The association between non-alcoholic fatty liver disease and cerebral hemodynamics arises from cardiovascular damage mechanisms such as endothelial dysfunction, arterial wall increased stiffness, high thickness of the intimate index of the internal carotid artery, left ventricular hypertrophy, left diastolic dysfunction, calcification coronary arteries and increased epicardial fat. The multidirectional relationship between systemic inflammation and lipid metabolism constitutes a common and simultaneous mechanism that causes vascular damage. This study aims to provide insight into the relationship between non-alcoholic fatty liver disease and the function of systemic circulation and cerebral circulation using Doppler ultrasound.
Patients and methodsIs an observational, cross-sectional, prospective, comparative study conducted at Medica Sur Hospital. Thirty-five patients were selected consecutively. The patients consulted neurological service for various symptoms without severity criteria, such as vertigo, primary headache and balance disturbances.
ResultsThere is a difference in the variables mean of the right MCA PI (p = 0.023), left MCA PI” (p = 0.004), and left VA PI (p = 0.036) between the control and NAFLD groups. The correlation analysis between these variables and the CAP showed a positive correlation of the three variables with the CAP, "right MCA PI" (r = 0.384), left MCA PI "(r = 0.509) and" left VA PI " (r = 0.551).
ConclusionsThis study demonstrates a subclinical process of the middle cerebral artery in subjects with NAFLD, which suggests it may be involved in the disease development and points the need to make decisions for this liver manifestation prevention and treatment.
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic fatty infiltration in the absence of significant alcohol consumption and other secondary causes [1]. Liver injury includes isolated steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, and finally cirrhosis [2].
NAFLD is a primary chronic liver disease and is estimated to become the main reason for liver transplantation in some years, especially in those with NASH [3]. On the other hand, cardiovascular disease has been described as the leading cause of death among patients with NASH [4]. The link between NAFLD and cardiovascular disease can be explained by the metabolic characteristics shared by both entities, such as abdominal obesity, systemic arterial hypertension, atherogenic dyslipidemia, and insulin resistance [5]. However, non-alcoholic fatty liver is recognized as an independent predictor of cardiovascular disease [6]. Recent clinical studies associate NAFLD with different mechanisms of cardiovascular damage, such as endothelial dysfunction [7], arterial wall increased stiffness [8], high thickness of the intima-media index of the internal carotid artery [9], left ventricular hypertrophy [10], left diastolic dysfunction [11], coronary arteries calcification [12] and increased epicardial fat [13].
The multidirectional relationship between insulin resistance, systemic inflammation, and lipid metabolism constitutes a common and simultaneous mechanism that causes vascular damage [14]. Oxidative stress is a precursor to vascular damage in patients with NASH due to mitochondrial dysfunction and the production of reactive oxygen species [15]. Also, due to increased insulin resistance and hepatic lipid synthesis, patients with NAFLD present high levels of triglycerides and low levels of Cholesterol-High Density Lipoprotein [16,17]. Furthermore, NAFLD and NASH are associated with prothrombotic states due to the increased concentration of factors VIII, IX, XI, and XII [18], tissue plasminogen activator-1 inhibitor (PAI-1) [19], and deficiency of antithrombotic factors such as protein C [20]. This study aims to provide knowledge about the relationship between NAFLD/NASH and the function of the systemic circulation and cerebral circulation using Doppler ultrasound.
2MethodsThis is an observational, cross-sectional, prospective, comparative study conducted at Medica Sur Hospital. Thirty-five patients were selected consecutively. The patients consulted neurological service for various symptoms without severity criteria, such as vertigo, primary headache and balance disturbances.
Patients with a history of stroke six months before or with severe or unstable systemic diseases, who had a non-vascular lesion on neuroimaging tests, uncontrolled arterial hypertension, known hematological alteration or secondary causes of the fatty liver such as significant alcohol consumption, steatogenic drug use or hepatitis virus infection were excluded. The patients were divided into two groups: NAFLD and control group. All subjects gave a written informed consent following ethical guidelines of the 1975 Helsinki Declaration.
2.1Neurovascular studyFor this protocol, the renal arteries, the abdominal aorta, Doppler ultrasound and the Willis polygon arteries evaluated both carotid and vertebral arteries by transcranial Doppler (TCD). The morphology of the arterial wall, the mean intimate thickness of the internal carotids, the characteristics of the atheroma plaque, and the hemodynamic parameters of the maximum systolic velocity (MSV), resistance index (RI), and pulsatility index (PI) were assessed in the peripheral arteries. In TCD evaluation, MSV, as well as PI and RI of the middle cerebral arteries (right and left) were measured. The morphology of the flow curve and its direction were assessed.
2.2Transition elastographyTransition elastography was performed in all patients after the neurovascular study, which was performed by an experienced physician, who was unaware of the clinical, laboratory, and neurovascular characteristics of the participants at the procedure time, which used a Fibroscan® device (Echosens, Paris). With the patient in a supine position and with the right arm in maximum abduction placed behind the head to facilitate access to the right lobe of the liver through an intercostal space, the tip of the probe transducer was placed; M or XL was suggested by the automatic device selection tool, and the measurements were started by pressing the probe button to start measurements (“shots”). At least ten successful acquisitions were made in each patient. Only the results obtained with a success rate of at least 60% and an interquartile range ≤30% of the mean value of the liver stiffness measurement (LSM) (IQR/med ≤ 30%) and an interquartile range of the controlled attenuation parameter (CAP) (IQR/med ≤ 40 dB/m) were considered reliable. The degree of hepatic steatosis was defined as absent (0), mild (1), moderate (2) and severe (3), based on the CAP values with the following cut-off points; S0: <232, S1: 232–256, S2: 257–290, S3;>290.
2.3Statistical analysisStatistical analysis was performed using IBM SPSS Statistics software (version 26.0). Data is expressed as mean and SD for continuous variables. The two groups of participants (patients with NAFLD versus subjects without NAFLD) were compared using t-tests for independent samples. Furthermore, the association of TCD parameters (PI, RI) with NAFLD was tested by bivariate analysis using the Pearson correlation coefficient. For all tests, a value of p < 0.05 was considered statistically significant.
3Results3.1Study population characteristicsFrom thirty-five patients, two were excluded due to secondary causes of fatty liver. Twenty-one patients were women (64%) and twelve patients were men (36%). The mean age of the patients was 64.36 ± 13.24 years. Fourteen patients presented a healthy body mass index (BMI) and nineteen were overweight or with some degree of obesity, presenting a mean BMI of 25.92 ± 4.39. Only three patients had a diagnosis of diabetes mellitus, of which two were in treatment and control of blood glucose values. Fourteen patients had a systemic arterial hypertension diagnosis and treatment with adequate blood pressure control. Some degree of liver steatosis was detected in eighteen patients (54%), while in fifteen patients (46%), NAFLD presence was discarded through Fibroscan®. Table 1 shows the means of total cholesterol, triglycerides, cholesterol-low density lipoprotein, cholesterol-high density lipoprotein, and glucose levels, as well as demographic and clinical characteristics between patients with NASH and patients without NASH.
Clinical, biochemical and cognitive evaluation of 33 patients, with and without NAFLD.
| Characteristic | With NAFLD (n = 18) | Without NAFLD (n = 15) | p value |
|---|---|---|---|
| Sex, women/men (%) | 9/9 (50%/50%) | 12/3 (80%/20%) | _ |
| Age (years) | 66.0 ± 12.6 | 62.4 ± 14.1 | 0.451 |
| BMI (kg/m²) | 28.22 ± 3.9 | 23.16 ± 3.2 | <0.001 |
| Glucose (mg/dL) | 98.8 ± 41.0 | 100.8 ± 58.2 | 0.118 |
| Total Cholesterol (mg/dL) | 170.6 ± 38.9 | 137.0 ± 9.5 | 0.015 |
| HDL (mg/dL) | 44.2 ± 8.3 | 52.0 ± 13.4 | 0.282 |
| LDL (mg/dL) | 104.66 ± 31.92 | 64.0 ± 19.9 | 0.004 |
| Triglycerides | 116.9 ± 65.2 | 119.4 ± 46.0 | 0.162 |
| MoCA | 24.67 ± 3.2 | 25.35 ± 4.0 | 0.605 |
Values are expressed as mean ± SD. Non-alcoholic fatty liver disease (NAFLD), body mass index (BMI), high density lipoprotein (HDL), low density lipoprotein (LDL), Montreal cognitive assessment (MoCA).
Bold values signifies that the values are significant.
Patients underwent carotid and transcranial Doppler ultrasound to measure the MSV, the PI, and the RI. (Table 2) The highest MSV was recorded in the left common carotid artery (69.97 ± 20.85) and the right common carotid artery (68.37 ± 16.85). Likewise, the highest PI and RI values were recorded in these arteries.
Transcranial and carotid Doppler parameters in the 33 patients.
| Minimum | Maximum | Mean ± SD | |
|---|---|---|---|
| Right MCA PI | 0.77 | 1.62 | 1.09 ± 0.20 |
| Right MCA RI | 0.52 | 0.79 | 0.64 ± 0.06 |
| Right MCA MSV | 20.8 | 104.0 | 60.94 ± 18.35 |
| Left MCA PI | 0.72 | 2.25 | 1.14 ± 0.32 |
| Left MCA RI | 0.48 | 0.87 | 0.64 ± 0.09 |
| Left MCA MSV | 20.0 | 131.0 | 61.65 ± 20.46 |
| Right ACC PI | 0.75 | 2.07 | 1.38 ± 0.32 |
| Right ACC RI | 0.54 | 0.82 | 0.70 ± 0.076 |
| Right ACC MSV | 33.6 | 103.3 | 68.37 ± 16.85 |
| Left ACC PI | 0.90 | 4.13 | 1.50 ± 0.62 |
| Left ACC RI | 0.54 | 0.91 | 0.72 ± 0.084 |
| Left ACC MSV | 32.8 | 138.0 | 69.97 ± 20.85 |
| Right VA PI | 0.63 | 3.60 | 1.33 ± 0.52 |
| Right VA RI | 0.43 | 1.46 | 0.70 ± 0.17 |
| Right VA MSV | 5.6 | 67.8 | 35.30 ± 13.69 |
| Left VA PI | 0.76 | 3.00 | 1.36 ± 0.51 |
| Left VA RI | 0.52 | 1.66 | 0.71 ± 0.19 |
| Left VA MSV | 8.8 | 76.5 | 35.00 ± 16.64 |
Pulsatility index (PI), resistance index (RI), maximum systolic velocity (MSV), middle cerebral artery (MCA), anterior cerebral carotid (ACC), vertebral arteries (VA).
While performing the mean comparison analysis, significant differences were demonstrated between the patients with NAFLD and the healthy subjects in the pulsation indexes of the left and right middle cerebral artery (MCA), of the left vertebral artery and in the RI of the left MCA (Table 3).
Transcranial and carotid Doppler parameters related to non-alcoholic fatty liver disease.
| NAFLD | N | Mean | Standard deviation | p value | |
|---|---|---|---|---|---|
| Right MCA PI | No | 15 | 1.0133 | 0.17508 | 0.023 |
| Yes | 18 | 1.1711 | 0.20295 | ||
| Right MCA RI | No | 15 | 0.6247 | 0.05693 | 0.087 |
| Yes | 18 | 0.6622 | 0.06504 | ||
| Right MCA MSV | No | 15 | 59.400 | 21.0994 | 0.674 |
| Yes | 18 | 62.233 | 16.2461 | ||
| Left MCA PI | No | 15 | 0.9820 | 0.21455 | 0.004 |
| Yes | 18 | 1.2867 | 0.33678 | ||
| Left MCA RI | No | 15 | 0.6033 | 0.08658 | 0.021 |
| Yes | 18 | 0.6767 | 0.08602 | ||
| Left MCA MSV | No | 15 | 60.320 | 25.8887 | 0.751 |
| Yes | 18 | 62.761 | 15.2861 | ||
| Right ACC PI | No | 15 | 1.3073 | 0.25432 | 0.231 |
| Yes | 18 | 1.4417 | 0.37363 | ||
| Right ACC RI | No | 15 | 0.6813 | 0.05927 | 0.157 |
| Yes | 18 | 0.7183 | 0.08638 | ||
| Right ACC MSV | No | 15 | 68.087 | 15.7023 | 0.928 |
| Yes | 18 | 68.622 | 18.2174 | ||
| Left ACC PI | No | 15 | 1.3113 | 0.29995 | 0.091 |
| Yes | 18 | 1.6589 | 0.76917 | ||
| Left ACC RI | No | 15 | 0.6987 | 0.05854 | 0.115 |
| Yes | 18 | 0.7439 | 0.09894 | ||
| Left ACC MSV | No | 15 | 67.227 | 15.4488 | 0.482 |
| Yes | 18 | 72.256 | 24.6966 | ||
| Right VA PI | No | 15 | 1.1727 | 0.28719 | 0.085 |
| Yes | 18 | 1.4739 | 0.63925 | ||
| Right VA RI | No | 15 | 0.6513 | 0.08823 | 0.087 |
| Yes | 18 | 0.7489 | 0.21082 | ||
| Right VA MSV | No | 15 | 34.153 | 7.7612 | 0.648 |
| Yes | 18 | 36.261 | 17.3646 | ||
| Left VA PI | No | 15 | 1.1767 | 0.19342 | 0.036 |
| Yes | 18 | 1.5294 | 0.63437 | ||
| Left VA RI | No | 15 | 0.6553 | 0.05805 | 0.092 |
| Yes | 18 | 0.7644 | 0.25268 | ||
| Left VA MSV | No | 15 | 33.133 | 10.0802 | 0.542 |
| Yes | 18 | 36.567 | 20.7866 |
Pulsatility index (PI), resistance index (RI), maximum systolic velocity (MSV), middle cerebral artery (MCA), anterior cerebral carotid (ACC), vertebral arteries (VA).
Bold values signifies that the values are significant.
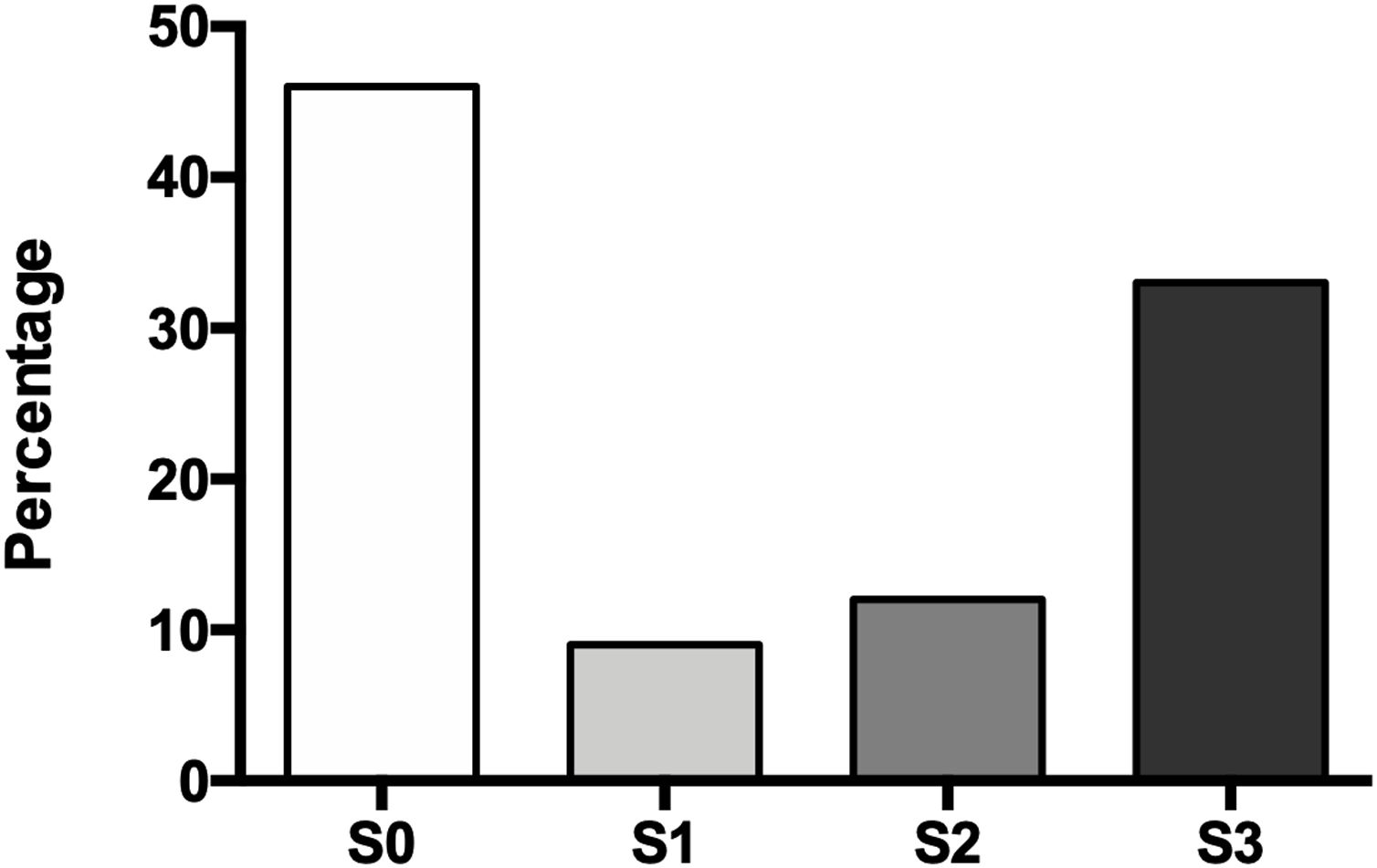
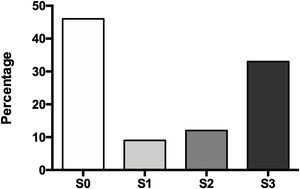
The degree of hepatic steatosis was mild in three patients (9%), moderate in four patients (12%), and severe in eleven subjects (33%); in the rest of the population, fatty liver was dismissed (46%). The mean age in the patients without NAFLD was 62.4 ± 14.1 years, while those with hepatic steatosis was 66.0 ± 12.6 years. Of the group of patients with NAFLD, half were women and half men (Fig. 1).
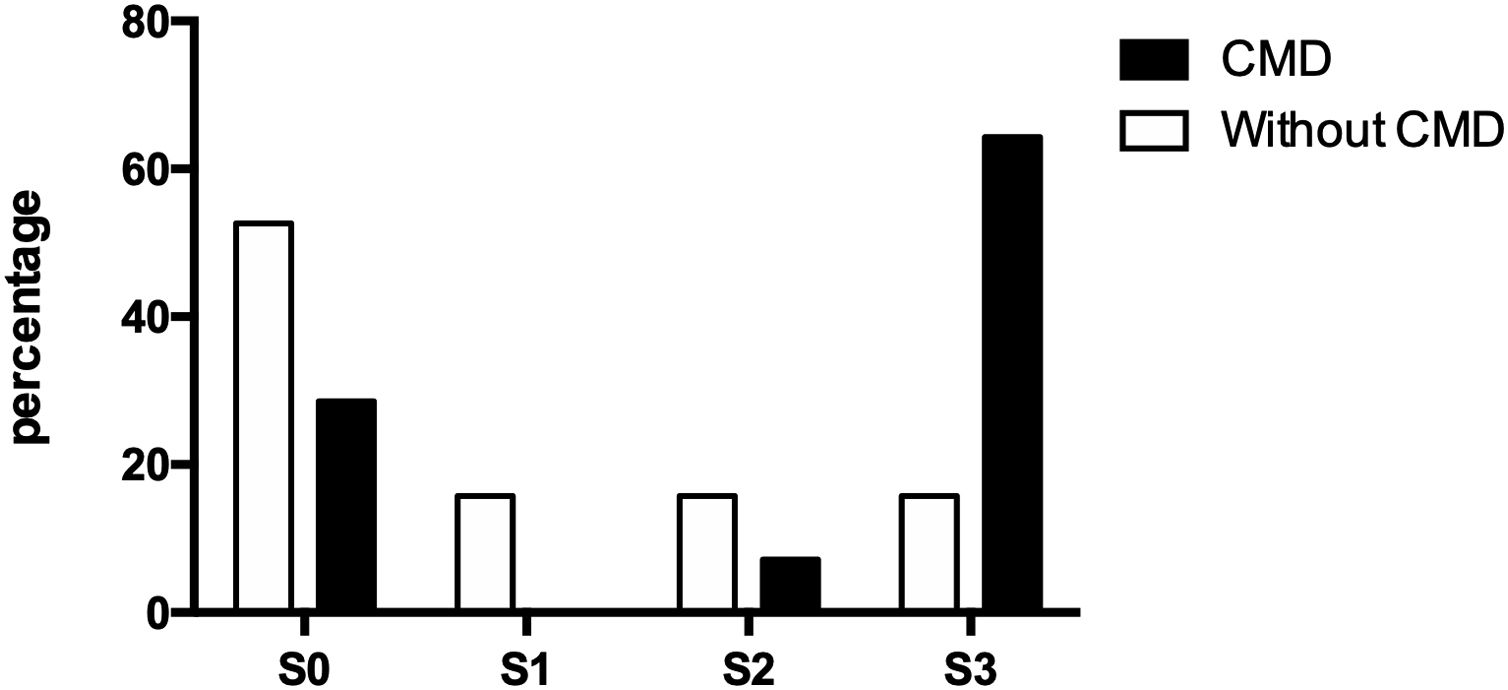
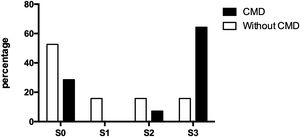
3.4Doppler values and NAFLD presenceOf the thirty-three participating patients, nineteen did not present cerebral microvascular disease, of which ten (52.63%) did not show hepatic steatosis and manifested alterations for each steatosis degree (S1, S2, and S3) three (15.79% each). On the other hand, fourteen patients suffered from a cerebral microvascular disease, of which, only four (28.57%) were ruled out with fatty liver, while one (7.14%) presented moderate steatosis and nine (64.29%) presented severe steatosis (Fig. 2).
PI elevation (>1.1) of the left MCA was presented in fourteen patients; eleven of them presented hepatic steatosis as well. The right MCA PI was elevated in ten subjects with the presence of steatosis in nine; while fifteen patients did not present NAFLD, an average PI (≤1.1) of the right MCA was observed in fourteen subjects (93%) and of the left MCA in twelve patients (80%).
The univariate analysis found that there is a difference in the variables mean of the right MCA PI (p = 0.023), left MCA PI” (p = 0.004), and left VA PI (p = 0.036) between the control and NAFLD groups. The correlation analysis between these variables and the CAP showed a positive correlation of the three variables with the CAP, "right MCA PI" (r = 0.384), left MCA PI "(r = 0.509) and" left VA PI " (r = 0.551). Table 4 describes the associated risks of having a high PI in patients with NAFLD. The left MCA with 6 times more risks (OR 6.28; 95% CI: 1.29, 30.53), the right MCA (OR 14; 95% CI: 1,507, 130.09) and the left vertebral artery (OR 3.14; 95% CI: 0.751, 13,159).
Analysis of the correlation between the pulsatility index and NAFLD (CAP) of the arteries in which there was a significant difference between NAFLD vs controls means and risk estimation in NAFLD presence for PI alteration.
| Pearson correlation | Odds ratio | CI of 95% | |||
|---|---|---|---|---|---|
| Coefficient r | p value | Inferior | Superior | ||
| Right MCA PI | 0.384 | 0.027 | 14.0 | ||
| Left MCA PI | 0.509 | 0.002 | 6.28 | 1.294 | 30.538 |
| Left VA PI | 0.551 | 0.001 | 3.14 | ||
Middle cerebral artery (MCA), vertebral arteries (VA), pulsatility index (PI).
This study shows that subjects with NAFLD present an alteration in the transcranial Doppler parameters, specifically in the PI of the right and left middle cerebral artery. PI measured by TCD, first described by Gosling and King [21], has been postulated in different studies as an indicator of distal cerebrovascular resistance degree [22–24].
One possible cause of increased resistance in the cerebral circulation is narrowing of the small vessels due to lipohyalinosis and microaterosclerosis [25]. In more recent investigations, it has been proposed that PI results from a more complex interaction involving various hemodynamic factors in addition to the cerebral vascular resistance itself, such as cerebral perfusion pressure, pulsatility of arterial blood pressure, elasticity of the cerebral arterial bed and heart rate [26]. However, the directly proportional increase in PI has been correlated with the increase in intracranial pressure, also observing this correlation between PI and cerebral perfusion pressure [27]. When the RI values are higher than 0.8, they are associated with intracranial pressure increase as well as with the PI. Its elevation is also associated with intracranial and cerebral perfusion pressure increase; however, when comparing both indices, the IR is less sensitive to variations in the intracranial pressure [28]. Thus, the pulsatility index has been used more frequently in various studies as an indirect measure of cerebrovascular resistance.
However, other authors have shown that PI changes are not directly related to microvascular disease demonstrated by Magnetic Resonance Image (MRI) [29]. On the other hand, increasing PI has been associated with cognitive decline, particularly with hypertensive patients. In our work, even though there was a significant PI difference between both groups, it was not related to cognitive dysfunction as demonstrated by the MoCA test [30,31].
The loss of vascular integrity in small vessel disease is due to different, non-specific and not fully understood mechanisms that contribute to normal aging [32]. The involvement of the cerebrovascular endothelium could be due to various systemic inflammatory processes; however, it is not clear what the mechanisms are for these alterations, there is the possibility that many cytokines circulate from the site of inflammation to the brain [33]. On the other hand, NAFLD is characterized by different inflammation degrees produced by cytokines secreted in the liver that pass into the systemic circulation and that can cause side effects on the cardiovascular system, by altering endothelial function, vascular tone, and coagulation [34].
The specific contribution of fatty liver to increased cerebral microvascular risk is difficult to separate from the risk factor combination that they share. However, this study is not entirely explained by the components of the metabolic syndrome, since only 3 of the 33 included patients have diagnosed type 2 diabetes mellitus and all patients with systemic arterial hypertension diagnosis have an adequate blood pressure control, in addition to the fact that the mean BMI of the population barely exceeds 25 kg/m² (25.92 ± 4.39) despite the existence of a significantly higher BMI in the group with hepatic steatosis, as expected.
In NAFLD presence and the alteration of the transcranial Doppler values, age was a crucial risk factor in both groups (66.0 ± 12.6 vs. 62.4 ± 14.1 years). An advantage of transcranial Doppler evaluation and Fibroscan® is that both allow patients to be monitored and see hemodynamic changes and intrahepatic fat content evolving. This way, it can be known whether changes in the style of life and improvement of dietary habits, for a significant weight reduction and hepatic steatosis, generate a change in said evolution of hemodynamic changes.
The main limitation of the study is related to the sample size. A higher participant number could provide more reliable data on the analyzed variables and better define its significance. However, results obtained can serve as a reference point for future research in larger populations.
5ConclusionThis study demonstrates a subclinical process of the middle cerebral artery in subjects with NAFLD, which is essential when knowing ischemic cerebral vascular disease epidemiology, in which we know that this artery is the most affected. The finding suggests that NAFLD may be involved in the disease development and points to the need to make decisions for the prevention and treatment of this liver manifestation. These results need to be confirmed with longitudinal studies to determine if these changes could evolve into a manifest disease, such as cerebral vascular disease and cognitive impairment.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Medica Sur Clinic & Foundation paid for the Doppler ultrasound and the transition elastography.
Ethical approvalThe study was approved by the ethics committee of the Medica Sur hospital, code number 2019-EXT-431.
Conflicts of interestThe authors have no conflicts of interest to declare.
Consent to participateAll patients signed an informed consent, where they agreed to participate in the study.
Authors contributionsAll authors have contributed to the realization and improvement of the article, also agreed on the content of the manuscript. Dr. Vidal-González, Dr. López-Sánchez, Dr. Concha-Rebollar, Dr. Rodríguez-Herrera, Dr. Morales-Ramirez and Dr. Nuño-Lámbarri design, carried out the study and wrote the article, Dr. Chávez-Tapia, Dr. Uribe and Dr. Nader-Kawachi revised, contributed with diverse ideas and corrected the final version of the manuscript. The final version has been read and approved by all authors.
Availability of data and materialsThe data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.AbbreviationsNAFLD
non-alcoholic fatty liver disease
NASHnon-alcoholic steatohepatitis
TCDtranscranial Doppler
MSVmaximum systolic velocity
RIresistance index
PIpulsatility index
LSMliver stiffness measurement
CAPcontrolled attenuation parameter
BMIbody mass index
MCAmiddle cerebral artery
We thank Medica Sur Clinic & Foundation for their support and for allowing us to make use of the hospital facilities.