Hepatitis C (HCV) screening is imperative to meet WHO elimination targets including increased detection and reduced mortality. An electronic medical record (EMR) system can be utilized in health care centers to indicate if a patient should be targeted for HCV screening, thus increasing the number of those offered testing.
Materials and methodsWe examined English language publications reporting on the impact of EMR system utilization on HCV screening and the HCV continuum of care. Relevant papers were identified using multiple search engines to search key terms. Clinical outcomes considered included any or no change in HCV screening rates following EMR system introduction, as well as any or no change in rates of patients progressing along the HCV cascade of care after diagnosis once an EMR system was implemented.
ResultsFrom a search pool of 18 studies, 11 meet inclusion criteria and reported on the selected clinical outcomes. Each outcome assessed indicated that use of an EMR system increased the proportion of patients offered and/or receiving HCV testing. We were unable to conclude if an EMR system had an impact on the number of patients progressing along the HCV cascade of care following a positive test result. Overall, all methods of implementation of an EMR system had the same outcome of increasing screening rates.
ConclusionsEMR system utilization had a positive impact on increasing HCV screening. However, the clinical effectiveness of utilizing an EMR system to help eliminate transmission and increase HCV treatment cure rates requires further study.
In the last decade, yearly hepatitis C (HCV) infection rates have increased in Canada [1]. Over 11,000 newly identified cases were reported in 2017 alone. HCV infection causes liver-specific and extrahepatic complications. This infection accounts for more years of life lost than any other infectious disease in Canada [2]. Infection often produces non-specific mild symptoms until advanced liver injury has developed. As a consequence, HCV often remains undiagnosed for decades after infection. HCV is curable with antiviral therapy [3]. A cure halts HCV disease progression, reduces the risk for liver-related morbidity and mortality, as well as all-cause mortality, and improves quality of life [3]. Identifying those who are undiagnosed and linking them with care and treatment before developing HCV complications will decrease the health consequences of HCV. After diagnosis, it is imperative for patients to continue along the cascade of care in order to monitor their infection and ultimately receive curative treatment. Unfortunately, most studies find that 25–35% of people who test positive for HCV antibodies never have follow-up HCV RNA testing. Furthermore, loss to follow-up rates are higher among priority populations living with HCV [3].
Electronic medical record (EMR) systems represent an enabling technology that may assist health care providers and systems to pursue quality improvement initiatives. Information technology is recognized as a valuable tool for improving patient safety and quality of care [4]. An EMR system could provide health care providers with reminders of HCV screening guidelines or directly indicate if a patient is eligible for HCV screening. Additionally, EMR systems could be utilized to increase the number of patients who progress along the HCV cascade of care [5–15].
To date, there has been no synthesis of evidence related to the use of an EMR for HCV screening and care. Our systematic review addresses this deficiency evaluating the clinical effectiveness of EMR system utilization to increase HCV screening in asymptomatic, treatment-naive adults, and assessing the clinical effectiveness of using an EMR system to increase progression along the HCV cascade of care.
2Methods2.1Aim and outcomes of interestThe objectives of this systematic review were to assess the published research evidence on the clinical effectiveness of EMR system utilization to increase HCV infection screening in asymptomatic, treatment-naive adults, and to evaluate the impact of an EMR on patient flow along the HCV cascade of care compared to pre- EMR system implementation. We assessed HCV screening rate change following EMR system introduction. We also evaluated rates of patient progression along the HCV cascade of care after diagnosis once an EMR system was implemented. Outcomes of interest included the number or proportion of patients screened for HCV before and after the implementation of an EMR system, as well as data on the number of patients continuing along each step in the HCV cascade of care.
2.2Eligibility criteriaStudies were considered for inclusion if an EMR was utilized by the clinic(s) and results of HCV screening were reported for asymptomatic, treatment-naïve adults who were at least 18 years old before and after implementation of an EMR system.
2.3DefinitionsHCV diagnosis was defined as a positive HCV antibody test confirmed by a positive HCV RNA test [10,14,15]. HCV screening was defined as receiving HCV antibody testing [5–10,14,15]. Cure [aka Sustained Virological Response (SVR)] was defined as HCV ribonucleic acid (RNA) negativity 12 weeks or more post cessation or completion of HCV antiviral treatment [10]. HCV cascade of care steps considered for our review included diagnostic HCV RNA testing to confirm a chronic infection, referral to a HCV care provider, HCV treatment initiation, and confirmation of cure [3]. The baby boomer birth cohort is defined as those born between 1945 and 1965 [5,7–15].
An EMR was defined as a digital version of patient characteristics and information pertaining to individual patient medical history, diagnoses and treatments. An electronic health record is a digital version of a patient’s chart, but it is a more inclusive snapshot of the patient’s medical history [16]. Best Practice Alerts (BPAs) are defined as automated alerts within an EMR that help expedite widespread communication of information to health care providers. They are used clinically to save time, identify patients for follow-up, and increase clinician efficiency [17]. Plan-Do-Study-Act (PDSA), is a four-stage problem-solving model. Each steps of this cycle is integrated into the next. The “plan” step consists of a detailed plan and the objectives. During the “do” phase any changes from the “plan” phase are implemented and data is collected. The data is analysed during the “study” phase., During the “act” phase one decides if the implemented change should be adopted, adapted or abandoned [11]. Electronic clinical decision support refers to health information technology systems designed to assist clinicians and other health care professionals in clinical decision-making with the aim of improving patient care [18]. Caradigm Intelligence Platform (CIP) combines medical information from various data systems into a single database. The aggregated data is comprised of laboratory test results for all HCV antibody tests, HCV RNA tests, and HCV genotypes [9].
2.4Literature search strategy and study selectionPublished literature was identified by searching the following bibliographic databases: PubMed, Medline and Google Scholar with no publication date restrictions. The publication search was restricted to English language. The search strategy used a combination of keywords and controlled vocabulary that included “hepatitis C”, “electronic medical record”, “screening rates”, and “cascade of care”. We conducted electronic searches of medical literature for reports that presented original research data on HCV screening and HCV care continuum while implementing an EMR system. Duplicate publications, companion reports, narrative reviews, and editorials were excluded from the systematic review. Grey literature such as case series, case reports, conference abstracts was hand searched. The reference lists of all reviewed materials were examined to identify cited articles not found by electronic searches.
After the removal of duplicate results, two reviewers (LB, CC) independently screened titles and abstracts from the literature search and selected articles that warranted further evaluation. Full texts of potentially relevant manuscripts identified through the initial screen were retrieved and independently assessed for inclusion based on our predetermined selection criteria.
Using inclusion and exclusion criteria, identified abstracts were assessed for relevance by the same reviewers. Data from the studies identified for inclusion were extracted by one reviewer and checked by a second reviewer with disagreements discussed until a consensus was reached.
2.5Data extraction and analysisFor all studies, the following variables were extracted by one reviewer: authors, publication year, study design, inclusion criteria, intervention description, and results pre-implementation and post-implementation. HCV cascade of care data were extracted from the papers. Quantitative results that were applicable to the outcomes of interest were extracted. Qualitative results were extracted when quantitative was not present or applicable. A second reviewer then critiqued the data extracted. Discrepancies between investigators were resolved through discussion.
Evidence was synthesized by reviewing the proportion of patients screened for HCV from each individual study. A narrative synthesis was conducted which involved presenting the results from each included study in qualitative, narrative and table formats.
ROBIS was used to assess the risk of bias in this systematic review [19]. The evaluation was completed by one reviewer with a second reviewer examining the assessment.
3ResultsOur search was conducted between April 20 and June 30, 2020. Based on the results of abstract screening, 18 papers were selected for full-text review. Ultimately, 11 papers fulfilled the inclusion criteria for this systematic review. A summary of data from included articles is reported in Tables 1 and 2. All of the studies were from the United States and published between 2015 and 2019. Grey literature was excluded from this systematic review as identified materials did not meet inclusion criteria. The type of EMR implementations were diverse and included: electronic clinical decision support (eCDS)/best practice alert (BPA) (n = 8 of 11), PDSA (plan-do-study-act) cycles (n = 2 of 11), and usage of CIP (Caradigm Intelligence Platform) database to identify patients then manually pre-enter orders into an EMR system (n = 1 of 11). All of the interventions to improve HCV screening using an EMR system targeted at least one primary care clinic. Most interventions were performed only in birth cohort patients (n = 10 of 11). All evaluated studies had an increase in screening rates of at least 10% after implementation of an EMR system [5–15]. The average increase in screening rates for eligible patients for the studies that used an eCDS/BPA and provided percentages was 45.81% (n = 8 of 11) [5,7,8,10,12–15]. Burrell et al. reported conducting 150 HCV tests within 364 days pre-implementation of BPAs and 1895 tests performed within 360 days post-implementation [6]. Papers pertaining to PDSA cycles (n = 2 of 11) had an average increase of 53.4% in screening rates and a 40.7% increase in test offering [13,11]. The study utilizing a CIP database to identify patients followed by manually pre-enter ordering into an EMR system noted a 17.5% increase in screening rates [9].
Characteristics of each paper included in the systematic review.
| Name of paper | Year | Location | Inclusion criteria | Type of implementation |
|---|---|---|---|---|
| Sidlow R, Msaouel P. Improving hepatitis C virus screening rates in primary care: a targeted intervention using the electronic health record. J Health Qual 2015;37:319–23. 10.1097/JHQ.0000000000000010 | 2015 | New York, USA | Persons born between 1945−1965 with no previous HCV result on file | HCV testing decision support |
| Trinh J, Turner N. Improving adherence to hepatitis C screening guidelines. BMJ Open Quality 2018;7:e000108. doi:10.1136/bmjoq-2017−000108 | 2018 | North Carolina, USA | Persons born between 1945−1965 and with no active or known liver disease as HCV screening may not have been obtained for screening purposes | Six total PDSA cycles were executed, with six interventions |
| Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis C screening and curative treatment for baby boomers. Hepatology 2017;66:1805–13. 10.1002/hep.29362 | 2017 | Michigan, USA | (1) was born between 1945 and 1965; (2) lacked a prior EHR International Classification of Diseases, Ninth or Tenth Revision, diagnosis code of HCV infection; and (3) lacked documented anti-HCV testing after 2009 in our EHR | BPA alert was developed in conjunction with their EHR Population and Health Management Ambulatory Care Services Team and consultation from several PCPs |
| Armstrong H, Gonzalez-Drigo M, Adeyemi O, Trick W, Diep L, et al. Electronic Clinical Decision Support (eCDS) intervention to Increase Hepatitis C Virus (HCV) Screening and Linkage to Care among Baby Boomers in Urban Safety-Net Health System. J Community Med Health Educ 9: 646. doi:10.4172/2161-0711.1000646 | 2019 | Illinois, USA | Patients born during 1945 through 1965, who had never had an anti-HCV test, and who had any order for a laboratory blood test | electronic clinical decision support tool implemented across all outpatient sites |
| Yeboah-Korang A, Beig MI, Khan MQ, Goldstein JL, Macapinlac DM, Maurer D, et al. Hepatitis C screening in commercially insured U.S. birth-cohort patients: Factors associated Med 2018; 6: 82-9. | 2018 | Illinois, USA | Age cohort patients who presented for at least one outpatient visit who had not been previously HCV-tested at their institution | Best Practice Alert (BPA) was generated and prominently displayed in the patient’s electronic medical record |
| Shahnazarian V, Karu E, Mehta P Hepatitis C: improving the quality of screening in a community hospital by implementing an electronic medical record intervention BMJ Open Quality 2015;4:u208549.w3409. doi: 10.1136/bmjquality.u208549.w3409 | 2015 | New York, USA | Patients born during 1945 through 1965 that were either admitted to the hospital or seen in outpatient clinic setting and is HCV testing naïve | Created an algorithm in the electronic medical records to measure how many admitted and clinic patients were eligible for testing. The program shows how many of those patients had an HCV test result in the computer. |
| Al-hihi E, Shankweiler C, Stricklen D, et al. Electronic medical record alert improves HCV testing for baby boomers in primary care setting: adults born during 1945–1965. BMJ Open Qual2017;6:e000084 10.1136/bmjoq-2017-000084 | 2017 | Kansas, USA | Patients born between 1945-1965 with no previous screening including: positive antibody, positive viral load, positive genotype, screened previously using anti-HCV | Developed a workflow which included visual reminders in the EMR as best practice alerts, health maintenance overdue items, along with vaccinations, and reminders for breast, colorectal and cervical cancer screenings that were developed by IT |
| Federman AD, Kil N, Kannry J, et al. An electronic health Record-based intervention to promote hepatitis C virus testing among adults born between 1945 and 1965: a cluster-randomized trial. Med Care 2017;55:590–7. 10.1097/MLR.0000000000000715 | 2017 | New York, USA | Scheduled visit with a consented clinician of a Birth Cohort patient who had no prior HCV antibody test, hepatitis C viral load result, or prior diagnosis of hepatitis C as documented in the EHR | best practice alert (BPA) programmed in the EHR to appear in yellow highlight on the EHR-clinician interface during visits to inform clinicians of the patients’ eligibility for HCV testing and to facilitate testing by providing a testing order set |
| Golden MR, Duchin J, Chew LD, et al. Impact of an electronic medical Record-Based system to promote human immunodeficiency virus/hepatitis C virus screening in public hospital primary care clinics. Open Forum Infectious Diseases 2017;4 10.1093/ofid/ofx075 | 2017 | Washington, USA | Born between 1945-1965 and had no record of HCV testing based on laboratory records | used CIP database to identify patients with scheduled clinic visits who met the criteria and pre-enter orders into the EMR for HCV testing |
| Castrejón M, Chew KW, Javanbakht M, Humphries R, Saab S, Klausner JD. Implementation of a Large System-Wide Hepatitis C Virus Screening and Linkage to Care Program for Baby Boomers. Open Forum Infect Dis. 2017;4(3):ofx109. Published 2017 May 27. doi:10.1093/ofid/ofx109 | 2017 | California, USA | Patients were eligible for inclusion if they (1) were born between 1945 and 1965, (2) had a primary care visit between start and end date of study, (3) were seen at one of the outpatient clinics within UCLA Health, and (4) were tested for HCV during their visit. Patients without previous documentation of positive HCV antibody or HCV RNA testing within the EHR or a diagnosis in their medical record, as determined by electronic chart review, were included in the analysis | HCV screening status was added to the routine health maintenance reminder, a clinical decision support (CDS) tool in the EHR, for patients born between 1945 |
| Burrell CN, Sharon MJ, Davis SM, Wojcik EM, Martin IBK. Implementation of a Collaborative HIV and Hepatitis C Screening Program in Appalachian Urgent Care Settings. West J Emerg Med. 2018;19(6):1057-1064. doi:10.5811/westjem.2018.9.39512 | 2018 | West Virginia, USA | HCV testing is recommended for those who: are born between 1945 and 1965, currently inject drugs or used to, received clotting factor concentrates produced before 1987, have HIV infection, ever on long term hemodialysis, with persistently abnormal alanine aminotransferase levels, received transfusion of blood, blood components, or an organ transplant before July 1992 or were notified they received blood from donor who later tested positive for HCV infection | BPAs were used to populate within the charts of registered patients who qualified to receive: 1) only an HIV screening; 2) only an HCV screening; or 3) both an HIV and HCV screening |
HCV= Hepatitis C Virus; PDSA = Plan Do Check Act; BPA = Best Practice Alert; EHR = Electronic Health Record; PCP = Primary Care Physician; EMR = Electronic Medical Record; IT = Information Technology; CIP = Caradigm Intelligence Platform; UCLA = University of California, Los Angeles; CDS = clinical decision support; HIV= Human Immunodeficiency Viruses.
Types of implementation, method and quantitative data of each paper.
| Name of paper | Type of implementation | Method: | % HCV screening pre-implementation: | % HCV screening post-implementation: |
|---|---|---|---|---|
| Sidlow R, Msaouel P. Improving hepatitis C virus screening rates in primary care: a targeted intervention using the electronic health record. J Health Qual 2015;37:319–23. 10.1097/JHQ.0000000000000010 | HCV testing decision support | A module was programmed to display both the phrase “HCV test offer” and associated Yes/No. If the offer was accepted, the note author was instructed to click “Yes,” whereon the EHR automatically generated a laboratory order for an HCV enzyme-linked immunosorbent assay blood test for that office visit | 11 | 46 |
| Trinh J, Turner N. Improving adherence to hepatitis C screening guidelines. BMJ Open Quality 2018;7:e000108. doi:10.1136/bmjoq-2017-000108 | six total PDSA cycles were executed, with six interventions | (1) A baseline survey of provider knowledge, (2) distribution of guidance for providers for discussing HCV screening with patients, (3) addition of an EMR prompt in the clinic’s annual visit template to remind providers to screen for HCV, (4) a petition to the institution’s EMR management board to include HCV as an automatic, age-specific, prompt within the Health Maintenance section and the addition of a modified prompt in the EMR that would 'force' a response to screening, (5) incorporation of HCV screening in the health maintenance section of the EMR and (6) individualized audit of provider’s HCV screening rates with rewards for those with the highest screening rates | Initial HCV screening rates were 24% | Clinic-wide screening rates exceeded 90% and documentation rates improved from 4% to 96%. |
| Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis C screening and curative treatment for baby boomers. Hepatology 2017;66:1805–13. 10.1002/hep.29362 | BPA alert was developed in conjunction with their EHR Population and Health Management Ambulatory Care Services Team and consultation from several PCPs | Patient educational flyers regarding the rationale for HCV screening in baby boomers were posted in primary care clinics and educational materials were also provided in the electronic patient portal to raise awareness and prime patients for potential discussion about HCV screening during their visit. The BPA would “fire” for any patient seen in primary care clinic who met the inclusion criteria | HCV screening was ordered in 28% of patients over a 3-year period | HCV screening was ordered in 71% of patients over 1-year period |
| Armstrong H, Gonzalez-Drigo M, Adeyemi O, Trick W, Diep L, et al. (2019) Electronic Clinical Decision Support (eCDS) intervention to Increase Hepatitis C Virus (HCV) Screening and Linkage to Care among Baby Boomers in Urban Safety-Net Health System. J Community Med Health Educ 9: 646. doi:10.4172/2161-0711.1000646 | Electronic clinical decision support tool implemented across all outpatient sites | Rule for testing was triggered for patients meeting the inclusion criteria | Number of newly tested patients each month was 2,874 | Number of newly tested patients each month was 12,756 (344% increase) and percent of eligible patients tested increased by 24% across all sites |
| Yeboah-Korang A, Beig MI, Khan MQ, Goldstein JL, Macapinlac DM, Maurer D, et al. Hepatitis C screening in commercially insured U.S. birth-cohort patients: Factors associated Med 2018; 6: 82-9. | Best Practice Alert (BPA) was generated and prominently displayed in the patient’s electronic medical record | EPIC alert was designed to identify age cohort patients who had not been previously HCV-tested at our institution of care. A BPA was generated and prominently displayed in the patient’s electronic medical record. The BPA prompted the physician to place an order for the HCV antibody, or to forego placing the test at their or the patient’s discretion | HCV testing rates were 0.68% pre-BPA (69/10,089) | HCV testing rates was 10.76% (5451/45188) |
| Shahnazarian V, Karu E, Mehta P Hepatitis C: improving the quality of screening in a community hospital by implementing an electronic medical record intervention BMJ Open Quality 2015;4:u208549.w3409. doi: 10.1136/bmjquality.u208549.w3409 | Created an algorithm in the electronic medical records to measure how many admitted and clinic patients were eligible for testing. The program shows how many of those patients had an HCV test result in the computer. | The implementation went through multiple PDSA cycles during the intervention period to fix problems that arose. The final product was a patient is flagged by EMR system if they are eligible. It then checks to see if the patient has either been offered HCV testing or if they have ever received it in the past. If they have ever received it, they are excluded. If they have never, an action box pops-up, asking the healthcare professional to offer testing to the patient and what the patient’s response is. Once a selection has been made, it is documented in the EMR. If they refuse, they are excluded from the EMR algorithm. If they accept or are unable to make a choice, the test is automatically ordered by the EMR system. | Dec 2013: testing offered was 47.2% | Feb 2015: testing offered was 87.9% test offering had statistically significant increase in testing in all months except Oct 2014 |
| Al-hihi E, Shankweiler C, Stricklen D, et al. Electronic medical record alert improves HCV testing for baby boomers in primary care setting: adults born during 1945–1965. BMJ Open Qual2017;6:e000084 10.1136/bmjoq-2017-000084 | Developed a workflow. This workflow included visual reminders in the EMR as best practice alerts, health maintenance overdue items, along with vaccinations, and reminders for breast, colorectal and cervical cancer screenings that were developed by IT | The best practice alerts and health maintenance alerts flagged all patients in the 1945–1965 birth cohort for HCV screening. Patients who had a history of HCV identified by positive antibody, positive viral load or positive genotype were considered to have received screening. In addition, patients who had been screened previously, using anti-HCV, also satisfied the measure. If a patient was identified in clinic as meeting the criteria for HCV screening, then the provider explained the reason for the screen to the patient, ordered the anti-HCV lab and instructed the patient to have the lab drawn. | 30% | 55% |
| Federman AD, Kil N, Kannry J, et al. An electronic health Record-based intervention to promote hepatitis C virus testing among adults born between 1945 and 1965: a cluster-randomized trial. Med Care 2017;55:590–7. 10.1097/MLR.0000000000000715 | best practice alert (BPA) programmed in the EHR to appear in yellow highlight on the EHR-clinician interface during visits to inform clinicians of the patients’ eligibility for HCV testing and to facilitate testing by providing a testing order set | Pathway 1: if a medical assistant opened the chart for an eligible patient before the clinician did, they would encounter a BPA prompting them to order an HCV test. The order would appear as “pended” when the clinician opened the chart, at which time the clinician could choose to discuss testing with the patient, sign the order, or delete it Pathway 2: if the clinician opened the chart before the medical assistant or if the medical assistant bypassed the alert presented, a different BPA appeared for the clinician. This BPA briefly outlined the Birth Cohort testing guidelines and suggested HCV testing for the patient. The alert presented an order for HCV testing which the clinician could accept or bypass. The HCV testing BPAs for both pathways would continue to appear at subsequent visits until an HCV testing order was placed. An order pended by the medical assistant could not be placed unless the clinician ultimately signing the order associated it with the appropriate diagnosis. The clinician could delete the pended test based on their clinical judgment. The BPA would reappear at subsequent visits until HCV testing was ordered or the clinician selected the option to delay or permanently exclude the patient from testing | 1.8% at control sites | 20.2% at intervention sites |
| Golden MR, Duchin J, Chew LD, et al. Impact of an electronic medical Record-Based system to promote human immunodeficiency virus/hepatitis C virus screening in public hospital primary care clinics. Open Forum Infectious Diseases 2017;4 10.1093/ofid/ofx075 | used CIP database to identify patients with scheduled clinic visits who met the criteria and pre-enter orders into the EMR for HCV testing | A list of people that were eligible was sent to a designated person in each clinic, every weekday morning and had pre-entered orders for HCV testing. Medical providers were required to electronically sign these orders before laboratory testing could be completed. | HCV: 18.0% | HCV: 35.5% Intervention had no impact on HCV or HIV case findings |
| Castrejón M, Chew KW, Javanbakht M, Humphries R, Saab S, Klausner JD. Implementation of a Large System-Wide Hepatitis C Virus Screening and Linkage to Care Program for Baby Boomers. Open Forum Infect Dis. 2017;4(3): ofx109. Published 2017 May 27. doi:10.1093/ofid/ofx109 | HCV screening status was added to the routine health maintenance reminder, a clinical decision support (CDS) tool in the EHR, for patients born between 1945 | HCV screening was noted as “due” among patients with no laboratory evidence of HCV testing (HCV antibody or HCV ribonucleic acid [RNA]) in their EHR or if testing was not marked as “completed” by the provider. The CDS tool was available in all charts, but it only appeared on the patient’s dashboard if previously activated by the provider. The care coordinator used an internal electronic message to contact outpatient providers to recommend the following: (1) quantitative HCV RNA testing with reflex to HCV genotype testing and (2) noninvasive serum fibrosis testing | 5676 patients tested | 13930 patients (145% overall increase in testing) |
| Burrell CN, Sharon MJ, Davis SM, Wojcik EM, Martin IBK. Implementation of a Collaborative HIV and Hepatitis C Screening Program in Appalachian Urgent Care Settings. West J Emerg Med. 2018;19(6):1057-1064. doi:10.5811/westjem.2018.9.39512 | BPAs were used to populate within the charts of registered patients who qualified to receive: 1) only an HIV screening; 2) only an HCV screening; or 3) both an HIV and HCV screening | Routine EMR-based HIV and HCV screenings were free of charge to all eligible patients. Placards were in care rooms, triage areas, and restrooms to inform patients of the current screenings, providing them the opportunity to opt out from testing. If eligible, providers and nursing staff discussed the options of screenings privately with patients. Other exclusions included providers' decisions on the populated BPAs, and patients refusing a venipuncture during their visit. Upon patient verbal consent, blood samples or oral swabs were obtained from eligible patients for HIV and/ or HCV testing. | 150 HCV screenings were conducted between June 2016-May 2017 | June 5, 2017- May 31, 2018: 1,895/7,465 (25.4%) of patients eligible according to BPA alert were screened for HCV |
HCV= Hepatitis C Virus; PDSA = Plan Do Check Act; BPA = Best Practice Alert; EHR = Electronic Health Record; PCP = Primary Care Physician; EMR = Electronic Medical Record; IT = Information Technology; CIP = Caradigm Intelligence Platform; CDS = clinical decision support; RNA = Ribonucleic Acid ; HIV= Human Immunodeficiency Viruses.
Of the eleven papers, four included data on the HCV continuum of care of those testing HCV seropositive (Table 3). Among the eligible reports that presented HCV care continuum outcomes, there was variance in the continuum steps and none of the studies provided sufficient data to compare pre and post implementation statistics on continuum of care.
Data from papers that included cascade of care results.
| Name of paper | Type of implementation | Statistics on cascade of care |
|---|---|---|
| Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis C screening and curative treatment for baby boomers. Hepatology 2017;66:1805–13. 10.1002/hep.29362 | BPA alert was developed in conjunction with their EHR Population and Health Management Ambulatory Care Services Team and consultation from several PCPs | Among the 56 patients with an initial detectable HCV RNA in the post-BPA period, 3 had low HCV RNA levels (<12 IU/mL) that were not confirmed on subsequent testing. All 53 patients with confirmed chronic hepatitis C were referred to specialty care. At the time of data analysis, a total of 46 had attended their specialty clinic appointment, 1 had an upcoming appointment, 2 declined referral, and 4 were lost to follow up. DAA therapy was prescribed in 31 patients, of whom 20 had started treatment. Of the 20 patients who started therapy, 9 had completed treatment and achieved SVR 12 and 11 had SVR labs upcoming, with 9 having end of treatment response |
| Armstrong H, Gonzalez-Drigo M, Adeyemi O, Trick W, Diep L, et al. (2019) Electronic Clinical Decision Support (eCDS) intervention to Increase Hepatitis C Virus (HCV) Screening and Linkage to Care among Baby Boomers in Urban Safety-Net Health System. J Community Med Health Educ 9: 646. doi:10.4172/2161-0711.1000646 | Electronic clinical decision support tool implemented across all outpatient sites | Due to restrictive Medicaid reimbursement policies, only 44% of patients with a fibrosis stage lower than F3 received HCV treatment |
| Castrejón M, Chew KW, Javanbakht M, Humphries R, Saab S, Klausner JD. Implementation of a Large System-Wide Hepatitis C Virus Screening and Linkage to Care Program for Baby Boomers. Open Forum Infect Dis. 2017;4(3): ofx109. Published 2017 May 27. doi:10.1093/ofid/ofx109 | HCV screening status was added to the routine health maintenance reminder, a clinical decision support (CDS) tool in the EHR, for patients born between 1945 | % of HCV antibody positive patients who received HCV RNA testing increased from 83% to 95%. HCV care coordinator was successful in facilitating linkage to care for 94% of those identified as HCV RNA positive |
| Burrell CN, Sharon MJ, Davis SM, Wojcik EM, Martin IBK. Implementation of a Collaborative HIV and Hepatitis C Screening Program in Appalachian Urgent Care Settings. West J Emerg Med. 2018;19(6):1057-1064. doi:10.5811/westjem.2018.9.39512 | BPAs, a clinical decision tool, were used to populate within the charts of registered patients who qualified to receive: 1) only an HIV screening; 2) only an HCV screening; or 3) both an HIV and HCV screening | Patients were referred for follow-up appointments with a university-based, infectious diseases clinic upon an initial HCV antibody-positive screening result. Thirty-one patients (1.6%) screened antibody-positive for HCV, with three (9.7%) subsequently having a positive RNA result. All patients with antibody-positive HCV results were referred to infectious diseases for follow-up through their PNs |
BPA = Best Practice Alert; EHR = Electronic Health Record; PCP = Primary Care Physician; HCV= Hepatitis C Virus; DAA = Direct-Acting Antiviral; SVR = Sustained Virologic Response; CDS = clinical decision support; HIV= Human Immunodeficiency Viruses.
Over the course of the Armstrong et al. study (pre and post implementation), 844 patients were determined to be HCV seropositive. Six hundred and five of 844 (71.7%) completed HCV RNA testing and 347 of 605 (57.4%) patients had detectable HCV RNA. Of these, 68 of 347 (19.6%) patients initiated HCV antiviral treatment [15]. In the Trinh et al. study, during implementation, 89.2% of HCV antibody positive patients were HCV RNA tested of which 62.6% were found to be HCV RNA positive. Of those diagnosed, 80.6% received follow up care [13]. Post implementation in the Konerman et al. study in which 28% of 56,220 Baby Boomers were screened for HCV, 53 patients were confirmed to have chronic HCV and 46 of these 53 (86.8%) attended a scheduled specialty appointment. HCV antiviral therapy was prescribed for 31 of these patients [10]. All patients that were screened and tested seropositive in the Burrell et al. study were referred to infectious diseases and had appointments completed or scheduled. Three of those 31 patients tested positive for HCV RNA.(6)
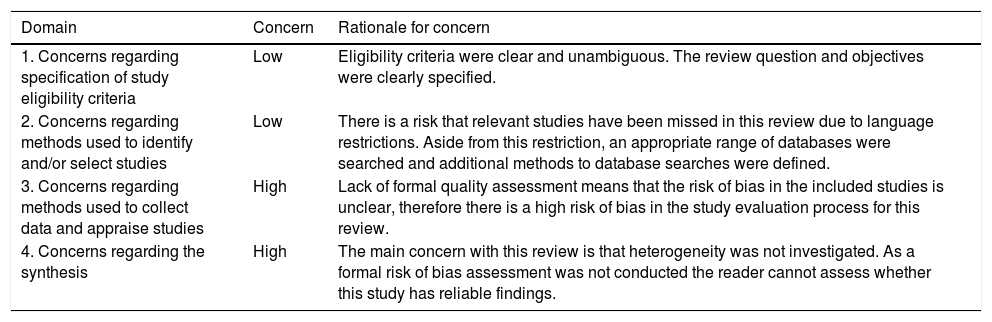
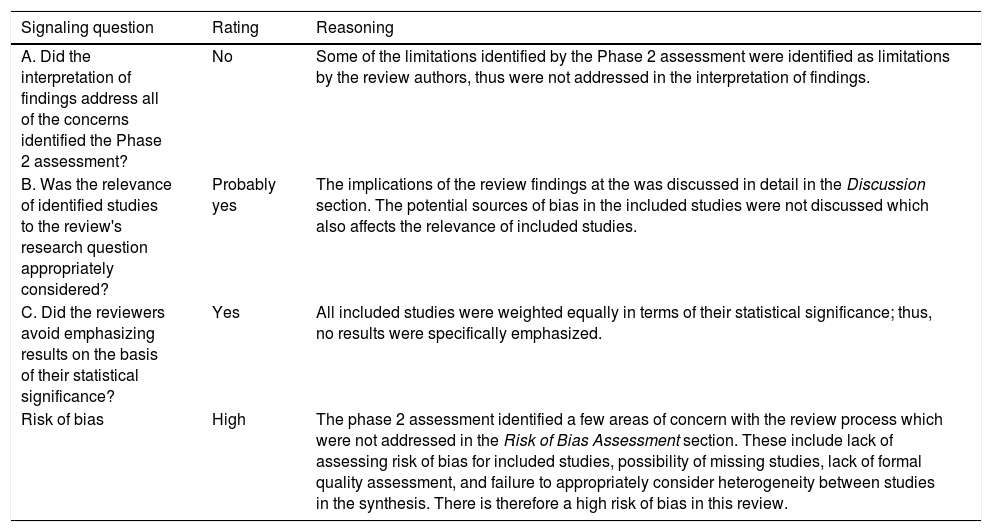
A summary of concerns identified during the assessment is provided in Table 4 and the rating for synthesis with information used to reach the judgment is reported in Table 5 [19]. An assessment utilizing the ROBIS tool is presented in Table 6.
Summary of concerns identified during the assessment.
| Domain | Concern | Rationale for concern |
|---|---|---|
| 1. Concerns regarding specification of study eligibility criteria | Low | Eligibility criteria were clear and unambiguous. The review question and objectives were clearly specified. |
| 2. Concerns regarding methods used to identify and/or select studies | Low | There is a risk that relevant studies have been missed in this review due to language restrictions. Aside from this restriction, an appropriate range of databases were searched and additional methods to database searches were defined. |
| 3. Concerns regarding methods used to collect data and appraise studies | High | Lack of formal quality assessment means that the risk of bias in the included studies is unclear, therefore there is a high risk of bias in the study evaluation process for this review. |
| 4. Concerns regarding the synthesis | High | The main concern with this review is that heterogeneity was not investigated. As a formal risk of bias assessment was not conducted the reader cannot assess whether this study has reliable findings. |
Rating for synthesis.
| Signaling question | Rating | Reasoning |
|---|---|---|
| A. Did the interpretation of findings address all of the concerns identified the Phase 2 assessment? | No | Some of the limitations identified by the Phase 2 assessment were identified as limitations by the review authors, thus were not addressed in the interpretation of findings. |
| B. Was the relevance of identified studies to the review's research question appropriately considered? | Probably yes | The implications of the review findings at the was discussed in detail in the Discussion section. The potential sources of bias in the included studies were not discussed which also affects the relevance of included studies. |
| C. Did the reviewers avoid emphasizing results on the basis of their statistical significance? | Yes | All included studies were weighted equally in terms of their statistical significance; thus, no results were specifically emphasized. |
| Risk of bias | High | The phase 2 assessment identified a few areas of concern with the review process which were not addressed in the Risk of Bias Assessment section. These include lack of assessing risk of bias for included studies, possibility of missing studies, lack of formal quality assessment, and failure to appropriately consider heterogeneity between studies in the synthesis. There is therefore a high risk of bias in this review. |
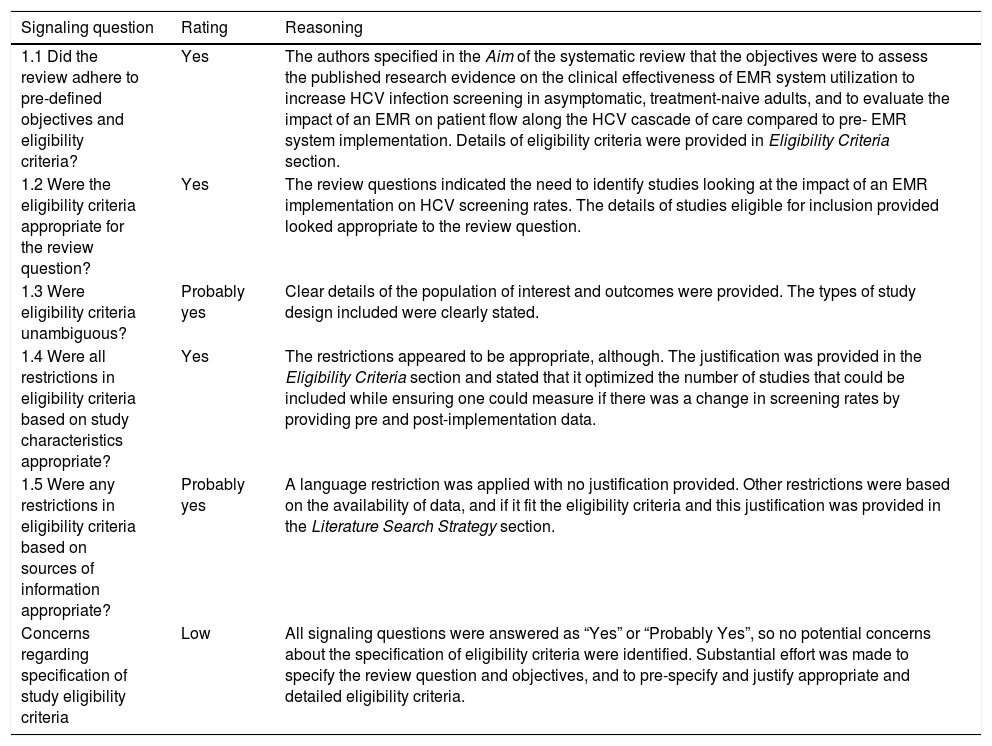
ROBIS Risk of Bias Assessment.
| Signaling question | Rating | Reasoning |
|---|---|---|
| 1.1 Did the review adhere to pre-defined objectives and eligibility criteria? | Yes | The authors specified in the Aim of the systematic review that the objectives were to assess the published research evidence on the clinical effectiveness of EMR system utilization to increase HCV infection screening in asymptomatic, treatment-naive adults, and to evaluate the impact of an EMR on patient flow along the HCV cascade of care compared to pre- EMR system implementation. Details of eligibility criteria were provided in Eligibility Criteria section. |
| 1.2 Were the eligibility criteria appropriate for the review question? | Yes | The review questions indicated the need to identify studies looking at the impact of an EMR implementation on HCV screening rates. The details of studies eligible for inclusion provided looked appropriate to the review question. |
| 1.3 Were eligibility criteria unambiguous? | Probably yes | Clear details of the population of interest and outcomes were provided. The types of study design included were clearly stated. |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | Yes | The restrictions appeared to be appropriate, although. The justification was provided in the Eligibility Criteria section and stated that it optimized the number of studies that could be included while ensuring one could measure if there was a change in screening rates by providing pre and post-implementation data. |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | Probably yes | A language restriction was applied with no justification provided. Other restrictions were based on the availability of data, and if it fit the eligibility criteria and this justification was provided in the Literature Search Strategy section. |
| Concerns regarding specification of study eligibility criteria | Low | All signaling questions were answered as “Yes” or “Probably Yes”, so no potential concerns about the specification of eligibility criteria were identified. Substantial effort was made to specify the review question and objectives, and to pre-specify and justify appropriate and detailed eligibility criteria. |
| Signaling question | Rating | Reasoning |
|---|---|---|
| 2.1 Did the search include an appropriate range of databases/ electronic sources for published and unpublished reports? | Yes | MEDLINE, PubMed and Google Scholar were searched. This was judged to be an appropriate range. |
| 2.2 Were methods additional to database searching used to identify relevant reports? | Probably yes | HCV conferences and abstract literature were also searched. |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | Yes | A detailed search strategy was provided in the Literature Search Strategy section. It appeared to have no inappropriate restrictions. |
| 2.4 Were restrictions based on date, publication format, or language appropriate? | No | The review was restricted to English language studies; there is therefore a potential for publication bias. |
| 2.5 Were efforts made to minimize errors in selection of studies? | Yes | Inclusion assessment is reported to have been conducted independently by at least two reviewers. It was explicit that this applied to both screening search results and assessing full text articles. |
| Concerns regarding methods used to identify and/or select studies | Low | Restriction of the review to English language articles means that we think that there is a risk that relevant studies have not been included in this review. Aside from that one potential area of bias, the review is likely to have included a high proportion of relevant studies. |
| Signaling question | Rating | Reasoning |
|---|---|---|
| 3.1 Were efforts made to minimize error in data collection? | Probably yes | Data extraction was performed by one reviewer and a second reviewer critiqued. Differences were resolved by agreement. |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | Yes | The summary tables included details on year of publication, type of implementation, method of implementation, proportion of patients being screened pre and post-implementation and data on patients continuing cascade of care. |
| 3.3 Were all relevant study results collected for use in the synthesis? | Probably yes | Detailed information was included in the Methods section on how results data were obtained when not reported in the format required for synthesis. Most included studies reported the data in the same format required for the synthesis. |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | No | Study quality was not formally assessed. |
| 3.5 Were efforts made to minimize error in risk of bias assessment? | No | Study quality was not formally assessed. |
| Concerns regarding methods used to collect data and appraise studies | High | Lack of formal quality assessment means that the risk of bias in the included studies is unclear, therefore there is a high risk of bias in the study evaluation process for this review. |
| Signaling question | Rating | Reasoning |
|---|---|---|
| 4.1 Did the synthesis include all studies that it should? | Probably yes | The author stated that 18 studies were relevant to the systematic review, of which 11 fit the inclusion criteria. |
| 4.2 Were all predefined analyses followed or departures explained? | No information | No analyses were predefined in an explicitly referenced protocol. No further information was given in the text. |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | Probably no | All studies were weighted equally, and results were pooled for studies that used the same type of EMR implementation. The author did not perform within-study comparisons although it appears that there is clinical diversity across the studies |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | Probably yes | A narrative synthesis was conducted on the basis that a statistical combination of all included studies was inappropriate. |
| 4.5 Was robustness of the finding(s) assessed e.g. through funnel plot or sensitivity analyses? | No information | Authors did not state whether sensitivity analyses were used to assess the robustness of their findings. |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | No | Risk of bias has not been assessed and reviewers have not incorporated it into findings/conclusions. |
| Concerns regarding the synthesis and findings | High | The author summed data from studies that used same type of EMR implementation. Risk of bias was not assessed for individual studies nor was potential bias considered for the synthesis. There was no discussion or assessment of heterogeneity in the analysis. |
The evaluation revealed a lack of assessing risk of bias for included studies, possibility of missing studies, lack of formal quality assessment, and failure to appropriately consider heterogeneity between studies in the synthesis. It was concluded that there is a high risk of bias present for the review.
4DiscussionBased on this analysis, utilization of an EMR system consistently results in increased offering of screening and testing for HCV infection in asymptomatic, treatment-naive adults. In all included studies, there was an increase in both test offering or screening rates by 10% or greater, regardless of the method of EMR implementation. Due to insufficient HCV cascade of care data, we were unable to conclude if EMR utilization increases the number of patients progressing along the HCV cascade of care. Specifically, some reports did not disclose the dates when cascade of care data was extracted. In other publications, the pre- and post-implementation data was combined.
This systematic review highlights the paucity of data related to the impact of EMR system usage on HCV screening rates in healthcare centers. As well, our review demonstrates the need for specific data on the impact of EMR on number of patients continuing along the HCV cascade of care post-implementation. None of the evaluations included in our systematic review were performed in Canada. Thus, evidence is required from EMR screening strategies relevant to the Canadian publicly funded, universal health care access system and to specific at-risk populations residing within Canada.
Most of the identified studies (10 of 11) targeted a specific birth cohort population and may have excluded those in high risk groups with HCV infection [5,7–15]. A testing strategy of at least one-time testing for Baby Boomers is likely to be cost-effective and lifesaving in Canada [3]. However, only targeting those in the birth cohort may diminish the number of newly diagnosed patients. Although Baby Boomers make up a large portion of those with current infections, newly diagnosed infections are increasing rapidly among those outside this birth cohort [3,6]. High risk groups should be targeted in order to have a greater impact on decreasing infection incidence and the number of people living with undiagnosed HCV [3]. In Canada, up to two thirds of those who inject drugs have or had an HCV infection, 35% of all those infected are immigrants and newcomers, and close to a quarter of Canadian prisoners have a current or past infection [3]. Thus, we believe that the utility for EMR screening in other priority populations should be assessed in future studies. Alternatively, universal HCV screening by EMR systems should be considered to maximize the diagnostic yield. This approach also reduces stigma related to targeted screening strategies.
The identified references in our systematic review focused on larger health care facilities. In the last 15 years, there has been a continuous transition toward the use of EMR systems in the primary care setting [4]. It is important to recognized that HCV screening utilizing EMR systems is an important service that primary care providers can offer [20]. We recommend further evaluation of this approach to HCV case identification.
EMR implementation has multiple benefits including reduced medical errors, facilitated patient information sharing with healthcare providers, and lower cost of administration [4]. Nonetheless, there are recognized challenges to implementation. A key barrier to EMR system adoption is the disconnect between the cost of implementing and maintaining the technology, the medical benefits to patients, and financial reimbursement for health care providers and/or medical facilities [4]. It is key to bridge these disconnects. Other concerns include privacy, security, and programming errors [4]. Transitioning to an EMR system requires a major investment in technology and considerable clinician training [4]. Implementation can initially reduce primary care facility productivity until workers are adjusted.
Despite these challenges, our work suggests that the benefits of EMR systems within the HCV realm are justified. Utilization of EMR screening reminder systems reduce the number of those living with undiagnosed HCV. Additional research is required to evaluate the impact of EMR-based screening on the numbers of people continuing along each step of the HCV cascade of care.
Authors’ contributionsCC: concept, analysis, writing; LB: data collection, analysis, writing, risk of bias assessment. Both authors read and approved the final manuscript.
FundingFunding to support this work was provided by a Canadian Network on Hepatitis C - 2020 CanHepC Summer Student Award provided to Lauren Barter.
Conflicts of interestThe authors have no conflicts of interest to declare.
Excluded papers (did not provide data pre-implementation of EMR)- •
Kasting ML, Giuliano AR, Reich RR, Duong LM, Rathwell J, Roetzheim RG, Vadaparampil ST. Electronic medical record-verified hepatitis C virus screening in a large health system. Cancer Med. 2019;8(10):4555-64.
- •
Geboy AG, Nichols WL, Fernandez SJ, Desale S, Basch P, Fishbein DA. Leveraging the electronic health record to eliminate hepatitis C: Screening in a large integrated healthcare system. PLoS One. 2019;14(5):e0216459.
- •
Turner BJ, Taylor BS, Hanson JT, Perez ME, Hernandez L, Villarreal R, Veerapaneni P, Fiebelkorn K. Implementing hospital-based baby boomer hepatitis C virus screening and linkage to care: Strategies, results, and costs. J Hosp Med. 2015;10(8):510-6.