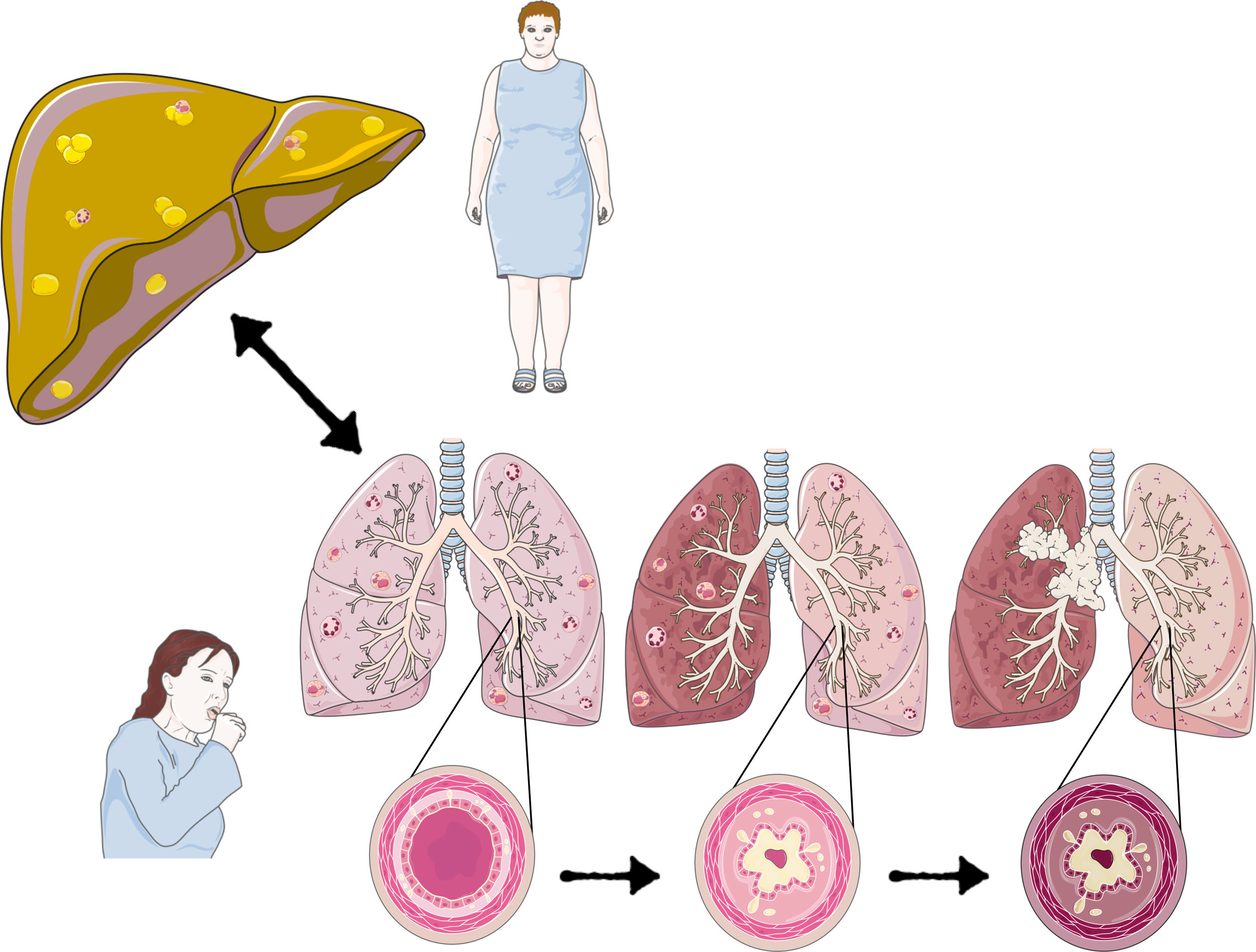
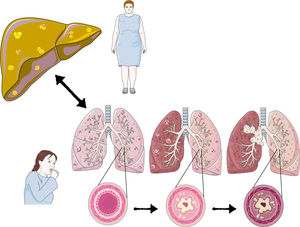
Non-alcoholic fatty liver disease is defined as hepatic fat accumulation in more than 5% of hepatocytes, without other liver steatosis causes. It comprises a broad spectrum that can range from benign steatosis and progress to non-alcoholic steatohepatitis, fibrosis, and ultimately hepatocellular carcinoma. Non-alcoholic fatty liver is considered a multisystemic disease since it is related to multiple disorders, such as type 2 diabetes mellitus, polycystic ovary syndrome, chronic kidney disease, psoriasis, osteoporosis, hypothyroidism, cardiovascular diseases, and obstructive sleep apnea syndrome; it is becoming increasingly clear that it is also a risk factor for developing certain respiratory diseases. This article aims to understand the liver and chronic obstructive pulmonary disease mechanisms, obstructive sleep apnea syndrome, asthma, and lung cancer. Given that non-alcoholic fatty liver disease has a considerable impact on the patient’s well-being and life quality, as well as on the costs they generate for the country’s health services, it is essential to continue research, especially in areas such as the respiratory tract, as there is much misinformation about it.
Non-alcoholic fatty liver disease (NAFLD) is defined as hepatic fat accumulation in more than 5% of hepatocytes, without secondary causes; mainly, excessive alcohol consumption, inherited liver diseases, viral infections such as hepatitis B and C, or steatogenic drugs consumption [1]. NAFLD comprises a broad spectrum of diseases, ranging from mild steatosis and progression to non-alcoholic steatohepatitis (NASH), fibrosis, and as the last outcome to hepatocellular carcinoma [2]. NAFLD has recently become the leading cause of chronic liver disease in developed countries, affecting 25% of the general population, reaching up to 70% in patients with obesity, diabetes mellitus, and metabolic syndrome [3]. Genetic and epigenetic mechanisms are considered vital factors for disease development; among them are insulin resistance, de novo lipogenesis, intestinal microbiota alterations, oxidative stress, and inflammatory factors [1].
When liver damage progression is observed, the association with other comorbidities such as cardiovascular diseases, type 2 diabetes mellitus, chronic kidney disease, polycystic ovary syndrome, psoriasis, osteoporosis, hypothyroidism, and obstructive sleep apnea syndrome (OSA) worsens and increases [4]. Little research has yielded exciting data linking NAFLD to certain respiratory tract diseases, so this is a subject that is not yet well known. This article aims to review the current literature to elucidate the NAFLD relationship with different respiratory tract diseases.
2NAFLD and respiratory diseasesNAFLD is a risk factor for developing cardiovascular disease, type 2 diabetes, and some forms of cancer; it is becoming increasingly clear that it is also a risk factor for developing certain respiratory diseases. This article discusses NAFLD’s impact on the association, development, and prognosis of various respiratory diseases.
2.1NAFLD and chronic obstructive pulmonary disease (COPD)Chronic obstructive pulmonary disease (COPD) is a severe public health problem and a significant cause of chronic morbidity and mortality worldwide. In most patients, it is associated with concomitant chronic diseases that increase their morbidity and mortality. The global initiative for chronic obstructive lung disease classifies COPD in four stages according to the fraction of forced expired volume in 1 s, which correlates with the dyspnea degree and is classified with the modified Medical Research Council scale dyspnea assessment [5]. The prevalence of metabolic syndrome in patients with COPD is estimated between 23–53% and depends mainly on the degree of a global initiative for chronic obstructive lung disease, the modified Medical Research Council, and the patient’s inflammatory state [6,7].
Patients with COPD often present an increase in visceral fat related to systemic inflammatory processes and cardiometabolic comorbidities [8–10], probably due to the pulmonary dysfunction and dyspnea that limits physical activity, contributing to sedentary lifestyle and accumulation of visceral adipose tissue, which could lead to the development of hepatic steatosis. However, there are no scientific studies to help us demonstrate this idea. It can be expected that pulmonary function rehabilitation will allow COPD patients to perform physical activity, obtaining a beneficial effect on patients suffering from any entity of the NAFLD spectrum.
The first study investigating NAFLD prevalence and severity in patients with COPD demonstrated that steatosis, NASH, and fibrosis prevalence were 41%, 37%, and 61%, respectively. Also, obesity and insulin resistance were found to associate COPD with systemic inflammation [11]. These metabolic disturbances are associated with an increased risk of adverse outcomes such as liver cirrhosis, hepatocarcinoma, and a significant mortality increase [12,13].
Liver fat accumulation and the sum of harmful factors such as lipotoxicity, oxidative stress, mitochondrial abnormalities, and increased inflammatory cytokines may result from simple steatosis to hepatocellular inflammation fibrosis. One of the most studied inflammatory cytokines in NAFLD is the tumor necrosis factor-alpha (TNF-α) since it is usually elevated; it is involved in oxidative stress and apoptosis [14]. In addition to its role in liver disease, it exerts biological effects on different tissues and multiple conditions [15]. Moreover, adipokines in NAFLD development have also been related. Leptin is the first adipokine described, and its relationship with NAFLD is due to its pro-steatogenic and profibrotic effect that triggers a detrimental effect [14,15]. Patients with COPD and NASH had elevated TNF-α and leptin levels, unlike patients with COPD without liver damage, probably due to visceral tissue inflammation [11].
Because NAFLD represents an independent factor in developing multiple comorbidities and increased mortality [3], it should be included among the pathologies to be investigated in a subject with COPD. The synergy that can trigger the chronic inflammatory state between both diseases can lead to a worse patient outcome. On the other hand, there is an association between COPD and NAFLD, sustained by COPD’s relationship with obstructive sleep apnea (OSA); another disease that has been positively related to the development and progression of liver damage, and untreated OSA in COPD patients has been shown to predispose to further injury and progression of NAFLD [11].
2.2NAFLD-obstructive sleep apnea (OSA)OSA is a highly prevalent disease in patients with elevated body weight, and it is characterized by episodes of partial or total upper airways obstruction during sleep with interruption or reduction of airflow (apnea) that lead to a transient decrease in oxygen saturation, elevation of carbon dioxide levels, followed by a brief awakening, which ultimately causes the restoration of upper airway patency [14]. The American Academy of Sleep Medicine classifies OSA’s severity according to the apnea-hypopnea index and the degree of daytime sleepiness as mild, moderate, and severe [15].
Obesity is one of OSA’s leading risk factors; however, reducing body weight helps decrease its severity [16]. OSA is related to multiple pathophysiological mechanisms such as the sympathetic nervous system hyperactivity, oxidative stress, vascular endothelial dysfunction, metabolic dysregulation, and selective inflammatory activation of the airways [17].
In humans, OSA’s clinical manifestations are associated with chronic exposure to intermittent hypoxia, leading to increased cardiovascular morbidity (systemic high blood pressure and increased risk of stroke) [18]. Periods of hypoxia trigger extracellular stimuli that activate signaling cascades, which lead to alteration in reactive oxygen species production such as superoxide anion and hydrogen peroxide, and the imbalance of endogenous antioxidants defense mechanisms. One of the most critical mechanisms in reactive oxygen species production is mitochondrial dysfunction due to hypoxia [19]. Reactive oxygen species activate an inflammation cascade that increases proinflammatory cytokines and adhesion molecules expression [20]. Furthermore, reactive oxygen species can affect the arterial endothelium by activating the nuclear factor-kB (NF-kB) due to leukocytes and platelet accumulation, promoting atherosclerosis and cardiovascular development diseases [20–22].
In experiments with C57BL/6 mice exposed to three hypoxic regimes of increasing severity, 580 differentially expressed genes were obtained from mice exposed to more severe hypoxia by microarray evaluation; suggesting that acute and chronic hypoxia causes transcriptional changes related to the immune system, carbohydrate and lipid metabolism, angiogenesis, and amino acid phosphorylation [20].
Different investigations have demonstrated the role of hypoxia in hepatic metabolic pathways and their injury mechanisms in NASH. Mice with hepatocellular deficiency of phosphatase and tensin homolog (PTEN), exposed to an atmosphere of 10% O2 (hypoxic) for seven days, present increased hepatic steatosis, increased glucose and plasma triglycerides, as well as decreased insulin sensitivity compared to mice that were exposed to 21% O2 (control). Furthermore, it was found that hypoxia increases the expression of several genes such as sterol regulatory element-binding protein 1-c (SREBP-1c), peroxisome proliferator-activated receptor gamma (PPAR-γ), acetyl-CoA carboxylase 1 and 2 (ACC1, ACC2). In counterpart, mitochondrial β-oxidation genes such as peroxisome proliferator-activated receptor alpha (PPAR-α) and carnitine palmitoyltransferase-1 (CPT-1) are significantly decreased [21].
A polymorphism in the IL6 gene (-G174C) and TNF-α (-G308A) promoter region affects their expression and is related to lipid metabolism alteration [23]. Furthermore, it has been identified in mice that intermittent hypoxia and consequent hypercapnia can decrease miR-34a expression [24], which is associated with IL-6 and TNF-α production [25] and is also related to NAFLD pathogenesis through multiple physiological processes, such as fatty acids oxidation and synthesis, triglycerides and cholesterol metabolism, and hepatocytes apoptosis [26]. The alteration of its levels and the relationship in lipid metabolism predisposes weight gain in OSA subjects, increasing the risk of metabolic disorders such as insulin resistance [23].
Inflammatory components such as IL-6, IL-8, TNF-α, vascular endothelial growth factor, leptin, and C reactive protein are found in high concentrations; therefore, they could be used as biological markers [17,27].
2.3NAFLD-asthmaAsthma is a potentially reversible chronic inflammatory airway disorder, where mast cells, eosinophils, T lymphocytes, macrophages, epithelial cells, and neutrophils play an essential role, especially in sudden onset cases exacerbations, occupational asthma, and smoking patients. In susceptible individuals, this inflammation causes recurrent coughing episodes, wheezing, dyspnea, and chest tightness, associated with generalized obstruction with airflow variability, often reversible spontaneously or with treatment [28].
Being overweight or obese has been described as a risk factor for developing asthma. Patients with asthma and obesity are often resistant to traditional asthma therapies, have a higher number of hospitalizations, and have worse outcomes than normal-weight asthmatics [29]; besides, the higher the body mass index, the greater the risk of asthma [30]. This suggests that patients with asthma and obesity may benefit from addressing weight loss as part of their therapy.
Based on epidemiological studies in the 1990s and 2000s, it is estimated that more than 250,000 new asthma cases per year in the United States may be related to obesity [30]. This is due to the chronic proinflammatory state triggered by obesity, which predisposes the subject to constant inflammatory pathways activation. This mechanism is probably carried out by free fatty acids and lipid accumulation in the body. Furthermore, NAFLD is strongly linked to obesity, with a reported prevalence of up to 80% and only 16% in individuals with a healthy BMI without metabolic risk factors [31,32]. An amount of visceral adipose tissue in obese individuals contributes to a high NAFLD prevalence. Free fatty acids derived from visceral adipose tissue and dietary sources and de novo lipogenesis are released to the portal venous system. This free fatty acid and chronic inflammation are considered two of the most critical factors contributing to liver injury in NAFLD progression. Furthermore, adipokine secretion from visceral adipose tissue and lipid accumulation in the liver promotes inflammation through the NF-kB signaling pathways activated by free fatty acids [33]. In the liver, the NF-kB inhibitor is activated by a high-fat diet and obesity associated with increased chronic inflammation, thus exacerbating hepatic steatosis [34].
Liver fat accumulation initiates a biochemical signaling cascade that includes oxidative stress, inflammatory response, mitochondrial dysfunction, lipid peroxidation, and dysfunction of the extracellular matrix balance; these signals, with continuous stimulation over time, consolidate fibrogenesis in the tissue, events in which the transforming growth factor-beta 1 (TGF-β1) plays a fundamental role. The latter is identified as an essential profibrotic cytokine that promotes collagen I expression by stellate cells and is possibly involved in inhibiting extracellular matrix metalloproteinases [35,36].
Free fatty acids rise in visceral adipose tissue induces its circulation in the liver, which leads to diacylglycerol accumulation, an increase in the activity of the protein kinase C-delta and monocyte chemoattractant protein 1 (MCP-1) plasma levels, as well as the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, leading to chronic inflammation and insulin resistance [37].
At the pulmonary level, TNF-α, IL-1β, and IL-6 have a role in asthma pathology, stimulating the inflammatory response by recruiting eosinophils and neutrophils, which activate mast cells and the pulmonary endothelium with consequent histamine release and stimulation of acute-phase protein production [38]. While TGF-β is vital in tissue repair, fibrosis, and modulation of the inflammatory immune response, it correlates with the degree of subepithelial fibrosis and the prominent eosinophilic infiltrates in patients with severe asthma [35].
The relationship between asthma and obesity is well known; however, NAFLD’s connection can only be inferred through the common inflammation mechanisms. Similarly, it can be deduced that due to the lack of physical activity that some patients with poor asthma control may have, they could lead a sedentary lifestyle that predisposes them to increase weight and body fat. Finally, as mentioned above, this is only an assumption, and more studies are needed to elucidate the possible association between these two entities.
2.4NAFLD and pulmonary cancerLung cancer has become a significant public health problem, being the leading cause of cancer death worldwide; there are different subtypes of lung cancer, each with characteristics that give it a different prognosis. Currently, lung adenocarcinoma is the most common and is characterized by the formation of glands, ducts, and significant mucus production; it is usually found in the periphery of the lung, unlike the small cell and squamous cell variant, which are generally located in the center [39]. Like any other malignancy, lung cancer prognosis depends on the stage in which it is found; however, lung adenocarcinoma has a better prognosis of the different histological variants [40]. The main risk factor for having any variant of lung cancer is tobacco use [41]. In recent decades, changes in eating habits and lifestyle have become a significant risk factor for developing obesity and different types of cancer; however, its role in lung neoplasms has not yet been thoroughly studied.
As well, NAFLD is associated with the risk of suffering from different cancer types such as hepatocellular carcinoma and colorectal carcinomas [42]; the reason why it is speculated that the prevention and NAFLD control can help to reduce hepatocellular carcinoma incidence and probably other malignant neoplasms like lung cancer, especially the histological variant of adenocarcinoma.
The epidermal growth factor receptor (EGFR) plays a vital role in regulating lipid levels in the liver and plasma [43]; EGFR has a high incidence of mutation in women and obese patients [44], probably due to increased levels of endogenous estrogens in adipose tissue, which is why it is similar to breast and endometrial neoplasms [45]. Reactive oxygen species caused by mitochondrial damage can cause deletions, insertions, and substitutions in the EGFR gene [46].
In a retrospective study, Zhu et al. showed for the first time that obesity and NAFLD are closely associated with lung adenocarcinoma, regardless of age, sex, smoking, and cholesterol levels. This is probably because lung adenocarcinoma is associated with genomic alterations different from other subtypes of lung cancer and EGFR mutation is one of the most critical factors in its development and treatment [47]. Meanwhile, the combination of liver X receptor agonist T0901317 and gefitinib (EGFR inhibitor) may help treat lung cancer by inhibiting cancer migration and invasion [48].
On the other hand, some results indicate that pulmonary adenocarcinoma induces hepatic metabolic reprogramming, caused by the tumor microenvironment since it functions as an organizer of the endogenous circadian cycle. The STAT3-SOCS3 inflammatory signaling axis is induced in mice with lung neoplasia, resulting in inhibition of hepatic insulin signaling, glucose intolerance, and impaired lipid metabolism, further increasing tumorigenesis [49].
These results suggest that cholesterol regulation, fatty acid metabolism, inflammatory responses, and glucose homeostasis may explain the relationship between lung adenocarcinoma and NAFLD. Furthermore, the findings define the need to continue studying the underlying pathophysiological mechanisms of cancer.
3ConclusionRespiratory diseases become more severe, more difficult to manage, and less responsive to medications when NAFLD is also suffered, directly impacting patients’ quality of life and those close to them. In addition to the standard pharmacological treatment for respiratory disease management, it is considered that an intervention in lifestyle through a healthy diet and regular physical activity is the standard of care in patients with both diseases. However, due to the impaired lung function that occurs in various respiratory diseases, most patients have a sedentary lifestyle that predisposes to the gain of body weight and to hepatic lipid storage; therefore, increasing systemic inflammation and oxidative stress. It could be assumed that good management of lung function will improve the patient’s respiratory capacity, so that physical activity could be performed with less effort to reduce body weight.
Studies carried out to date reveal NAFLD’s relationship with respiratory diseases such as OSA and COPD due to pathophysiological triggering mechanisms such as obesity, insulin resistance, and systemic inflammation, which predispose individuals to exacerbate these respiratory diseases. On the other hand, its relationship with asthma is not scientifically established; however, knowing NAFLD’s systemic inflammatory component makes it possible to infer its interaction in different organs and body systems. Inflammatory cytokines released due to liver inflammation can interact and affect pathologies with a clear inflammatory component such as asthma; the lack of research on the subject leaves us with an idea without scientific support.
The primary mechanism that relates NAFLD to different diseases is due to the chronic inflammation it triggers. NAFLD’s oxidative stress is an essential component in its pathophysiology and one of the mechanisms by which it can damage different organs and systems. Therefore, it is essential to consider NAFLD as a pathology to be ruled out in inflammatory respiratory pathologies. A decrease in steatosis and liver fibrosis probably improves the symptoms and prognosis of these respiratory pathologies correcting the inflammatory state (Fig. 1).
The inflammatory component caused by NAFLD can perpetuate oxidative stress and inflammation of the bronchus. In turn, patients’ difficulty to perform physical activity can aggravate the fatty liver process. Hypercholesterolemia and NAFLD may contribute to an increased risk of pulmonary adenocarcinoma.
With the complexity of respiratory diseases and NAFLD association, addressing a patient with the proper treatment just in time can be challenging for the clinician. An optimal intervention should be composed of many components, in which a multidisciplinary team could define a personalized program for each condition and patient type, where the approach to treat and prevent the disease is through the consideration of each subject's genetic variability, environment, and lifestyle, thereby improving multiple outcomes.
Because respiratory diseases and NAFLD are chronic diseases that present certain risk factors with a multifactorial evolution, they have a considerable impact on the patient well-being, as well as on the costs they generate for the country's health services, which is why it is highly essential to continue research with in vitro, in vivo and human models, to elucidate the molecular and genetic mechanisms involved, and thus understand and explain the processes by which the diseases progress. This kind of research is vital, given that there are no specific guidelines for the management of respiratory diseases and NAFLD association in current clinical practice. Soon, it could be used as a tool to elucidate the severity of various diseases and give a much better and accurate early prognosis, facilitating the doctor’s work; however, plenty of research is still needed.
In a five-year vision, the clarification of the factors that genetically and environmentally predispose a person to experience disease and its possible comorbidities could lead researchers to discover non-invasive biomarkers for early diagnosis, allowing new preventive strategies; likewise, adequate strategies would be created to permit clinical mediation, reducing costs and preventing complications, which could improve the patient’s quality of life. Finally, it is expected to have a better strategy to reach personalized medicine, which will be of much help to the treating specialist since there will be various tools available to facilitate their work; also, the patient will be able to obtain a more favorable prognosis.
New questions arise regarding NAFLD, its broad-spectrum, and its physiological components denote a possible relationship with multiple diseases. Thanks to research advances, some questions have been clarified; however, many aspects of this pathology are still unknown.AbbreviationsNAFLD Non-alcoholic fatty liver disease non-alcoholic steatohepatitis obstructive sleep apnea syndrome chronic obstructive pulmonary disease tumor necrosis factor-alpha nuclear factor-kB phosphatase and tensin homolog sterol regulatory element-binding protein 1-c peroxisome proliferator-activated receptor gamma acetyl-CoA carboxylase 1 and 2 peroxisome proliferator-activated receptor alpha carnitine palmitoyltransferase-1 transforming growth factor-beta 1 monocyte chemoattractant protein 1 epidermal growth factor receptor
Alán E. Botello-Manilla, Guillermo N. López-Sánchez and Natalia Nuño-Lámbarri design and wrote the article. Norberto C. Chávez-Tapia, and Misael Uribe revised, contributed with diverse ideas, and corrected the manuscript final version. All authors have contributed to the article's realization and improvement and agreed on the content of the document.
Conflict of interestAll authors have read the authorship agreement and the dissemination policy of possible conflicts of interest of the journal and certify that they have no commercial associations (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements) that might pose a conflict of interest in connection with the submitted article. This work has not been published previously or is not under consideration for publication elsewhere.
We appreciate the support of Medica Sur Clinic & Foundation so that this article could be made. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.