerides (mg/dL) | |||
| Before | 169.7 ± 17.5 | 179.0 ± 18.8 | 0.719 |
| After | 153.6 ± 16.2 | 170.0 ± 14.7 | 0.436 |
| Difference % (CI 95%) | -16.0 (-42.3 to 10.2) | -8.22 (-42.9 to 26.5) | 0.688 |
| p | 0.232 | 0.643 |
Variables described as mean ± standard deviation and confidence interval (CI 95%). ALT, alanine aminotransferase, AST, aspartate aminotransferase, GGT, gamma-glutamyl transferase, HbA1C, glycosylated hemoglobin, HDL, high-density lipoprotein, HOMA-IR, homeostasis model assessment for insulin resistance, LDL, low-density lipoprotein
The evaluation of the CVR by clinical scores in qualitative (considering only high-risk cases) form before and after the 24 weeks of intervention can be seen in Table 4. There was a significant decrease in CRI and AC in the placebo group (p = 0.045 and 0.048, respectively) and a smaller decrease in the probiotics group (p = 0.058 for both). There were no differences in the absolute value of CRI-I, CRI-II and AC between the groups (Supplementary Table 6). Applying ASCVD, SCORE and Framingham, there was no difference between groups (Supplementary Fig. 2). Endothelial lesion markers and microRNAs before and after intervention are shown in Table 5. There was no significant difference between the two groups. However, from the baseline to the end of the study, PAI-1 levels significantly (p < 0.001) decreased in both groups, as well as miR-122. After the intervention, the placebo and probiotic groups had no alteration of the CVR by ASCVD and SCORE. The variation of miR-122 and PAI-1 resulted in a decrease in CVR by 95% and 72.7%, respectively, in the probiotic group, without significance. However, in the placebo group, there was a decrease in CVR in 82.4% and 95.5% of patients with a variation of miR-122 and PAI-1, respectively, without significance.
Evaluation of the cardiovascular risk by clinical scores before and after intervention considering high-risk patients
| Variables# | Placebo (n = 23) | Probiotic (n = 23) | p |
|---|---|---|---|
| CRI-I: high risk | |||
| Before | 18 (78.3) | 22 (95.7) | 0.070 |
| After | 14 (63.6) | 18 (81.8) | 0.167 |
| Difference % (CI 95%) | -14.6 (-28.9 to 0.00) | -13.8 (-28.2 to 0.00) | 0.377 |
| p | 0.045 | 0.058 | |
| CRI-II: high risk | |||
| Before | 4 (17.4) | 0 (0.0) | 0.028 |
| After | 7 (31.8) | 3 (13.6) | 0.140 |
| Difference % (CI 95%) | 14.4 (-4.5 to 33.3) | 13.6 (-0.7 to 27.9) | 1.000 |
| p | 0.134 | 0.062 | |
| AC: high risk | |||
| Before | 19 (82.6) | 22 (95.7) | 0.146 |
| After | 15 (68.2) | 18 (81.8) | 0.290 |
| Difference % (CI 95%) | -14.4 (-28.7 to -0.00) | -13.8 (-28.2 to 0.00) | 0.428 |
| p | 0.048 | 0.058 |
Endothelial lesion markers and microRNAs before and after intervention
| Variables# | Placebo (n = 23) | Probiotic (n = 23) | p |
|---|---|---|---|
| PAI-1 (pg/ml) | |||
| Before | 5379.5 ± 108.1 | 5457.8 ± 86.4 | 0.572 |
| After | 4617.2 ± 230.4 | 4500.5 ± 179.3 | 0.689 |
| Difference % (CI 95%) | -762.3 (-1196.4 to -327.7) | -957.3 (-1229.4 to -685.2) | 0.456 |
| p | 0.001 | <0.001 | |
| VCAM-1 (ng/ml) | |||
| Before | 10.0 ± 0.54 | 9.73 ± 0.72 | 0.706 |
| After | 15.7 ± 1.05 | 15.8 ± 1.47 | 0.977 |
| Difference % (CI 95%) | 5.67 (3.86 to 7.48) | 6.06 (3.99 to 8.13) | 0.779 |
| p | <0.001 | <0.001 | |
| ICAM-1 (ng/ml) | |||
| Before | 0.59 ± 0.28 | 0.66 ± 0.39 | 0.174 |
| After | 0.80 ± 0.21 | 0.77 ± 0.03 | 0.434 |
| Difference % (CI 95%) | 0.21 (0.13 to 0.28) | 0.11 (0.005 to 0.21) | 0.126 |
| p | <0.001 | 0.038 | |
| miR-122 | |||
| Before | 2.43 ± 0.92 | 1.69 ± 0.63 | 0.509 |
| After | 0.35 ± 0.99 | 0.38 ± 0.12 | 0.838 |
| Difference % (CI 95%) | -2.08 (-3.93 to -0.22) | -1.30 (-2.56 to -0.04) | 0.515 |
| p | 0.028 | 0.042 | |
| miR-33a | |||
| Before | 1.53 ± 0.48 | 1.73 ± 0.43 | 0.759 |
| After | 2.09 ± 0.50 | 2.84 ± 0.59 | 0.342 |
| Difference % (CI 95%) | 0.56 (-1.03 to 2.16) | 1.10 (-0.55 to 2.76) | 0.306 |
| p | 0.488 | 0.191 |
During the study, one patient in the placebo group had an acute myocardial infarction, one had stable angina and one patient had subepicardial ischemia (p = 0.223). No cardiovascular events were observed in the probiotics group.
4DiscussionIn this study, oral supplementation with probiotics for a 24-week period did not promote any significant changes in CVR markers in comparison to placebo. Indeed, PAI-1 and miR-122 decreased after the intervention, but this difference occurred in both groups, not only in those who received probiotics. To the best of our knowledge, the present study is the first triple-blinded random control trial with probiotics in biopsy-proven NASH patients intended to evaluate CVR. This study fulfills the expectation regarding random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), and blinding of outcome assessment (detection bias). Thus, it seems to have internal validity and, since it included common patients in clinical practice, external validity.
Probiotics are promising for NAFLD therapy due to their relatively easy availability, low cost, and absence of serious side effects [48], but their use is still quite controversial. Recently, a randomized, double-blind, placebo-controlled study evaluated whether probiotic supplementation for six months is able to improve hepatic steatosis, fibrosis, and other clinical markers in NAFLD patients [49]. This study demonstrated that the use of probiotics did not promote significant clinical improvement in the patient with NAFLD; however, at the microenvironment level, their use was effective in controlling the increase in intestinal permeability [49]. In fact, the use of probiotics, prebiotics and symbiotics has been considered a potential and promising strategy to regulate the intestinal microbiota. However, they are not able to play a healing role. Its use can be an adjuvant therapy in pathological processes involving NAFLD and its spectra, either by improving the intestinal barrier or preventing the formation of metabolites toxic to the liver, or acting on the immune system [50]. More studies with larger sample sizes, longer duration and different strains are needed to assess the real benefit of probiotics in NAFLD, as their therapeutic use is not supported by high-quality clinical studies to date [51]. There are previous random control trials using different strains and doses of probiotics that demonstrated some interesting effects on blood glucose, insulin resistance, lipid profile and MS [52, 53]. However, even meta-analysis differs on the usefulness of probiotics in patients with NAFLD [21, 53, 54].
Regarding demographic and clinical variables, such as age, gender and comorbidities, the population included was quite consistent with previously published studies [3, 55-58]. Except for the history of CVD and the presence of T2DM, which were higher in placebo, all other baseline variables were similar between the groups. All included patients had biopsy-proven NASH (NAS ≥ 4). This is important not only to verify the liver injury but also because CVR seems to strongly depend on the presence of more advanced fatty liver disease [59]. However, most of the patients in both groups presented grade 1 fibrosis, and this mild disease could probably exert an influence on the CVR.
Our research group has been developing studies with the objective of evaluating the cardiovascular manifestations associated with NAFLD since CVD is the main cause of death and is an outcome that has been poorly evaluated [16, 45]. In fact, CVD associated with NAFLD takes a long time to cause clinical consequences, so we chose, in this study, to carry out CVR assessments through molecular analysis and scores applied in clinical practice. As there is a lack of studies that assess CVR as the main outcome in patients with NASH who underwent probiotic supplementation, and considering that CVR is related to the presence of fibrosis, the sample size calculation was based on the reduction of hepatic fibrosis. Inflammation and oxidative stress seem to be involved in the onset and progression of NAFLD [88] and the production of PAI-1 may be over-regulated by inflammatory factors [60]. These mechanisms and mutual interactions seem to explain the association between NAFLD and CVD [61]. Increased levels of PAI-1 are predictors of future cardiovascular events and have been reported in patients with coronary artery disease [62]. Our study shows an improvement in PAI-1 in both groups, more pronounced in the probiotics group. Our finding may show an improvement in CVR based on the study by Jung et al., where PAI-1 levels were higher in a patient who had major cardiovascular events [60]. Besides that, Tofler et al. concluded that the analyses of PAI-1 are predictive of CVD after considering the established risk factors [63]. Although the patients in this study had a diagnosis of NASH, most had mild or absent fibrosis, preventing the performance of a stratified analysis of the impact of probiotic supplementation on CVR according to the NAS score as would be advisable [64].
Due to the role of microRNAs in regulating metabolic pathways (like lipogenesis, glycolysis, gluconeogenesis) and also the association of microRNAs with oxidative stress, they are considered biomarkers and potential therapeutic targets for NAFLD [9]. Especially, miR-122 is quite important in liver diseases, including NAFLD [65]. In our study, it decreased after intervention in both groups. This finding is consistent with the results we observed in PAI-1 since miR-122 has also been considered a potential biomarker for the diagnosis and prognosis of CVD, mainly with the presence and severity of coronary artery disease, independent of other CVR factors [66]. No deaths occurred during the study, perhaps due to the reduced number of participants and the length of clinical follow-up. However, during the study in the placebo group, we can observe the appearance of some cardiovascular events, highlighting the high CVR these patients often present.
In this randomized clinical trial, there was no benefit from the use of probiotics over CVR. Studies with a number of patients and time similar to ours have shown discrepant results, some showing improvement and another worsening of profiles related to CVR and use of probiotics. Duseja et al. [26] conducted a randomized, double-blind, multicenter study with several strains of probiotics for 12 months in 39 Indian patients with NAFLD without T2DM. Patients who used probiotics had a significantly greater reduction in ALT and inflammatory cytokines compared to placebo. In the study by Wong et al. [67], biopsy-proven NASH patients were randomized to receive probiotics (multiple strains of 20 × 106 CFU; 10 patients) or no medication (10 patients) for six months. Metabolic parameters were evaluated but without any other evaluation of the RCV. The use of probiotics was not associated with changes in BMI, waist circumference, glycemic and lipid profile. Therefore, this relationship between probiotics with RCV is still open.
This study has some strengths, such as its triple blinding, the strict monitoring of patients, the high adherence index, the inclusion of only biopsy-proven NASH, and the investigative approach regarding different possibilities of CVR assessment, such as inflammatory markers, microRNAs, and also the evaluation through questionnaires and physical examination. However, it does present some limitations, like being a single-center study, the number of patients, the short period of treatment, and the low severity of NASH patients included. Furthermore, this study does not apply to the suggested concept of MAFLD, meaning that there were excluded patients with other causes of liver diseases, such as viruses or alcohol, among others [45].
5ConclusionsIn summary, in this double-blind placebo-controlled randomized clinical trial, probiotics supplementation was not able to significantly decrease CVR markers in comparison to placebo in NASH patients.
Author contributionsBarcelos STA, Silva-Sperb A, Moraes HA, Longo L, Moura BC, Uribe-Cruz C, Silveira TR, Dall'Alba V and Álvares-da-Silva MR performed the conceptualization, methodology, formal analysis, investigation, data curation, writing of the original draft, writing-review, and editing; Michalczuk MT and Cerski CT performed the methodology and formal analysis; writing review and editing.
Declaration of InterestNone.
FundingThis study is financed by the Research and Events Fund from the Hospital de Clínicas de Porto Alegre (FIPE), Coordination for the Improvement of Higher Education Personnel (CAPES/PROAP), National Council for Scientific and Technological Development – Brazil (CNPq, Universal 1/2016). We thank the Farmoquimica Company for the probiotics donated. No funding sources are involved in the study design, data collection, analysis, and interpretation of data.