Patients with non-alcoholic fatty liver disease (NAFLD) are at risk for cardiovascular and chronic kidney diseases. Liver steatosis and fibrosis were assessed using the fatty liver index and fibrosis-4 index, respectively. This study aimed to examine the association between these two parameters in patients with atherosclerosis and chronic kidney disease.
Materials and MethodsThe two parameters were calculated for 11,867 adults who participated in the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study. Intima-media thickness and estimated glomerular filtration rate were also measured. Logistic regression models were used to estimate the odds ratios (OR).
ResultsOverall, 4257 (35.9%) and 4733 (39.9%) participants had a higher probability of liver steatosis and fibrosis, respectively. The adjusted OR of higher fatty liver index compared to lower fatty liver index for atherosclerosis and chronic kidney disease were 0.98 (95% confidence interval [CI], 0.77–1.24) and 1.79 (95% CI, 1.19–2.69), and those of higher FIB-4 compared to lower FIB-4 were 1.03 (95% CI, 0.82–1.30) and 0.79 (95% CI, 0.52–1.19) for atherosclerosis and chronic kidney disease, respectively.
ConclusionsA higher FLI was associated with CKD independent of other risk factors. Further research is required to identify the causal relationship between liver fat accumulation and CKD.
The number of patients with non-alcoholic fatty liver disease (NAFLD) has been increasing, and NAFLD is now the most prevalent chronic liver disease [1]. Some patients with NAFLD develop non-alcoholic steatohepatitis (NASH), which leads to cirrhosis, hepatocellular carcinoma, and liver failure [2]. In addition to liver-related outcomes, cardiovascular disease (CVD) is a major cause of mortality in patients with NAFLD [3,4].
Several studies have reported that atherosclerotic vascular damage, a major risk factor for CVD, is associated with NAFLD. For example, a meta-analysis examining the association between NAFLD diagnosed by liver ultrasonography, computed tomography, or biopsy and carotid atherosclerosis, detected by carotid intima-media thickness (cIMT) or the presence of carotid plaque or stenosis, showed that the risk of atherosclerosis in patients with NAFLD was significantly greater than that in patients without NAFLD (odds ratio [OR] of 3.20) [5]. Liver fibrosis, as defined by liver biopsy, is also associated with increased cIMT in patients with NAFLD [6]. Although liver biopsy remains the diagnostic gold standard for NASH, several non-invasive biomarkers for predicting NASH and assessing liver fibrosis in NASH have been studied in addition to assessing liver steatosis [7,8]. Some studies have reported a relationship between the non-invasive biochemical index of liver steatosis and fibrosis with atherosclerotic vascular damage [9–11]. The association between non-invasive markers and atherosclerosis in the general population is yet to be investigated, considering the convenience of non-invasive markers and their applicability to populations without specific health concerns.
NAFLD has also been linked to impaired renal function and failure. The presence of NAFLD, defined by ultrasonography, is independently associated with chronic kidney disease (CKD) in adults with type 2 diabetes [12], obesity [13], and in the healthy population [14]. Among patients with NAFLD, the liver fibrosis stage was significantly correlated with the deterioration of renal function, as measured by the estimated glomerular filtration rate (eGFR) [15]. As in the case of atherosclerosis, the association of CKD and worsening renal function with non-invasive markers in the general population is yet to be explored [16,17].
There is accumulating evidence of a relationship between NAFLD and pathological changes in each extrahepatic organ; however, investigation of the extrahepatic consequences of pathological changes in the liver via other tissues or organs is insufficient. For example, both NAFLD and CKD have been reported to be risk factors for CVD [18]; however, the mechanistic links between NAFLD, CKD, and cardiovascular diseases are poorly understood. To further elucidate the multi-organ effect of NAFLD, it is necessary to investigate the extrahepatic effect of NAFLD on multiple organs in a single study. In this cross-sectional study using large population-based cohort data, we evaluated the association of non-invasive liver steatosis and fibrosis markers with atherosclerosis and renal function. We aimed to evaluate the association of both markers with each organ and address the link between the extrahepatic effects of NAFLD and cardiovascular and kidney diseases.
2Methods2.1Study PopulationThis cross-sectional study was part of the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study (TMM BirThree Cohort Study), which recruited 73,529 participants between July 19, 2013, and March 31, 2017, in Miyagi and Iwate prefectures, Japan. The Institutional Review Board of Tohoku University Graduate School of Medicine approved this cohort study (May 27, 2013; Approval No:2013-1-103-1). The details of the TMM BirThree Cohort Study have been described in earlier studies [19–22]. As follow-up is ongoing, the present study implemented cross-sectional analyses of the baseline data.
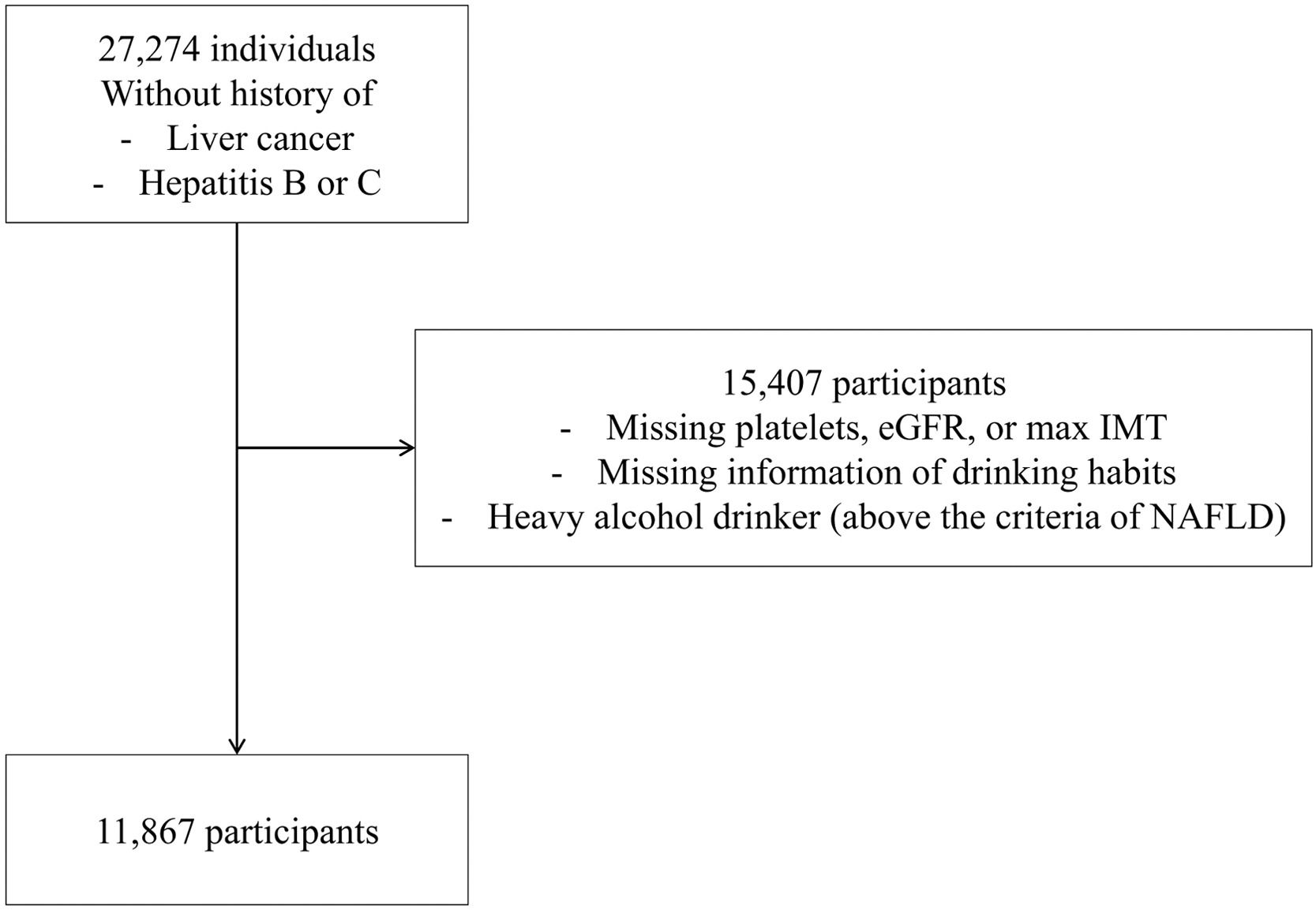
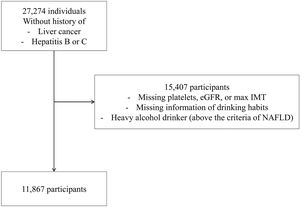
Among the cohort participants, 27,274 individuals aged ≥ 20 years who did not retract informed consent and underwent blood testing between June 2017 and January 2019 were included in this study. Information regarding medical history and drug, alcohol and cigarette use was also collected. Exclusion criteria included known liver diseases, including liver cancer, hepatitis B and C, missing information on platelets, eGFR, maximum intima-media thickness (IMT), and questionnaire answers related to alcohol consumption and excessive alcohol consumption, which are equivalent to the criteria for alcoholic liver disease. Data from 11,867 participants were analyzed in this study (Fig. 1).
2.2Measurements2.2.1cIMTCarotid ultrasound was performed using a real-time B-mode ultrasound imaging unit (Toshiba Sonolayer SSA-250A; Toshiba, Tokyo, Japan) with a 7.5 MHz annular array probe with an axial resolution of 0.25 mm. Ultrasonography was performed by specially trained doctors using a standardized technique. The study procedure involved scanning the near and far walls of both common carotid arteries, approximately 1 cm proximal to the carotid bulb on the longitudinal view. During each examination, different scanning angles (anterior, lateral, and posterior) were used to identify the maximum IMT on each wall. All participants were examined in a sitting position. Maximum IMT of ≥ 1.1 mm was used as an index of atherosclerosis.
2.2.2Laboratory measurementsBlood samples were collected and measured, as previously described [19,23]. We calculated the eGFR as an index of renal function. The new Japanese equation for glomerular filtration rate estimation [24] was used to calculate eGFR.
eGFR (mL/min/1.73 m2) = 194 × Scr −1.094 × age−0.287 (× 0.739 if female).
The serum creatinine level (Scr) was determined using the enzymatic method.
An eGFR < 60 mL/min/1.73 m2 was used as an index of renal impairment.
2.2.3Noninvasive indexes for liver fat accumulation and fibrosisThe formula for fatty liver index (FLI) was as follows [25]: FLI = [e0.953 × loge (triglycerides) + 0.139 × BMI + 0.78 × loge (γ-glutamyltransferase) + 0.053 × waist circumference - 15.745)]/[1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (γ-glutamyltransferase) + 0.053 × waist circumference - 15.745] × 100; where BMI represents the body mass index; triglyceride levels are presented in mg/dL, γ-glutamyltransferase levels in U/L, and waist circumference measurements in cm. The scores ranged from 0 to 100. An FLI value of ≥30 was used as the cutoff for suspected participants with hepatic steatosis.
The fibrosis-4 index (FIB-4) was calculated as follows: (age × AST)/(platelet count × [square root of ALT]), where AST represents aspartate aminotransferase and ALT represents alanine aminotransferase [26]. FIB-4 ≥ 1.30 was used as the cutoff for suspected participants with liver fibrosis [27].
In this study, individuals with higher FLI values (≥ 30) were assumed to have liver steatosis. Analyses were conducted to investigate the association between liver fibrosis, atherosclerosis, and CKD in individuals with liver steatosis.
In supplemental analyses, stratification of participants using higher cutoff values (60 for FLI and 2.67 for FIB-4) was performed for sensitivity analyses.
2.3Data analysesContinuous data were expressed as means ± standard deviation (SD) and compared using a two-tailed t-test. Categorical variables were compared using the χ2 test. Logistic regression analysis adjusted for confounders was used to determine the association between noninvasive liver markers and organ damage, vascular atherosclerosis, and renal impairment. Information regarding medical intervention of the participants, including the alimentary regimen, was not included in the analyses owing to the limitation of data availability. Statistical significance was set at a two-tailed P value of 0.05. All data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.4Ethical considerationsThis study was approved by the institutional review board of the Tohoku University Tohoku Medical Megabank Organization on September 14, 2020 (registration number:2020-4-061). Informed consent was obtained from the TMM BirThree Cohort Study participants [20,22], and those who withdrew their consent before the start of the study were excluded from the analyses.
3ResultsThe clinical and biochemical features of the study groups are presented in Table 1. The mean age of the study participants was 56.9 years (SD, 15.1 years) (Table 1). The prevalences of type 2 diabetes, hypertension, dyslipidemia, and smoking at baseline were 3.8%, 22.5%, 10.5%, and 50.2%, respectively. The proportion of participants with a higher probability of liver steatosis (FLI ≥ 30) was 35.9% (n = 4257) while that of those with a higher probability of liver fibrosis (FIB-4 ≥ 1.30) was 39.9% (n = 4733). Among participants with a higher probability of liver steatosis, the proportion of males was significantly higher than that among those with a lower probability of liver steatosis. Compared to participants with a lower probability of liver steatosis, those with a higher probability of liver steatosis were older; had higher BMI; systolic and diastolic blood pressure; total and low-density lipoprotein (LDL) cholesterol, blood glucose, hemoglobin A1c (HbA1c), AST, and ALT levels; lower high-density lipoprotein (HDL) cholesterol levels; and included more smokers and patients with type 2 diabetes, hypertension, and hyperlipidemia (Table 1). Among participants with a higher probability of liver fibrosis, the proportion of males was significantly higher than that among those with a lower probability of liver steatosis. Participants with a higher probability of liver fibrosis were older, had higher systolic and diastolic blood pressure, and total cholesterol, blood glucose, HbA1c, AST, and ALT levels compared to those with a lower probability of liver steatosis. Participants with a higher probability of liver fibrosis also included more smokers and patients with type 2 diabetes, hypertension, and hyperlipidemia compared to those with a lower probability of liver fibrosis (Table 1).
Anthropometric and biochemical characteristics of the study subjects by liver steatosis and fibrosis risk scores.
| All study population (n=11,867) | Lower probability of liver steatosisFLI < 30(n=7610) | Higher probability of liver steatosisFLI ≥ 30(n=4257) | Lower probability of liver fibrosisFIB-4 < 1.30(n=7134) | Higher probability of liver fibrosisFIB-4 ≥ 1.30(n=4733) | |
|---|---|---|---|---|---|
| Male, n(%) | 5904 (49.8) | 2944 (38.7) | 2960 (69.5) a | 2790 (39.1) | 3144 (66.4) d |
| Age, year | 56.9 (15.1) | 55.1 (15.6) | 60.0 (13.6) a | 48.8 (13.5) | 69.0 (6.9) d |
| BMI, kg/m2 | 23.1 (3.4) | 21.5 (2.3) | 26.0 (3.0) a | 23.1 (3.5) | 23.2 (3.1) |
| smoker, n(%) | 5917 (50.2) | 3259 (43.1) | 2658 (62.9) a | 3448 (48.7) | 2469 (52.4) d |
| Systolic blood pressure, mmHg | 127.4 (18.0) | 123.9 (18.1) | 133.6 (16.2) a | 122.9 (17.3) | 134.1 (17.0) d |
| Diastolic blood pressure, mmHg | 77.7 (10.9) | 75.8 (10.5) | 81.2 (10.7) a | 77.3 (11.1) | 78.4 (10.6) d |
| Total cholesterol, mg/dL | 200.8 (34.6) | 198.1 (33.9) | 205.6 (35.4) a | 200.2 (35.6) | 201.6 (33.0) b |
| HDL cholesterol, mg/dL | 62.5 (16.6) | 67.3 (16.3) | 53.8 (13.5) a | 62.3 (16.5) | 62.7 (16.8) |
| LDL cholesterol, mg/dL | 115.8 (30.0) | 112.9 (28.8) | 120.9 (31.3) a | 115.8 (31.0) | 115.6 (28.3) |
| Blood glucose, mg/dL | 89.7 (18.4) | 86.7 (14.5) | 95.0 (22.8) a | 87.4 (16.5) | 93.1 (20.4) d |
| HbA1c, % | 5.5 (0.5) | 5.5 (0.4) | 5.7 (0.6) a | 5.5 (0.5) | 5.6 (0.5) d |
| AST, IU/L | 22.8 (9.0) | 20.8 (5.9) | 26.3 (12.0) a | 20.6 (6.9) | 26.2 (10.6) d |
| ALT, IU/L | 21.5 (14.9) | 17.0 (7.6) | 29.5 (20.5) a | 21.1 (15.2) | 22.1 (14.6) c |
| Type 2 diabetes, n(%) | 419 (3.8) | 195 (2.6) | 224 (5.3) a | 167 (2.2) | 252 (5.9) d |
| Hypertension, n(%) | 2513 (22.5) | 1116 (14.7) | 1397 (32.8) a | 991 (13.0) | 1522 (35.8) d |
| Hyperlipidemia, n(%) | 1172 (10.5) | 614 (8.1) | 558 (13.1) a | 522 (6.9) | 650 (15.3) d |
Data are means ± SD. Categorical variables were compared by χ2 test. ALT = alanine aminotransferase; AST = aspartate aminotransferase; FLI = Fatty Liver Index; HbA1c = hemoglobin A1c; HDL = high density lipoprotein; LDL = low density lipoprotein.
Compared to participants with a lower probability of liver steatosis, those with a higher probability of liver steatosis had significantly higher maximum IMT and lower eGFR (P < 0.001, Table 2). The proportions of participants with atherosclerosis (max IMT ≥ 1.1 mm) and renal impairment (eGFR < 60 mL/min/1.73 m2) were higher among participants with a higher probability of liver steatosis than among those with a lower probability of liver steatosis (P < 0.001, Table 2). Compared to participants with a lower probability of liver fibrosis, those with a higher probability of liver fibrosis had significantly higher maximum IMT and lower eGFR (P < 0.001, Table 2). The proportions of participants with atherosclerosis and renal impairment was higher among participants with a higher probability of liver fibrosis than among those with a lower probability of liver fibrosis (P < 0.001, Table 2).
Correlation between intima-media thickness and renal function and liver steatosis and fibrosis risk scores.
| All study population (n=11,867) | |||||
|---|---|---|---|---|---|
| Lower probability of liver steatosis FLI < 30 (n=7610) | Higher probability of liver steatosis FLI ≥ 30 (n=4257) | Lower probability of liver fibrosis FIB-4 < 1.30 (n=7134) | Higher probability of liver fibrosis FIB-4 ≥ 1.30 (n=4733) | ||
| Max IMT | Mean | 0.72 (0.18) | 0.79 (0.18) a | 0.69 (0.16) | 0.84 (0.18) b |
| ≥1.1 mmn(%) | 255 (3.4) | 269 (6.3) a | 149 (2.1) | 375 (7.9) b | |
| eGFR | Mean | 113.3(24.6) | 101.6 (21.9) a | 118.8 (23.3) | 94.5 (17.7) b |
| <60 mL/min/1.73m2n(%) | 71 (0.9) | 99 (2.3) a | 36 (0.5) | 134 (2.8) b | |
Data are means ± SD. eGFR = estimated glomerular filtration ratio; FLI = Fatty Liver Index; IMT = intima-media thickness.
A logistic regression model adjusted for age, sex, smoking history, BMI, systolic blood pressure, and HbA1c and LDL cholesterol levels was used to estimate the risk of atherosclerosis and renal impairment in participants with a higher probability of liver steatosis and fibrosis. As shown in Table 3, a higher probability of liver steatosis (FLI ≥ 30) significantly correlated with renal impairment (1.8-fold OR). In contrast, a higher probability of liver fibrosis (FIB-4 ≥ 1.30) was not significantly correlated with atherosclerosis or renal impairment, and a higher probability of liver steatosis was not significantly correlated with atherosclerosis.
Adjusted risk of atherosclerosis and renal impairment according to probability of liver steatosis and fibrosis risk scores in logistic regression model.
| max IMT ≥ 1.1 mm | eGFR < 60 mL/min/1.73 m2 | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Higher probability of liver steatosisFLI ≥ 30 | 0.976 (0.771–1.236) | 0.841 | 1.786 (1.186–2.690) | 0.006 |
| Higher probability of liver fibrosisFIB-4 ≥ 1.30 | 1.029 (0.816–1.298) | 0.809 | 0.785 (0.517–1.193) | 0.257 |
eGFR = estimated glomerular filtration ratio; FLI = Fatty Liver Index; IMT = intima-media thickness; OR = odds ratio.
The models is adjusted for age, sex, smoking history, BMI, systolic blood pressure, HbA1c, and LDL cholesterol.
Sensitivity analyses were conducted using higher cut-off values to identify individuals with a higher probability of liver steatosis or fibrosis (Supplemental Table 1). As in the analyses using lower cutoff values, individuals with a high probability of liver steatosis had significantly higher maximum IMT and lower eGFR than those with a low probability of liver fibrosis (Supplemental Table 2). The proportions of participants with atherosclerosis and renal impairment were higher among those with a higher probability of liver steatosis than among those with a lower probability of liver steatosis (Supplemental Table 2). In the logistic regression model, only a high probability of liver steatosis was independently associated with lower eGFR, which was consistent with the logistic regression model using lower cutoff values of FLI and FIB-4 (Supplemental Table 3). In subgroup analyses by age and sex, the observed results were consistent with those of the overall study population (Supplemental Tables 5 and 6). In addition, the analyses using cutoff values of the outcomes to include earlier stages of cardiovascular thickening and renal function decrease (maximum IMT ≥ 0.6 mm and eGFR < 90 mL/min/1.73 m2) also showed results that were consistent with those of the original analyses (Supplemental Table 7). When participants were grouped based on eGFR values (> 90, ≥ 60 and < 90, and < 60), the prevalence of higher FLI significantly increased in accordance with worsening renal function (Supplemental Table 8).
Among participants with a higher probability of liver steatosis (liver steatosis group), the prevalence of higher probability of liver fibrosis (FIB-4 ≥ 1.30) was 42.9% (n=1828, Supplemental Table 6). Compared to participants with a lower probability of liver fibrosis in the liver steatosis group, participants with a higher probability of liver fibrosis had significantly higher maximum IMT and lower eGFR (P < 0.001, Supplemental Table 5). The proportion of participants with atherosclerosis and renal impairment was higher among individuals with a higher probability of liver fibrosis than among those with a lower probability of liver fibrosis (P < 0.001; Supplemental Table 5). In a logistic regression model adjusted for age, sex, smoking history, BMI, systolic blood pressure, and HbA1c and LDL cholesterol levels, a higher probability of liver fibrosis (FIB-4 ≥ 1.30) was not independently correlated with either atherosclerosis or renal impairment (Supplemental Table 6).
4DiscussionTo the best of our knowledge, this is the first study to evaluate the associations of non-invasive markers of liver steatosis (FLI) and liver fibrosis (FIB-4) with atherosclerosis and renal impairment in a large cohort of Japanese men and women of a wide age range. The main finding of this study was that a higher liver steatosis risk score was independently associated with the presence of renal impairment, although the liver fibrosis risk score did not show such an association. Surprisingly, neither the liver steatosis risk score nor the fibrosis risk score showed an independent association with atherosclerosis, detected using ultrasound vascular echo.
Patients with NAFLD are at risk of CVD, and several studies have suggested that liver fibrosis may be associated with CVD risk [28–30]. Atherosclerosis, one of the most well-investigated causes of CVD, is also linked to NAFLD and liver fibrosis, as determined by both definitive diagnostics [5,6] and non-invasive biochemical markers [9–11]. In this study, atherosclerosis determined by cIMT was significantly more prevalent among individuals with higher FLI or FIB-4; however, the association between atherosclerosis and FLI or FIB-4 was not independent when adjusted for sex, age, and metabolic factors that may affect atherosclerosis. Some studies have reported an independent association between FLI and cIMT [10,31]. The inconsistency in the results from this study and previous reports may be due to differences in the included population; one study included patients who were admitted to the hospital for cardiovascular-related concerns [10] and another included patients with type 2 diabetes [31], in contrast to the general population included in this study. These differences in results suggest that FLI may not be an independent predictor of increased cIMT in the general population. Regarding non-invasive markers for liver fibrosis, several studies have reported the independent association of FIB-4 or NAFLD fibrosis score with cIMT in patients with NAFLD or obesity [32–34]. In this study, FIB-4 was not independently associated with atherosclerosis, which limits the application of non-invasive liver fibrosis markers as an indicator of atherosclerosis in the general population. In this study, FIB-4 did not show an independent association with cIMT in individuals with higher FLI values, presumably in the group with NAFLD. It is known that liver fat decreases with the progression of liver fibrosis, called “burn-out” NASH [35]. There is a possibility that some individuals who developed liver fibrosis as a consequence of NAFLD were included in the lower FLI group, which may be the reason for the difference in our results compared to those of studies that included biopsy-proven NAFLD [32,34]. In contrast to studies reporting an independent association between NAFLD and CVD or subclinical precursory signs, a few recent studies have reported that the risk of CVD was not independently associated with NAFLD itself or its histopathological indices; rather, it was dependent on other metabolic risk factors for CVD [34,36]. The link between NAFLD and cardiovascular diseases should be carefully considered, and our study suggests that noninvasive markers of liver steatosis and fibrosis cannot be considered indicators of atherosclerosis in the general population.
Attenuated renal function and CKD are also extrahepatic complications of NAFLD [28]. In the current study, a higher FLI was independently associated with CKD, as determined by eGFR. Previous reports have indicated an independent association between FLI and the presence or future incidence of CKD [37–39], and the results of the current study are in line with these reports. In this study, non-invasive markers of liver fibrosis were also tested in a large population-based cohort study, which did not show an independent association with the presence of CKD. A previous study demonstrated an independent association between a higher FIB-4 and CKD in NAFLD patients detected using ultrasound echo [40]. In contrast, the current study suggests that the noninvasive marker of liver steatosis is useful for estimating the presence of CKD in the general population rather than that of liver fibrosis. In the current study, an independent association between FIB-4 and CKD was not observed in participants with higher FLI values. This might be due to the difference in population with liver steatosis identified by either ultrasonography or non-invasive biochemical markers, as in the case of the relationship between FIB-4 and atherosclerosis in our study. The current study suggests that risk characterization by FIB-4 in individuals with liver steatosis determined by FLI is not an efficient screening procedure for CKD.
In the current study, we tested the association of FLI and FIB-4 with two extrahepatic organ damages that are well investigated for their relationship, atherosclerosis, and CKD, in the same study population and using the same method. This is the first study to show that only the association between FLI and CKD was observed in combinations of two non-invasive markers of liver pathophysiological changes and two types of organ damage. Several mechanisms have been hypothesized to link liver steatosis with CKD. Cardiometabolic risk factors, such as obesity, insulin resistance, and dyslipidemia, which are related to NAFLD, also increase the risk of CKD [41]. In the current study, although FLI showed an independent association with CKD, no independent association was observed with BMI, and HbA1c and LDL cholesterol levels. This might indicate that the accumulation of liver fat affects organ damage in the kidney independent of other cardiometabolic factors. Although a direct link between NAFLD and CKD has not yet been established, inflammatory and fibrogenic factors, including fibroblast growth factor-21 and feturin-A, which are increased in patients with NAFLD, have been reported to potentially promote kidney injury [42–45]. Population-based studies have shown that both morbidity and mortality from CVD are increased in patients with NAFLD [3,4,28] and that CKD is another well-established risk factor for CVD [46]. In this study, an independent association between CKD and FLI was observed; however, the association between atherosclerosis and FLI was not independent after adjusting for cardiometabolic risk factors. This suggests that the morbidity and mortality caused by CVD among patients with NAFLD are, at least in part, due to CKD, which is associated with NAFLD. Genetic factors are also involved in the development and exacerbation of NAFLD [47]. The reason for the association of FLI with CKD, independent of other risk factors of CKD and cardiometabolic risk factors, and the absence of an association with atherosclerosis, may be attributed to genetic polymorphisms associated with an increased risk of NAFLD. Recent studies have indicated that patatin-like phospholipase domain-containing protein 3 (PNPLA3), which is one of the most well-studied genes related to NAFLD risk, is associated with reduced eGFR or CKD independent of other renal risk factors and NAFLD [48,49]. In contrast, a recent study showed that genetic components are not involved in the atherosclerotic damage associated with NAFLD [50]. Further studies, including observational studies with longer follow-up periods and interventional studies for CKD in patients with NAFLD, are required to confirm this hypothesis. However, the current study highlights the importance of multi-organ screening for cardiovascular and renal diseases in patients with NAFLD.
This study has certain limitations. First, as FLI and FIB-4 are indirect risk markers for pathological changes in the liver tissue, the results of this study require careful evaluation. To establish the relationship between liver steatosis and damage to other organs, imaging assessment of hepatic steatosis represented by ultrasonography might be necessary; similarly, for liver fibrosis, assessment by magnetic resonance imaging or elastography is necessary. In this study, these evaluations were not conducted because of operational and logistical limitations when applied to large cohorts, including a healthy population. Because the FLI is an index that uses some variables, it is possible that each component of the formula might be associated with CKD [38]. In our analyses, none of the components used to calculate FLI showed a meaningful association with CKD (Supplemental Table 4 for BMI and Supplemental Table 9 for other parameters). Second, this was a cross-sectional observational study; the findings should therefore be interpreted carefully, and no causal relationship can be confirmed. Further prospective and mechanistic studies are required to determine the relationship between FLI, CKD, atherosclerosis, and CKD. Third, the study used data from a large population-based cohort in which family members were recruited [19–22]. Supplemental analyses based on age groups (Supplemental Table 6) showed that results were consistent across both younger and older age groups, even though an independent association of higher FLI with renal function was not observed because of the small number of participants with renal impairment in the younger age group. This finding supports the fact that the effect of the affinity relationship on the study results is limited, even though the possibility of the effect cannot be fully excluded. Fourth, in the present study, although we excluded patients with other chronic liver diseases, including hepatitis B and C and liver tumors, a questionnaire was used to identify these participants, and no diagnostic procedures were performed to identify these liver diseases. In addition, patients with other rare types of chronic liver diseases, such as autoimmune hepatitis, might have been included in the analyses, although the number of such patients is likely to be small. Fifth, although several covariates were included in the adjustment, other potential mediators such as food intake, physical activity level, and medicines that influence renal diseases need to be taken into consideration.
5ConclusionIn conclusion, this cross-sectional study using a large cohort database showed that FLI was associated with CKD independent of other risk factors of CKD and cardiometabolic risk factors, although it was not associated with atherosclerosis. Overall, FLI was identified as a potential CKD marker. Further investigations using a prospective design, quantitation of liver fat, and mechanistic studies are required to identify the causal relationship between liver fat accumulation and renal disease.
FundingThe TMM BirThree Cohort Study was supported by the Japan Agency for Medical Research and Development (AMED), Japan [grant number: JP21tm0124005].
Author contributionsToshiya Machida: the conception and design of the study, analysis and interpretation of data, and drafting of the article. Taku Obara: the conception and design of the study, acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Mami Ishikuro: acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Keiko Murakami: acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Fumihiko Ueno: acquisition and analysis of data, revising the article, and final approval of the version to be submitted. Aoi Noda: acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Tomomi Onuma: acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Fumiko Matsuzaki: acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Jun Inoue: study supervision, revising important intellectual content of the article, and final approval of the version to be submitted. Shinichi Kuriyama: study supervision, acquisition and analysis of data, revising important intellectual content of the article, and final approval of the version to be submitted. Nariyasu Mano: study supervision, revising important intellectual content of the article, and final approval of the version to be submitted. All of the authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Declaration of interestToshiya Machida is an employee of Pfizer R&D in Japan. Toshiya Machida is a research collaborator at Tohoku University, and he contributed to the present study independently from the companies to which they belong. Nariyasu Mano received honoraria from Daiichi Sankyo Co. Ltd. The other authors have no conflicts of interest to declare.
The authors wish to express their appreciation to the participants of the TMM BirThree Cohort Study and staff members of the Tohoku Medical Megabank Organization. The list of members is available at https://www.megabank.tohoku.ac.jp/english/a210901/.