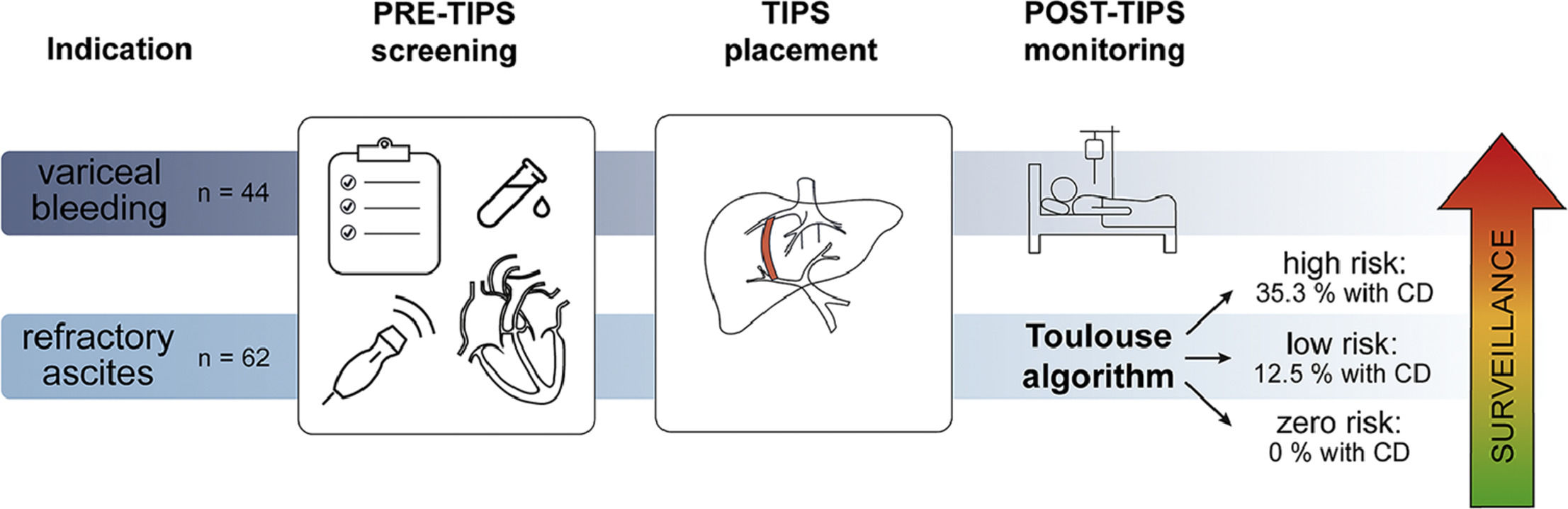
Transjugular intrahepatic portosystemic shunt (TIPS) is a well-established treatment for portal hypertension-related complications such as refractory ascites and variceal bleeding. The TIPS stent creates a shunt between the splanchnic and systemic circulation resulting in a brisk and effective reduction of the increased portal pressure. Unfortunately, shunt placement might result in complications such as hepatic encephalopathy, ischemic hepatitis and cardiac decompensation. Cardiac decompensation (CD) after TIPS is a direct consequence of the volume shift after shunt formation which results in an increased venous return and therefore cardiac preload. Failure to adapt to these hemodynamic changes could trigger CD after TIPS [1,2].
An impaired inotropic and chronotropic response of the left ventricle to stress is a specific characteristic of the cardiac dysfunction seen in patients with cirrhosis defined by the term ‘cirrhotic cardiomyopathy’ (CCMP) [3]. Other characteristics associated with CCMP include impaired diastolic relaxation and electrophysiological abnormalities (e.g. QT prolongation) in the absence of other underlying cardiac diseases [3]. TIPS placement may represent a clear stressor for the heart, especially in those with preexisting cirrhotic heart disease.
Patient selection is key in the prevention of complications, such as CD, and mortality post-TIPS [4–8]. However, the decision-making process to do so is still cumbersome and often ill-defined. Cardiac dysfunction in patients receiving TIPS has previously been studied, but results regarding the link between diastolic dysfunction and survival have been contradictory [1,9,10]. To our knowledge, only two studies to this day have looked at predictors specifically for CD post-TIPS [11,12]. In 2017, in a large TIPS database from the Cleveland Clinic, Modha et al. identified higher pre-TIPS right atrial and portal vein pressures, higher albumin level, longer prothrombin time (PT) and older age as possible predictors for symptomatic heart failure post-TIPS. In 2019, Billey et al. defined the ‘Toulouse algorithm’, a combination of (N-terminal pro-) brain natriuretic peptide ((NT-pro)BNP) and echocardiographic parameters that could aid patient selection for TIPS by identifying those at risk for CD [11].
In this retrospective study, we wanted to (I) investigate the incidence of CD post-TIPS, (II) study the relation between baseline variables including echocardiographic parameters with CD post-TIPS, and (III) assess if the Toulouse algorithm could have predicted CD in our population.
2Patients and Methods2.1Study populationAll patients with decompensated cirrhosis who underwent TIPS for either variceal bleeding or refractory ascites between 2011 and 2021 at the University Hospital of Leuven, Belgium, were included. A total of 219 patients underwent TIPS placement, of which 106 patients had both echocardiographic parameters and NT-proBNP results available.
Clinical data, lab results, imaging results and all hemodynamic measurements pre- and post-TIPS were extracted from the patient's medical file. The presence of CD was based on documentation of CD post-TIPS in the medical file. The need for intravenous diuretics to relieve acute lung oedema in the immediate postoperative period after TIPS was also recorded as CD. All complications were recorded from TIPS placement until the occurrence of liver transplantation, death or loss-to-follow-up.
2.2TIPS procedureAll patients received a covered TIPS stents (Gore Viatorr TIPS endoprosthesis®, Gore & Associates, Newark, Delaware, USA). Initially, all patients received self-expanding Viatorr stents. However, after the introduction of the controlled expansion Viatorr stents, the latter gradually replaced the former. From March 2019 onwards, all patients received controlled expansion stents. TIPS stents were dilated to 6, 8 or 10 mm at the discretion of the interventional radiologist while aiming for a post-TIPS portosystemic gradient of 12 mmHg or less [13]. Every patient was admitted to our intensive care unit for at least 24 h after TIPS placement. Patency of the stents was typically assessed by Doppler ultrasound 3 to 6 months after TIPS placement and every 6 months thereafter at the occasion of scheduled hepatocellular carcinoma (HCC) screening or revised earlier upon clinical indication.
2.3Echocardiography and nt-proBNPNT-proBNP was measured via an automated immunoassay (Roche Diagnostics, Mannheim, Germany) (unit ng/L or pg/mL) with normal value less than 125 ng/L. When a 12-lead electrocardiogram was available, the QT interval was measured and corrected according to the Bazett formula (QTc = QT/RR1/2) and Fridericia's formula (QTc = QT/(3.02 x RR1/2)). QTc was considered prolonged if >440 ms in men or >460 ms in women. Echocardiography was performed using a Vivid E9 or E95 ultrasound system (GE Vingmed, Harten, Norway) and digitally stored for offline analysis using the EchoPAC software (version 204; GE Healthcare, Horten, Norway). When available, the following measurements were extracted from the medical file: left ventricle ejection fraction (LVEF), presence of aortic valve stenosis (AoS), systolic pulmonary artery pressure (sPAP) and measures of diastolic dysfunction such as E/A ratio, E/e’ ratio and the left atrial volume index (LAVI). LVEF was calculated by the Simpson's biplane method using the apical 4- and 2-chamber views. Presence and grade of AoS were classified according to the valve area with >3.0 cm2 indicating absence of an AoS, 1.5–3.0 cm2 grade 1 AoS, 1.5–1.0 cm2 grade 2 AoS and <1.0 cm2 grade 3 AoS. sPAP (unit mmHg) was estimated from the peak tricuspid regurgitation velocity. The peak early (E wave) and late (A wave) transmitral velocities were measured by pulsed wave Doppler from the apical 4-chamber view with the sample volume placed at the tip of the mitral leaflets. The early peak myocardial relaxation velocity (e’) was measured using pulsed wave tissue Doppler obtained in the apical 4-chamber view at the septal mitral annulus. The E wave, A wave and e’ were used to calculate the E/A ratio and E/e’ ratio. The left atrial volume was assessed by the biplane area-length method from apical 4- and 2-chamber views and indexed to body surface area (unit ml/m2) resulting in the LAVI. All measurements were performed in accordance with international guidelines [14,15].
2.4Statistical analysisContinuous variables were reported as mean values ± standard deviation (SD), or median values with interquartile range (IQR) as indicated. Categorical variables were recorded as numbers and/or percentages. The Chi-square test was used to compare categorical variables. Continuous variables were compared with the Student t-test, one-way analysis of variance (ANOVA) or the Mann-Whitney U test as appropriate. Paired tests were used when comparing data within subjects. Correlation between variables was studied using Spearman's rho. Subgroup analysis was used to investigate patients receiving TIPS for refractory ascites and TIPS for variceal bleeding separately.
Univariate Cox regression was applied to assess variables associated with CD post-TIPS. Individual biochemical variables were used in the regression analysis, rather than compound scores such as the MELD and Child-Pugh score. Variables with p < 0.05 on univariate analysis were entered in the multivariate model. We used the Fine and Gray subdistribution hazard function with liver transplantation and death as competing risks to study the association with post-TIPS complications. Kaplan-Meier curves were compared using the log-rank test.
Only cases with available NT-proBNP and echocardiography reports were included, nevertheless, some values were missing, especially in cases performed before 2019, when structured reporting of echocardiography, including detailed hemodynamic measurements, was not yet implemented as standard of care. Missing data was handled by listwise deletion.
P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 28.0, IBM, Armonk, NY, USA) and R statistics (version 4.1.2, Vienna, Austria).
2.5Ethical statementsThis retrospective study was approved by the local research Ethics Committee of the University Hospital of Leuven, Belgium (MP017025). Need for an informed consent was waived by the Ethics Committee Research UZ/KU Leuven. Research was conducted in accordance with the Declarations of Helsinki.
3Results3.1Patient characteristicsA total of 219 patients underwent TIPS placement, of which 106 patients had both echocardiographic parameters and NT-proBNP available. Out of these 106 patients, 62 received TIPS for refractory ascites and 44 for variceal bleeding. Fig. 1 shows the inclusion flowchart. Patients with variceal bleeding underwent TIPS either as a rescue procedure (47.7%), a pre-emptive procedure (36.4%) or as secondary prophylaxis (15.9%). Included patients were mainly men (69.8%) with a mean age of 60.6 years old. Alcohol-related liver disease was the predominant aetiology (72.6%). Table 1 gives an overview of the study population demographics.
Study population demographics and comparison of baseline characteristics of patients receiving TIPS for refractory ascites and variceal bleeding.
| Entire cohort(n = 106) | TIPS for refractory ascites(n = 62) | TIPS for variceal bleeding(n = 44) | p-value | |
|---|---|---|---|---|
| Male sex | 69.8 % | 67.7 % | 72.7 % | 0.670 |
| Age (years) | 63.0 (54.7–68.0) | 61.5 (53.7–67.0) | 63.5 (55.2–68.7) | 0.634 |
| BMI (kg/m2) | 24.2 (22.1–27.0) | 23.6 (21.3–26.8) | 24.9 (22.8–27.1) | 0.230 |
Etiology
| 72.6 %12.3 %5.7 %9.4 % | 74.2 %12.9 %8.1 %4.8 % | 70.5 %11.4 %2.3 %15.8 % | 0.481 |
| Actively drinking at TIPS placement | 45.3 % | 41.9 % | 50.0 % | 0.411 |
| Controlled expansion TIPS stent | 34.0 % | 36.2 % | 37.0 % | 0.846 |
| PSPG before TIPS (mmHg) | 15.0 (13.0–19.0) | 14.5 (12.0–17.2) | 16.5 (14.0–21.7) | 0.012 |
| PSPG after TIPS (mmHg) | 5.0 (4.0–7.0) | 5.0 (4.0–7.0) | 5.5 (4.0–7.0) | 0.915 |
| Creatinine (mg/dL) | 1.06 (0.73–1.30) | 1.11 (0.76–1.31) | 0.89 (0.67–1.30) | 0.837 |
| Albumin (g/L) | 32.3 (27.9–37.0) | 32.6 (28.1–36.7) | 31.9 (27.0–38.0) | 0.640 |
| Platelets (x109/L) | 122.5 (78.7–176.0) | 134.5 (99.2–192.2) | 100.0 (67.5–145.5) | 0.002 |
| Bilirubin (mg/dL) | 1.36 (0.79–2.12) | 1.0 (0.65–1.69) | 1.7 (1.0–3.7) | < 0.001 |
| INR | 1.4 (1.2–1.5) | 1.3 (1.2–1.5) | 1.4 (1.3–1.9) | 0.002 |
| AST (U/L) | 43.0 (29.0–70.2) | 36.0 (26.7–48.5) | 64.0 (35.2–130.0) | < 0.001 |
| ALT (U/L) | 24.0 (16.0–37.0) | 20.0 (14.0–28.0) | 35.0 (24.0–81.0) | < 0.001 |
| White cell count (x109/L) | 6.2 (4.6–8.6) | 5.8 (4.8–7.7) | 7.9 (4.5–12.9) | 0.029 |
| Sodium (mmol/L) | 136.0 (132.4–139.1) | 135.0 (131.3–137.4) | 138.4 (135.1–141.1) | < 0.001 |
| Ammonia (μmol/L) | 73.0 (56.0–96.0) | 68.0 (56.0–82.0) | 85.0 (57.0–138.7) | 0.034 |
| MELD score | 12.3 (10.2–16.5) | 11.8 (10.1–14.4) | 14.1 (10.2–20.6) | 0.040 |
| Child-Pugh score | 8.0 (7.0–9.2) | 8.0 (7.0–9.0) | 8.0 (7.0–11.0) | 0.230 |
| CLIF-C OF score | 6.0 (6.0–8.0) | 6.0 (6.0–6.0) | 8.5 (6.0–12.0) | < 0.001 |
| ACLF at TIPS placement | 20.8 % | 4.8 % | 43.2 % | < 0.001 |
| NT-proBNP (ng/L) | 154.0 (98.0–587.0) | 150.0 (98.0–639.5) | 171.0 (98.5–533.0) | 0.864 |
| QTc (Bazett formula, msec) | 459.0 (441.0–482.5) | 457.0 (441.0–479.5) | 468.0 (441.0–490.0) | 0.178 |
| QTc (Fridericia formula, msec) | 438.5 (420.0–458.5) | 436.7 (419.2–455.7) | 441.5 (420.5–471.2) | 0.107 |
LV EF
| 0 %20.9 %79.1 % | 0 %24.6 %75.4 % | 0 %15.9 %84.1 % | 0.207 |
Aortic valve stenosis
| 81.4 %13.7 %4.9 %0% | 83.0 %11.9 %5.1 %0 % | 79.0 %16.3 %4.7 %0 % | 0.814 |
| sPAP (mmHg) | 25.0 (20.0–30.0) | 24.0 (20.0–27.0) | 26.5 (21.5–32.5) | 0.142 |
| E/A | 1.0 (0.8–1.2) | 0.9 (0.7–1.18) | 1.1 (0.85–1.35) | 0.132 |
| E/e’ | 7.3 (5.6–9.6) | 7.0 (5.4–9.5) | 7.6 (6.2–10.6) | 0.168 |
| LAVI (ml/m2) | 33.0 (27.4–40.4) | 30.4 (25.3–37.9) | 39.0 (32.7–48.2) | 0.002 |
| Cardiac decompensation | 11.3 % | 12.9 % | 9.1 % | 0.757 |
| Mortality one year after TIPS | 43.4 % | 43.5 % | 43.2 % | 0.970 |
| Liver transplantation after TIPS | 10.4% | 11.3 % | 9.1 % | 1.000 |
Numbers represent medians (IQR) and percentages. ACLF, Acute-on-Chronic Liver Failure; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BMI, Body Mass Index; CLIF-C OF, Chronic Liver Failure Consortium Organ Failure; E/A, Ratio of early (E) to late (A) ventricular filling velocities; E/e’, Ratio of early (E) mitral inflow velocity to early (e’) diastolic mitral annular velocity; INR, International Normalized Ratio; LAVI, Left Atrial Volume Index; LV EF, Left Ventricular Ejection Fraction; MELD, Model for End-Stage Liver Disease; NT-proBNP, N-terminal pro B-type Natriuretic Peptide; PSPG, Portal-Systemic Pressure Gradient; QTc, Corrected QT interval; sPAP, Systolic Pulmonary Arterial Pressure; TIPS, Transjugular Intrahepatic Portosystemic Shunt.
Patients receiving TIPS for variceal bleeding had more severe liver disease compared to the refractory ascites group illustrated by the significantly higher mean MELD score, CLIF-C OF score and higher portosystemic pressure gradient (PSPG) before TIPS placement (see Table 1).
The majority of included patients had an increased NT-proBNP (54.7%) and prolonged QTc (65.5%). There were no patients with a reduced ejection fraction in our population (i.e. LVEF <45%). Furthermore, there were no patients receiving TIPS with pre-existing severe AoS (i.e. grade 3). Half of the patients (50.7%) had pulmonary hypertension determined by an estimated sPAP >25 mmHg on echocardiography. Cardiac parameters did not differ between the refractory ascites and variceal bleeding group, except for LAVI which was significantly higher in the latter group (see Table 1).
Median time between TIPS and lab results was 1 day before TIPS (IQR −1 to 0 days), between TIPS and ultrasound 3 days before TIPS (IQR −28.25 to 1 day) and between TIPS and echocardiography 6 days before TIPS (IQR −41.75 to 0 days). Before 2019 (year of publication of the Toulouse algorithm), NT-proBNP was only occasionally determined. Thereafter, it became standard of care. Median time between TIPS and NT-proBNP analysis was 1 day before TIPS (IQR −14.25 to 0.5 days).
To assess possible selection bias, we compared baseline features of patients receiving TIPS between 2011 and 2021 with and without echocardiography and NT-proBNP results. Incidence of cardiac decompensation in patients without echocardiography and NT-proBNP was not significantly different from that in the current cohort (6.2% vs. 11.4%, p 0.178). Patients of whom echocardiography results were available more often underwent TIPS placement for refractory ascites (57.1% vs 25.0%, p 0.001), more frequently received controlled expansion stents (39.7% vs 14.8%, p 0.012), less often had active alcohol abuse (46.6% vs 67.9%, p 0.036), had higher albumin levels (32.4 vs 29.1 g/L, p 0.009) and lower sodium levels (135.8 vs 138.8 mmol/L, p 0.004) compared to patients without echocardiography results available. Patients of whom NT-proBNP measurement was available, were often older (60.4 vs 57.3 years old, p 0.051). All other baseline variables did not show significant differences (see Supplemental Table 1).
3.2Incidence of cardiac decompensation post-TIPSDuring a median follow-up time of 328 days (IQR 52.25 to 933.5 days) 12 patients (11.3%) developed CD. The median time between TIPS and CD was 11.5 days (IQR 4 to 56.5 days). Two patients (16.7%) experienced CD within 48 h, and nine patients (75%) experienced CD within 30 days post-TIPS.
Patients who developed CD were significantly older than patients who did not (66.8 vs 59.8 years old, p 0.045). Other baseline parameters were not significantly different, specifically MELD score, PSPG before/after TIPS, and inferior vena cava (IVC)/right atrium (RA) pressure before/after TIPS were similar (see Table 2).
Comparison of baseline characteristics of patients with or without cardiac decompensation after TIPS placement.
| No cardiac decompensation(n = 94) | Cardiac decompensation(n = 12) | p-value | |
|---|---|---|---|
| TIPS for refractory ascites | 57.4 % | 66.7 % | 0.757 |
| Male sex | 70.2 % | 66.7% | 0.751 |
| Age (years) | 61.5 (52.7–67.2) | 67.0 (61.0–69.7) | 0.045 |
| BMI (kg/m2) | 23.9 (21.9–26.8) | 25.3 (23.4–28.2) | 0.274 |
| Alcohol-related liver cirrhosis | 73.4 % | 66.7 % | 0.732 |
| Actively drinking at TIPS placement | 46.8 % | 33.3 % | 0.377 |
| Controlled expansion TIPS stent | 32.6 % | 50.0 % | 0.333 |
| PSPG before TIPS (mmHg) | 15.0 (13.0–19.0) | 14.5 (12.0–16.7) | 0.268 |
| PSPG after TIPS (mmHg) | 5.0 (4.0–7.0) | 4.0 (3.2–6.0) | 0.114 |
| IVC/RA pressure before TIPS (mmHg) | 9.0 (6.0–12.0) | 11.0 (5.7–16.5) | 0.363 |
| IVC/RA pressure after TIPS (mmHg) | 12.0 (9.0–16.0) | 14.0 (6.5–21.7) | 0.327 |
| MELD score | 11.9 (10.1–16.5) | 13.3 (10.5–20.1) | 0.389 |
| Child-Pugh score | 8.0 (7.0–10.0) | 8.0 (7.0–9.0) | 0.634 |
| CLIF-C OF score | 6.0 (6.0–8.0) | 6.0 (6.0–7.7) | 0.938 |
| ACLF at TIPS | 21.3 % | 16.7 % | 1.000 |
| NT-proBNP (ng/L) | 150.0 (97.0–512.0) | 539.5 (135.0–1255.0) | 0.492 |
| QTc (Bazett formula, msec) | 459.0 (441.5–482.2) | 457.0 (437.0–497.0) | 0.589 |
| QTc (Fridericia formula, msec) | 438.0 (419.5–458.0) | 439.0 (427.0–480.0) | 0.121 |
LVEF
| 0%23.4 %76.6 % | 0%8.3 %91.7 % | 0.457 |
Aortic valve stenosis
| 82.2 %13.3 %4.4 %0% | 75.0 %16.7 %8.3 %0% | 0.787 |
| sPAP (mmHg) | 25.0 (20.0–30.0) | 25.0 (22.0–31.0) | 0.816 |
| E/A | 1.0 (0.8–1.2) | 0.9 (0.6–1.3) | 0.725 |
| E/e’ | 7.4 (5.5–9.5) | 6.3 (7.0–10.6) | 0.886 |
| LAVI (ml/m2) | 32.6 (26.5–40.0) | 39.3 (29.3–48.5) | 0.140 |
| Mortality one year after TIPS | 44.7 % | 33.3 % | 0.455 |
Numbers represent medians (IQR) and percentages. ACLF, Acute-on-Chronic Liver Failure; BMI, Body Mass Index; CLIF-C OF, Chronic Liver Failure Consortium Organ Failure; E/A, Ratio of early (E) to late (A) ventricular filling velocities; E/e’, Ratio of early (E) mitral inflow velocity to early (e’) diastolic mitral annular velocity; IVC/RA, Inferior Vena Cava/Right Atrium; LAVI, Left Atrial Volume Index; LVEF, Left Ventricular Ejection Fraction; MELD, Model for End-Stage Liver Disease; NT-proBNP, N-terminal pro B-type Natriuretic Peptide; PSPG, Portal-Systemic Pressure Gradient; QTc, Corrected QT interval; sPAP, Systolic Pulmonary Arterial Pressure; TIPS, Transjugular Intrahepatic Portosystemic Shunt.
Echocardiographic parameters were not different between the two groups. Grade 1 and 2 AoS were slightly more frequent in the CD group (two grade 1 AoS and one grade 2 AoS), though the difference did not reach statistical significance. Two out of the twelve patients who developed CD had grade 1 aortic stenosis. Both patients received IV diuretics which relieved heart failure symptoms. Out of the five included patients with grade 2 aortic stenosis only one (male, 65yo) developed CD 25 days post-TIPS, which sadly progressed to recurrent heart failure episodes. He underwent surgical valve replacement 7 months post-TIPS as part of the work-up for liver transplantation and developed a delayed intrathoracic bleed post-surgery leading to hemorrhagic shock, multiorgan failure and eventually death.
When reviewing the 12 cases with CD, we saw that most patients were still in-hospital when symptoms of CD developed, which were managed with an increased dose or initiation of diuretics. Only two patients were rehospitalised and three patients were transferred to the intensive care unit. One required non-invasive and one invasive mechanical ventilation because of acute pulmonary oedema. One single patient, who also developed ischemic hepatitis immediately after TIPS, needed TIPS reduction. One patient receiving TIPS for acute variceal bleeding with ACLF grade 3 at time of TIPS placement, experienced cardiac arrest and subsequently died immediately after TIPS which was deemed due to acute pulmonary oedema and was therefore also allocated to the cardiac decompensation group. However, overall, mortality one year after TIPS was not elevated in patients with CD (with CD: 33.3% vs. without CD: 44.7%, p 0.455).
3.3Variables associated with cardiac decompensation post-TIPSFactors associated with CD were studied separately for patients with TIPS for either refractory ascites or variceal bleeding for two reasons. The first being the baseline differences between these two populations as shown in Table 1. Secondly, since the circumstances for TIPS placement in these two populations are very different, with TIPS for variceal bleeding being mostly performed in (the prevention of) life-threatening situations and TIPS for refractory ascites performed in non-urgent settings to improve quality of life.
In patients receiving TIPS for treatment of refractory ascites, competing risk univariate analysis found a significant association between CD and older age, higher albumin, higher NT-proBNP and higher LAVI. After multivariate regression, we found age, albumin and NT-proBNP predicted CD after shunt placement. The link between LAVI and CD presumably disappeared on multivariate analysis because higher LAVI correlated with higher NT-proBNP values (Spearman's rho 0.40, p 0.008).
Although NT-proBNP is significantly associated with CD, the hazard ratio (HR) is only 1.00 (CI 1.00–1.00), which is a result of the small unit (ng/L) of this measure. When we calculate the HR for every 100 ng/L increase in NT-proBNP the HR becomes 1.04 (CI 1.00–1.07) or for every 1000 ng/L increase the HR is 1.43 (CI 1.05–1.93). Fig. 2 illustrates the influence of a baseline NT-proBNP greater than 125 ng/L on the incidence of CD post-TIPS in the entire study population.
In TIPS for variceal bleeding, higher creatinine and International Normalised Ratio (INR), but lower albumin levels were linked to CD on univariate competing risk analysis. However, none of these variables remained significant on multivariate analysis. Again, significant correlation was found between higher creatinine and higher INR (Spearman's rho 0.45, p 0.002), and between higher INR and lower albumin (Spearman's rho −0.43, p 0.004), likely explaining why significance disappeared on multivariate analysis. See Table 3 for an overview of the uni- and multivariate Fine and Gray analysis.
Univariate and multivariate Fine and Gray regression analysis for cardiac decompensation after TIPS placement.
| TIPS for refractory ascites | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | p-value | aHR | 95% CI | p-value | |
| Age | 1.05 | 1.01 - 1.09 | 0.013 | 1.06 | 1.01 - 1.11 | 0.019 |
| Creatinine | 1.74 | 0.97 - 3.10 | 0.062 | |||
| Bilirubin | 1.32 | 0.70 - 2.48 | 0.39 | |||
| INR | 5.04 | 0.41 - 61.8 | 0.21 | |||
| Sodium | 1.05 | 0.91 - 1.23 | 0.49 | |||
| Albumin | 1.13 | 1.07 - 1.19 | < 0.001 | 1.10 | 1.03 - 1.18 | 0.009 |
| NT-proBNP | 1.00 | 1.00 - 1.00 | 0.022 | 1.00 | 1.00 - 1.00 | 0.023 |
| QTc (Baz) | 1.01 | 0.98 - 1.03 | 0.53 | |||
| QTc (Fri) | 1.02 | 0.98 - 1.00 | 0.065 | |||
| AoS grade | 1.59 | 0.64 - 3.95 | 0.31 | |||
| sPAP | 1.09 | 0.97 - 1.20 | 0.060 | |||
| E/A | 0.73 | 0.05 - 10.5 | 0.82 | |||
| E/e’ | 1.11 | 0.91 - 1.36 | 0.31 | |||
| LAVI | 1.08 | 1.02 - 1.15 | 0.014 | 1.04 | 0.99 - 1.09 | 0.16 |
| TIPS for variceal bleeding | ||||||
| Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | p-value | aHR | 95% CI | p-value | |
| Age | 1.13 | 0.96 - 1.34 | 0.15 | |||
| Creatinine | 3.57 | 1.61 - 7.89 | 0.002 | 4.35 | 0.31 - 60.5 | 0.27 |
| Bilirubin | 1.08 | 0.93 - 1.25 | 0.32 | |||
| INR | 2.53 | 1.34 - 4.77 | 0.004 | 0.63 | 0.06 - 6.89 | 0.71 |
| Sodium | 0.92 | 0.79 - 1.07 | 0.26 | |||
| Albumin | 0.92 | 0.86 - 0.99 | 0.031 | 0.95 | 0.88 - 1.02 | 0.17 |
| NT-proBNP | 1.00 | 0.99 - 1.00 | 0.71 | |||
| QTc (Baz) | 1.00 | 0.97 - 1.03 | 0.86 | |||
| QTc (Fri) | 1.01 | 0.97 - 1.04 | 0.65 | |||
| AoS grade | 1.02 | 0.16 - 6.44 | 0.98 | |||
| sPAP | 0.92 | 0.81 - 1.05 | 0.22 | |||
| E/A | 1.78 | 0.04 - 70.5 | 0.76 | |||
| E/e’ | 0.97 | 0.72 - 1.31 | 0.85 | |||
| LAVI | 1.02 | 0.88 - 1.18 | 0.76 | |||
AoS, Aortic Valve Stenosis; E/A, Ratio of early (E) to late (A) ventricular filling velocities; E/e’, Ratio of early (E) mitral inflow velocity to early (e’) diastolic mitral annular velocity; INR, International Normalized Ratio; LAVI, Left Atrial Volume Index; NT-proBNP, N-terminal pro B-type Natriuretic Peptide; QTc (Baz), Corrected QT interval (Bazett formula); QTc (Fri), Corrected QT interval (Fridericia formula); sPAP, Systolic Pulmonary Arterial Pressure; TIPS, Transjugular Intrahepatic Portosystemic Shunt.
Following the algorithm designed by Billey et al. [11], 90 patients could be classified into three risk groups, namely: 48 patients (53.3%) in the zero-risk group, 10 patients (11.1%) in the low-risk group and 32 patients (25.6%) in the high-risk group (see Table 4). The algorithm was able to identify a significant difference in the risk for CD (p 0.047), with the high-risk group identifying more patients that eventually would develop CD. Almost 80% of patients experiencing post-TIPS CD were classified in the high-risk group. However, 2 patients within the zero-risk group also developed CD. Both these patients received TIPS for variceal bleeding. Therefore, we revisited the Toulouse classification for each indication separately. As a result, we observed that the algorithm worked well in the refractory ascites population but failed in the variceal bleeding population (see Table 4).
There were only 3 patients with TIPS for variceal bleeding developing CD of which 2 were allocated to the zero-risk group. The first of these two patients (male, 70yo) received a pre-emptive TIPS for acute variceal bleeding and went on to develop CD 67 days post-TIPS needing initiation of diuretics. NT-proBNP was 100 ng/L, classifying him as low-risk according to the algorithm. Echocardiography was performed 6 months prior to TIPS insertion and showed E/A less than 1.5, E/e’ less than 10 but a LAVI more than 34 ml/m2. The second patient (male, 68yo) underwent rescue TIPS for a variceal bleed. NT-proBNP on the day of TIPS measured 52 ng/L. He received an echocardiography on the same day showing no clear abnormalities, however, due to lack of echogenicity and the acute setting no detailed measurements were taken. The patient developed subacute heart failure with preserved ejection fraction 6 months after TIPS placement needing diuretics. In both cases CD was well managed with diuretics only, mechanical ventilation or TIPS reduction was not indicated.
4DiscussionIn this observational retrospective cohort study, we witnessed CD after TIPS placement in 11.3% of included patients, occurring mostly in the first weeks after shunt placement. Multivariate regression demonstrated that older age, higher albumin and higher NT-proBNP values at baseline were linked to CD in patients receiving TIPS for refractory ascites. However, no clear predictors in patients receiving TIPS for variceal bleeding were found. Correspondingly, the Toulouse algorithm could identify patients at risk for CD, but only in the population receiving TIPS for refractory ascites.
In their study, the authors of the Toulouse algorithm described a very high incidence of CD after TIPS, namely 20%. The authors even restricted the definition of the primary outcome to severe CD, i.e. needing hospital admission for intravenous diuretics, implying an even higher number if mild-moderate CD would be encompassed. Studies describing the incidence of CD post-TIPS are scarce. In a large retrospective study including 882 patients, Modha et al. [12]. reported a CD incidence of only 0.9%. However, only cases in whom signs of CD developed during the first week after TIPS, were recorded in this study. In our cohort, cases of CD occurred mostly in the first month after TIPS. Because of the relation in time, we believe there is a clear link with shunt placement. In patients experiencing CD more than one month after TIPS placement or patients in need of aggressive volume resuscitation like with variceal hemorrhage, this link can be debated as other factors might have precipitated decompensated heart failure. In the Billey et al. study median time to CD was 30 days, from this we deduct that half of CD cases occurred in the first month, thus corresponding to an incidence of 10%. Incidence of CD within one month after TIPS was 8.5% in our cohort. We believe the incidence of TIPS-related CD was likely underreported in the study of Modha et al. since it only accounted for CD in the first week, and, correspondingly, might have been overreported in the study of Billey et al. since half of cases occurred more than one month post-TIPS.
Multivariate regression showed a significant association between CD after TIPS for refractory ascites and older age, higher albumin and higher NT-proBNP levels. While a higher NT-proBNP in patients at risk for CD was also described in the Toulouse study, higher age and albumin were not significant in this study. Modha et al. did find an older age and higher albumin level in patients with CD after TIPS, but they did not assess (NT-pro)BNP. It seems plausible that a higher age predisposes to CD as well as a higher albumin which reflects oncotic pressure. In patients receiving TIPS for variceal bleeding, none of the cardiologic variables were significantly associated with CD.
When applying the Toulouse algorithm, we discovered it worked well in patients with refractory ascites, but failed in those with cirrhosis and variceal bleeding. This finding might be explained by the different inclusion criteria in the Toulouse study, which included preoperative TIPS and excluded emergency and pre-emptive TIPS for variceal bleeding. Only a minority of patients included in our study received TIPS for secondary prevention of variceal bleeding. As a consequence of including urgent TIPS indications, some patients in our variceal bleeding group received echocardiography and NT-proBNP in an urgent setting where use of vasopressors, resuscitation with IV fluids and blood products, protective intubation, etc. might have influenced measurements. Based on our findings, we subsequently conclude that the Toulouse algorithm should not be applied in patients needing pre-emptive or emergency TIPS for variceal bleeding.
Besides the aforementioned differences with the Toulouse study, our study population had comparable baseline characteristics, particularly mean age, MELD score, PSPG before and after TIPS, NT-proBNP, QTc, E/A, E/e’, LAVI values and main aetiology were similar.
It should be mentioned that many patients identified as high-risk by the Toulouse algorithm did not experience CD post-TIPS, therefore the algorithm should merely be used to increase awareness and monitoring for signs of CD rather than as an exclusion criterium for TIPS placement. TIPS placement in patients with AoS remains a much-debated topic. Since CD post-TIPS was well managed with diuretics and did not lead to increased mortality in our series, TIPS appears safe in mild and moderate AoS. In our centre, TIPS insertion is avoided in patients with severe AoS. This cohort contained one case with moderate AoS who developed recurrent CD and died in the aftermath of surgical valve replacement. This case, in addition to the data from the Toulouse trial, does call for caution when TIPS is considered in patients with moderate to severe AoS, especially in a non-urgent setting.
This study has a few limitations inherent to the retrospective nature of our database. Cardiac assessment was not routinely performed before 2019 (year of publication of the Toulouse algorithm). Indeed, since not all patients receiving TIPS before 2019 had both NT-proBNP and echocardiography performed, it is possible that both investigations were only performed in those considered at higher risk by the treating physician, thus introducing selection bias in our database. This is reflected by the lower sodium and higher albumin values (possibly indicating clinical fluid overload) and by the older age of individuals with echocardiography and NT-proBNP results available. Echocardiography was also more frequently missing in the variceal bleeding group, presumably owing to the urgent setting of this TIPS indication. Furthermore, timing and circumstances of echocardiography and NT-proBNP measurements were not standardized. As mentioned before, clinical factors such as use of vasopressor, IV fluids (e.g. albumin substitution), etc. could have influenced the measurements. Nevertheless, our findings are important since this is the first study to confirm the efficacy of the Toulouse algorithm in patients needing TIPS for refractory ascites.
While we included patients receiving TIPS during a large timeframe, our cohort only involves single-centre data. More data in this field are welcomed which will likely be expedited by international collaborations and the introduction of artificial intelligence. Future studies should focus on the different indications for TIPS placement separately and should also focus on patients receiving preoperative TIPS. The Toulouse algorithm remains to be validated in this latter non-urgent indication, in which cardiac evaluation and risk-benefit assessment are all the more important.
5ConclusionsWe can conclude that CD is not an infrequent complication post-TIPS occurring in around 1 out of 10 patients and mostly in the first month post-TIPS. When TIPS is considered to relieve symptoms of refractory ascites, evaluation should include echocardiography and NT-proBNP, and the Toulouse algorithm can be applied to identify those at risk of CD requiring close monitoring for signs of heart failure. As CD can be well managed with diuretics in most cases, allocation to the high-risk group should not preclude shunt placement but does call for increased surveillance. Furthermore, in patients needing TIPS for variceal bleeding, the Toulouse algorithm failed to identify those at risk for developing CD. In these patients, TIPS should not be avoided based on cardiac parameters - such as diastolic dysfunction, prolonged QTc or mild-moderate AoS - especially when TIPS could be a life-saving or life-altering procedure.
Author contributionsConceptualization, methodology and project administration were performed by EV and WL. Data curation was performed by EV, YB and JC. Formal analysis, visualization and writing of the original draft was performed by EV. GM, EC and LB performed the TIPS procedures. Supervision was provided by GC and WL. All authors reviewed and commented on the manuscript. All authors approved the final manuscript.
FundingWL received funding from the MICROB-PREDICT (project ID 825694). The manuscript reflects only the authors’ views, and the European Commission is not responsible for any use that may be made of the information it contains. The funders had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript. YB received funding through the Flanders Research Foundation (FWO - T004420N). No other funding was withheld.