Non-alcoholic fatty liver disease (NAFLD) currently represents an epidemic worldwide. NAFLD is the most frequently diagnosed chronic liver disease, affecting 20–30% of the general population. Furthermore, its prevalence is predicted to increase exponentially in the next decades, concomitantly with the global epidemic of obesity, type 2 diabetes mellitus (T2DM), and sedentary lifestyle. NAFLD is a clinical syndrome that encompasses a wide spectrum of associated diseases and hepatic complications such as hepatocellular carcinoma (HCC). Moreover, this disease is believed to become the main indication for liver transplantation in the near future. Since NAFLD management represents a growing challenge for primary care physicians, the Asociación Latinoamericana para el Estudio del Hígado (ALEH) has decided to organize this Practice Guidance for the Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease, written by Latin-American specialists in different clinical areas, and destined to general practitioners, internal medicine specialists, endocrinologists, diabetologists, gastroenterologists, and hepatologists. The main purpose of this document is to improve patient care and awareness of NAFLD. The information provided in this guidance may also be useful in assisting stakeholders in the decision-making process related to NAFLD. Since new evidence is constantly emerging on different aspects of the disease, updates to this guideline will be required in future.
Non-alcoholic fatty liver disease (NAFLD) currently represents an epidemic worldwide. NAFLD is the most frequently diagnosed chronic liver disease, affecting 20–30% of the general population [1]. Furthermore, its prevalence is predicted to increase exponentially in the next decades, concomitantly with the global epidemic of obesity, type 2 diabetes mellitus (T2DM), and sedentary lifestyle. NAFLD is a clinical syndrome that encompasses a wide spectrum of associated diseases and hepatic complications such as hepatocellular carcinoma (HCC) [2]. Moreover, this disease is believed to become the main indication for liver transplantation in the near future [3]. Since NAFLD management represents a growing challenge for primary care physicians, the Asociación Latinoamericana para el Estudio del Hígado (ALEH) has decided to organize this Practice Guidance for the Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease, written by Latin-American specialists in different clinical areas, and destined to general practitioners, internal medicine specialists, endocrinologists, diabetologists, gastroenterologists, and hepatologists. The main purpose of this document is to improve patient care and awareness of NAFLD. The information provided in this guidance may also be useful in assisting stakeholders in the decision-making process related to NAFLD. Since new evidence is constantly emerging on different aspects of the disease, updates to this guideline will be required in future.
2MethodologyIn order to rate the recommendations provided by authors, we used the Delphi method. Its objective is to obtain a degree of consensus among the consulted experts regarding the available recommendations on a given subject. Its rationale is based on the premise that a collective group judgment should be superior to individual expert opinion. This is a useful tool in controversial topics, where strong recommendations are not feasible. For its implementation, each recommendation was submitted to a vote by the expert reviewers. Reviewers had to select their degree of concordance with each statement, considering the following options: (a) complete agreement; (b) agreement with minor comments/doubts; (c) agreement with major comments/doubts; (d) rejection with some comments/doubts; (e) complete rejection. Consensus was defined when >70% of participants expressed complete agreement/agreeing with minor comments/doubts with a statement. When there was a lack of positive consensus on the first round, recommendations were reviewed with co-authors in concordance with observations of reviewers, and they were re-written and re-submitted to expert revision. If consensus was not reached, the third round of revision was undertaken. Finally, in case of a lack of consensus despite three rounds of revisions, this was informed in the corresponding recommendation in the final draft of the manuscript [4].
3Definition and epidemiologyNAFLD is defined as the presence of hepatic steatosis, either by histology or imaging, in the absence of secondary causes of hepatic fat accumulation, such as excessive alcohol consumption, prolonged use of steatogenic medications, hepatitis C (genotype 3), or monogenic hereditary disorders. Histologically, it is confirmed when macrovesicular steatosis is present in ≥5% of hepatocytes [5]. NAFLD comprises two conditions with different prognosis and distinction between them can only be done by histology: (1) non-alcoholic fatty liver (NAFL), also called isolated steatosis, defined as the presence of ≥5% hepatic steatosis without evidence of hepatocellular injury in the form of hepatocyte ballooning, and (2) non-alcoholic steatohepatitis (NASH), defined as the presence of ≥5% hepatic steatosis, associated with hepatocyte ballooning and lobular inflammation, with or without fibrosis [6]. NASH is more prone to evolve to advanced fibrosis, cirrhosis, and its complications than NAFL. Patients with NAFLD usually precede or follow other features of metabolic syndrome, such as obesity, type 2 diabetes, dyslipidemia, or arterial hypertension.
Regarding epidemiology, a recent meta-analysis estimated the global prevalence of NAFLD, including data from 86 studies with a sample size of 8,515,431 individuals from 22 countries. The pooled global prevalence of NAFLD in adults was estimated to be 25.2% (95% CI, 22.1–28.6) [1]. The highest prevalence of NAFLD was reported in South America [30.4% (95% CI, 22.7–39.4)] and the Middle East, whereas Africa had the lowest rate of this disease. However, only two South American studies were included in this meta-analysis. Both studies included subjects undergoing health screenings: the first was a Brazilian study that enrolled middle-aged patients [7], detecting NAFLD in 35.2% of them, whereas the second study was performed in Colombia, including 263 young males [8]: NAFLD was found in 26.6% of them and was associated with higher insulin levels.
Only a few more Latin American studies assessing the prevalence of NAFLD have been published [9]. In a retrospective analysis of 2503 records of individuals who underwent a pre-occupational evaluation in Mexico City, NAFLD was detected in 14.3% [10]. Being overweight or obese and dyslipidemia were the main factors associated with NAFLD. Another population-based study performed in Santiago, Chile showed NAFLD in 23.4% of 832 Hispanic subjects, associated with a body mass index (BMI) >26.9, abnormal AST, increased levels of HOMA, and C-reactive protein [11].
Most of these studies were performed over a decade ago, thus they may underestimate the current NAFLD prevalence. Other studies including more specific and sensitive non-invasive procedures able to detect steatosis or fibrosis at earlier stages have been undertaken. In a recent Mexican study that investigated women with polycystic ovary syndrome, the prevalence of NAFLD in the control group determined by Fibroscan’s controlled attenuation parameter (CAP) was 34.6%. In another Mexican study, 57% of 505 young adults had at least one risk factor for NASH; 171 of these at-risk patients were assessed for fibrosis. In 106 patients that underwent transient elastography, abnormal liver stiffness was found in 54%, whereas in 65 obese patients that underwent liver biopsy, 90.8% had NASH and liver fibrosis [12].
3.1Metabolic associated fatty liver disease (MAFLD)Recently, a new proposal for modification of the NAFLD acronym to metabolic dysfunction-associated fatty liver disease (MAFLD) has been suggested to more accurately reflect its pathogenesis and to avoid the use of a nosological definition based on negative grounds [13]. Suggested MAFLD diagnostic criteria are described in the diagnosis section. In this new definition, the presence of other liver diseases including excessive alcohol intake does not exclude the presence of MAFLD. This definition reflects a better pathogenic knowledge and dynamic characteristics of the disease, abandoning the traditional need for exclusion of other liver disease and adopting inclusion criteria, in concordance with its elevated prevalence and coexistence with other liver diseases. However, there is still a lack of consensus regarding its widespread use of the new acronym, and consensus between the main scientific associations, including ALEH, needs to be reached.
3.2Recommendations- -
The prevalence of NAFLD ranges from 14.3% to 35.2% in Latin American population-based studies, but most of them were performed more than 10 years ago.
- -
The variances in the prevalence of NAFLD/NASH may be related to differences in both genetic and environmental risk factors. As the frequency of obesity and metabolic syndrome continues to increase, further studies on NAFLD/NASH prevalence should be performed in the general population of Latin America.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement or agreement with minor comments.
4Pathogenesis, genetics, and genetic testingNAFL and NASH develop due to a complex interaction among epigenetic, genetic, and environmental factors (i.e. Western diet) that result in disturbed lipid homeostasis and an excessive hepatocellular accumulation of triglycerides and other lipid species (i.e. diacylglycerol, saturated fatty acids, free cholesterol, and ceramides among others) [14]. Emergence and progressive worsening of insulin resistance (IR) is a central pathophysiological mechanism in NAFLD that is closely connected to its development and progression [15]. The exact mechanisms of IR in NAFLD are unknown but a number of phenomena, such as adipose tissue inflammation as well as uninhibited lipolysis in this compartment, leading to increased serum free fatty acids may be a root cause. Also, an increase in hepatic de novo lipogenesis and changes in gut microbiota with a dysfunctional gut barrier may contribute to hepatic fat loading and may trigger hepatic inflammation and hepatic stellate cell activation, which in turn leads to progressive fibrogenesis and disease progression first to NASH and eventually to cirrhosis [16].
Regarding genetic factors, initial studies of family aggregation and heterogeneous prevalence of NAFLD among different ethnic groups have confirmed the genetic role of the disease. Genome-wide association study (GWAS) has identified powerful and reproducible associations with NAFLD, including variants in patatin-like phospholipase domain-containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase domain containing 7 (MBOAT7), and more recently in the 17-beta hydroxysteroid dehydrogenase 13 (HSD17B13) genes, contributing to a better understanding of NAFLD pathogenesis [17]. PNPLA3 I148M variant (rs738409C>G) is the most studied genetic determinant of NAFLD. This variant modifies the remodeling of triglycerides and phospholipids in hepatic lipid deposits, which is associated with susceptibility to the whole spectrum of NAFLD. This is the strongest genetic risk factor for NAFLD and its severity, with odds ratios (ORs) ranging from 2.1 to 18.2 in different populations. The frequency of the (G) risk allele is 23.1% in the general population, with higher frequencies reported in Hispanic/Latino populations (47.2%) than in European American (22.8%) and African American (13.7%) [18].
Due to the presence of greater Hispanic ancestry held by Latin American countries, PNPLA3 can be an important genetic factor to be considered in the evolution of NAFLD in Latin America. However, it is important to consider the heterogeneous genetic makeup among different countries, with a greater contribution of African genetic ancestry in the Brazilian population and of native American ancestry in Bolivia, Peru, Mexico, Ecuador, Chile, and Colombia [19]. In Chile, an elevated frequency of G allele has been reported, reaching 59% in a preliminary population-based study [20]. In a Brazilian study, it was observed that PNPLA3 CG+GG increased the risk of NAFLD among subjects, and PNPLA3 GG was associated with liver enzyme elevation and fibrosis in NASH patients [21]. In Brazilian individuals with diabetes and NAFLD, the G allele was a marker for the risk of significant liver fibrosis and cardiovascular disease [22]. In a case-control association study from Argentina, the G allele was significantly associated with NAFLD, independent of other main contributing factors [23]. Recently, in a study that used electronic health records from a large multiethnic biobank suggested that stratifying risk within populations known to have an enhanced risk of liver disease, such as Hispanic carriers of the rs738409 variant, would be effective in earlier identification of those who would benefit most from NAFLD prevention and treatment strategies [18]. Thus, PNPLA3 genotyping might have a role in the Latin American population due to Hispanic ancestrality, which causes more risk to NAFLD development and progression.
4.1Recommendations- -
Carriers of the PNPLA3 I148M and the TM6SF2 E167K variants have a higher liver fat content and increased risk of NASH.
- -
Genotyping might be considered in the Latin American population, however, it still has not proven to be able to guide therapy on an individual basis and is costly. Thus, its role in clinical practice is yet to be established.
Delphi consensus: Achieved on the second round – all experts expressed complete agreement or agreement with minor comments.
5How should we diagnose NAFLD? (screening, clinic, laboratory, and images)5.1Clinical featuresNAFLD is usually diagnosed in three clinical scenarios: (a) after incidental abnormal liver tests in a clinical check-up, (b) when abdominal imaging is conducted for other reasons and it detects steatosis or (c) when NAFLD screening is undertaken in high-risk populations. Diagnosis is based on demonstrating the presence of liver steatosis of more than 5% of hepatocytes in the absence of other liver diseases, particularly excluding significant alcohol intake (males >30g/day, females >20g/day) [5]. NAFLD should be suspected in subjects with obesity or features of metabolic syndrome such as T2DM, dyslipidemia, arterial hypertension, etc. This is based on the growing evidence that supports a bidirectional relationship between NAFLD and metabolic syndrome [24,25].
5.2ScreeningThere is currently no consensus for recommending NAFLD screening in the general population, due to the low cost-effectiveness of this practice and the associated risks of invasive tests [26]. Screening seems suitable for patients with increasing age (>50-year-old), one or more features of metabolic syndrome, or obesity (BMI>30) due to their higher prevalence of NAFLD (over 60%) and their higher risk of steatohepatitis, advanced fibrosis, and liver-related mortality [27,28].
5.3Laboratory and imaging5.3.1NAFLD diagnosisLiver ultrasound is the most recommended technique as the first approach because of its wide availability, low-cost, and safety. However, reports assessing its accuracy vary greatly: its sensitivity ranges from 53% to 100% and its specificity from 77% to 98%, with higher accuracy when evaluating only patients with moderate to severe hepatic steatosis (histologic grade 30%–33%) and lower values when all grades of hepatic steatosis are considered [29]. Additionally, its performance is less accurate when assessing obese patients [30]. Computer tomography has a similar steatosis detection rate than ultrasound. Magnetic resonance imaging (MRI) spectroscopy and MRI proton density fat fraction (MRI-PDFF) have better sensitivity and can accurately quantify liver steatosis but they are less accessible methods. Controlled attenuation parameters (CAP) is an ultrasound-based method for liver steatosis quantification that is associated with vibration-controlled transient elastography (VCTE) with excellent accuracy for the detection of liver steatosis [AUROC 0.87 (CI 95% 0.82–0.92] [31]. Serological scores such as the fatty liver index, NAFLD liver fat score (NAFLD-LFS), Hepatic Steatosis Index (HSI), and SteatoTest-2 have been proven useful tools for steatosis diagnosis in large cohorts or population-based studies [32,33].
5.3.2Concomitant or competing chronic liver disease diagnosisInitial workup should aim at the diagnosis of other etiologies that may present with hepatic steatosis, such as significant alcohol consumption, chronic viral hepatitis, autoimmune liver disease, or others. Some abnormalities that may be present do not always reflect concomitant liver disease: elevated serum ferritin and/or elevation in serum autoantibodies are frequently found and should be interpreted after the diagnosis algorithm for hemochromatosis or autoimmune liver disease are carried out.
5.3.3MAFLD diagnostic criteriaThis syndrome includes the presence of liver steatosis associated with metabolic risk factors such as T2DM, obesity, or metabolic dysfunction. The latter is defined as the presence of at least 2 of the following criteria: hypertriglyceridemia (≥150mg/dL), low high-density lipoprotein (≤40mg/dL), high blood pressure (≥130/85mmHg), increased waist circumference, pre-diabetes, high HOMA-IR (≥2,5) or elevated high-sensitivity C reactive protein (≥2mg/L) [13].
5.4Recommendations- -
NAFLD screening is not recommended in the general population. NAFLD screening is recommended for patients with repeatedly altered liver enzymes, features of metabolic syndrome, or obesity (BMI>30). In patients with a high-risk profile (type 2 diabetes or metabolic syndrome and >50 years old) NASH and liver fibrosis diagnosis should be undertaken.
- -
The recommended method for initial screening for NAFLD in clinical practice is an abdominal ultrasound.
Delphi consensus: Achieved on the second round – all experts expressed complete agreement or agreement with minor comments.
6How should we use non-invasive assessment and images in NAFLD? (risk stratification)The estimation of the degree of liver inflammation and fibrosis is key to establishing NAFLD prognosis since it predicts the progression to cirrhosis, liver-related and all-cause mortality, and even HCC [34,35]. The gold standard method is liver biopsy, an invasive and expensive method, subjected to sampling error, interobserver variability, and potential complications. Thus, several non-invasive tests have been analyzed for the assessment of liver fibrosis in NAFLD, namely scores based on clinical and laboratory parameters, as well as tests based on physical or imaging characteristics of the liver. More sophisticated biomarkers such as proteomics, lipidomics, microRNAs, and microbiome-related products are beyond the scope of this document and are reviewed elsewhere [36].
6.1Scores based on clinical and laboratory parametersThe following scores can be calculated with inexpensive and widely available clinical and biochemical parameters. Their variables, Area Under the Receiver Operating Characteristic (AUROC) curve values and respective cutoffs for the detection (rule-in) of advanced fibrosis are shown in Table 1.
- -
Aspartate aminotransferase (AST) to Platelet ratio (APRI): Originally developed and validated in hepatitis C, its accuracy has been questioned in the context of NAFLD [36].
- -
BARD score: this score was developed specifically for NAFLD fibrosis assessment. In its validation cohort, it reached a negative predictive value (NPV) of 96% for advanced fibrosis with a result <2 [37].
- -
NAFLD fibrosis score (NFS): In the original work by Angulo et al. this index showed an accuracy of 82% and 88% to detect and exclude advanced fibrosis respectively [38].
- -
FIB-4: with a threshold of 2.67 and 3.25, the sensitivities and specificities of the FIB-4 score for advanced fibrosis were 26.6% and 96.5%, and 31.8% and 96.0%, respectively [39].
| Score | Components | AUROC (advanced fibrosis) | Lower cut-off (RULE OUT AF) | Higher cut-off (RULE IN AF) |
|---|---|---|---|---|
| NFS | Age, impared fasting glucose/diabetes, BMI, platelets, albumin, AST/ALT ratio | 0.84 | <−1.455 (36−64 years) | > 0.675 |
| <0.12 (65 years or older) | ||||
| FIB-4 | Age, AST,ALT, Platelets | 0.84 | <1.3 (36−64 years) | > 2.67 |
| <2 (65 years or older) | ||||
| BARD score | BMI, AST/ALT ratio, type 2 diabetes mellitus | 0.76 | ≤1 | > 2 |
| APRI | AST, platelets | 0.77 | <0.4 | > 1.5 |
| Hepamet fibrosis score | Age, gender, HOMA score, presence of diabetes, AST, albumin, platelets | 0.85 | <0.12 | > 0.47 |
| Fibroscan | ||||
| - M probe | Transient elastography | 0.88 | <5.8 | >8.7 |
| - XL probe | 0.85 | <4.8 | >5.7 | |
| SWE | Shear-wave elastography | 0.95 | <2.6 | >3.02 |
| MRE | Magnetic resonance elastography | 0.96 | <3.4 | >3.62 |
Online calculators:
HFS: https://www.hepamet-fibrosis-score.eu.
NFS: https://nafldscore.com.
FIB-4 and APRI: https://www.rccc.eu/calculadoras/Fib4.htm.
When these biomarkers were compared in a recent meta-analysis assessing accuracy for the detection of advanced fibrosis in NAFLD, both NFS and FIB-4 yielded an AUROC of 0.84; being superior to BARD and APRI score [40]. It has been found that both NFS and FIB-4 scores have lower specificity when used in individuals >65 years old (35% for FIB-4 and 20% for NFS). Mc Pherson et al. proposed and validated new cutoffs in this particular age group, increasing specificity to 70% without affecting sensitivity [41].
In a retrospective study that included diabetic patients, NFS, FIB-4, and APRI had lower accuracy for the prediction of cirrhosis and liver-related outcomes. Despite that FIB-4 yielded an acceptable accuracy for the detection of advanced fibrosis, authors found that diabetic patients with a low NAFLD-FS, FIB4, or APRI score had a 15%–21% 5-year rate of liver decompensation and a 10%–27% 5-year rate of HCC, demonstrating that an isolated assessment with these algorithms may not be ideal in this population [42].
- -
Hepamet fibrosis score (HFS): This recently validated score [43] had an AUROC of 0.85 for advanced fibrosis, compared to 0.8 obtained for NFS and FIB-4. It showed homogeneity across different ethnic populations without the need for age-adjusted cutoffs, with uniform performance across all BMI categories and aminotransferase levels. However, it has not been validated in Latin America.
- -
Vibration controlled transient elastography (Fibroscan®): the most commonly utilized point-of-care technique in clinical practice for the evaluation of liver fibrosis. It can achieve a diagnostic accuracy (AUROC) over 0.92 for advanced fibrosis in NAFLD patients [39]. It is less precise for intermediate stages. Liver stiffness measurements can be overestimated by the ingestion of a meal, inflammation, vascular congestion, or cholestasis [44].
- -
Acoustic radiation force impulse (ARFI) and shear wave elastography (SWE): These ultrasound-based techniques integrate ultrasound imaging and liver stiffness information, they both yield an accuracy comparable to Fibroscan [36]. However, there is still insufficient data regarding their quality criteria, confounders, or accuracy during follow-up [39].
- -
Magnetic resonance elastography (MRE): this method has the advantage of accurately assessing morphologic details and liver stiffness simultaneously, with an AUROC of 0.9 for cirrhosis. However, it is not widely available, is costly, and not easy to perform in claustrophobic patients [36].
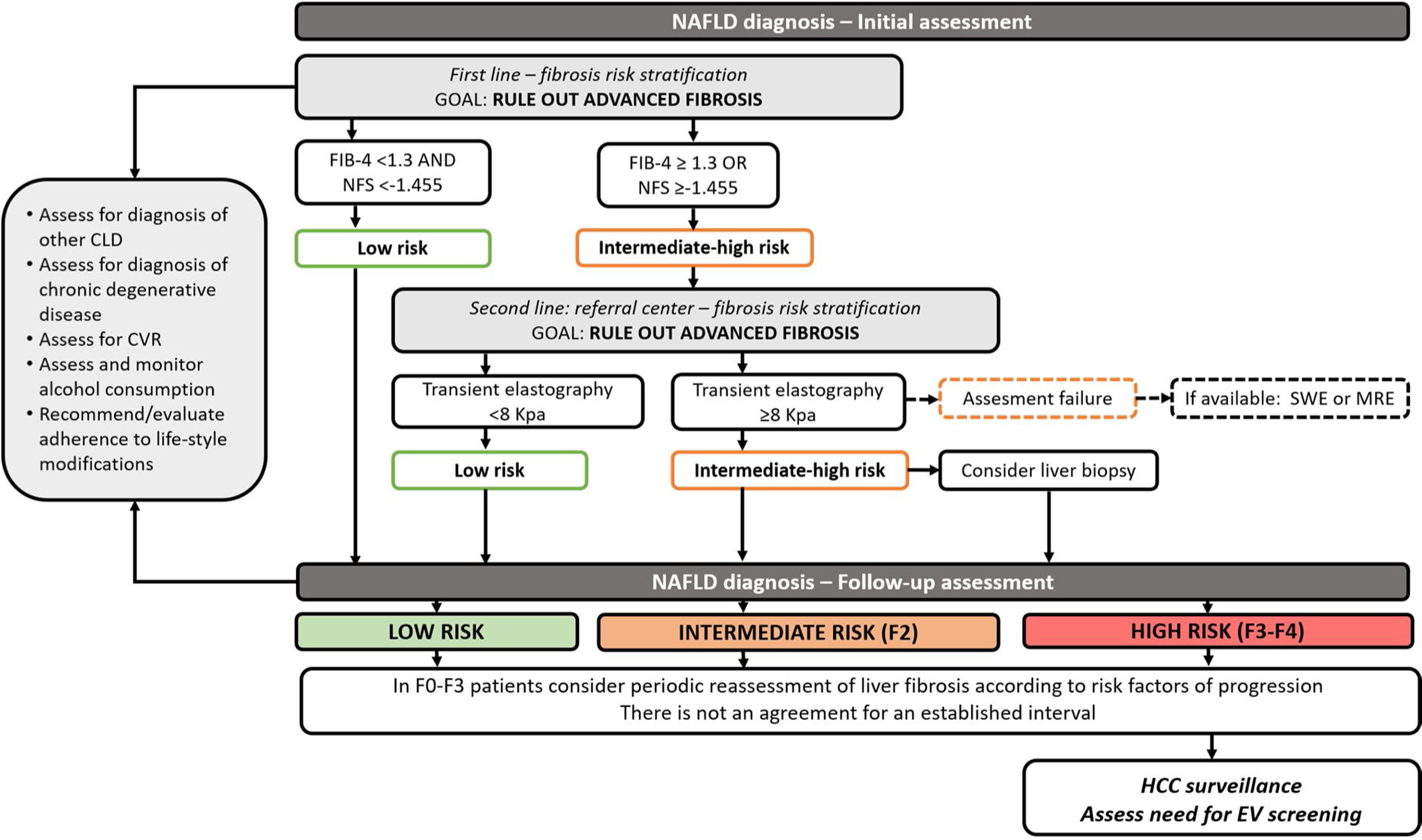
In clinical practice, it has been proposed to use non-invasive tests for initial risk stratification. Due to their optimal NPV, serum biomarkers such as FIB-4 and NFS could be used initially to rule out advanced fibrosis. Patients with intermediate or high risk for fibrosis should undergo a further assessment of an elastography-based technique such as Fibroscan (other elastography techniques may be considered according to local availability). Whenever advanced fibrosis cannot be excluded, patients should be considered for liver biopsy [39] (Fig. 1).
Algorithm for the follow-up of patients with NAFLD. MI; myocardial infarction, CV; cardiovascular, HCC; hepatocellular carcinoma.
Notes. Initial assessment and risk stratification for NAFLD patients (adapted from Castera and Yoneda). Patients with NAFLD should be screened for differential diagnosis, undergo assessment for cardiovascular risk and alcohol consumption. Initial NAFLD fibrosis risk assessment should be performed using non-invasive scores (NAFLD fibrosis score and FIB-4). When advanced fibrosis is not excluded, other non-invasive tests such as transient elastography should be performed. If this test is unavailable or fails to obtain results, other elastography techniques such as shear-wave elastography or magnetic resonance elastography can be selected. In patients with intermediate-high risk for advanced fibrosis, a liver biopsy should be considered. There is no current consensus regarding an interval for the follow up of these patients. It can be tailored according to the presence of risk factors for fibrosis progression. In patients with cirrhosis HCC and esophageal varices screening must be undertaken. In patients with advanced fibrosis, HCC screening should be considered.
- -
NAFLD Fibrosis Score and FIB-4 are useful tools for the initial assessment of fibrosis in NAFLD patients
- -
When NFS score and FIB-4 score are unable to exclude advanced fibrosis, an elastography based method is the suggested method in the risk stratification algorithm. Vibration controlled transient elastography is the best validated and available tool; other elastography techniques should be considered upon availability.
- -
When non-invasive methods are unable to exclude significant fibrosis, liver biopsy should be considered.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement or agreement with minor comments.
7When should we perform a liver biopsy in NAFLD?Liver biopsy remains the gold standard for differentiate patients presenting isolated steatosis from those with steatohepatitis (steatosis, lobular inflammation, hepatocytes ballooning) and for fibrosis staging. Although there have been advancements in noninvasive testing and methods, these tests are still unable to diagnose NASH and to display the full range of findings provided by liver biopsy. However, performing a liver biopsy in patients with suspected NAFLD is still controversial in daily practice due to several limitations of this procedure: it requires expertise for accurate interpretation, involves risk, and entails a high cost [45]. Additionally, the elevated NAFLD prevalence worldwide and lack of effective medical therapy makes the routine indication of liver biopsy unfeasible, thus it requires careful indication.
The most frequent indications for performing a liver biopsy in patients with NAFLD are to confirm or exclude NASH diagnosis and to stage the severity of the disease, as inclusion criteria of patients in clinical trials or to perform a differential diagnosis when another liver disease is suspected.
Performing a liver biopsy can be useful to perform a differential diagnosis or to detect concomitant liver disease and to stage the severity of each condition. In a study that analyzed 354 biopsied patients with unexplained abnormal liver tests, 66% were found to have steatosis, one half of them presented with NASH, and 19% had other liver disease diagnosed by histologic evaluation including autoimmune hepatitis, primary biliary cholangitis, hereditary hemochromatosis and alcohol-associated liver disease [46]. On the other hand, several studies have proven the benefits of a liver biopsy in NAFLD by demonstrating its presence in association with diseases such as hepatitis C, autoimmune liver disease, hemochromatosis, drugs, and occupational exposures [47,48]. Data obtained from decision-tree modeling suggests that early liver biopsy with interventions guided by the specific liver histology leads to a decrease in mortality as well as a decrease in progression to NASH with cirrhosis [49].
The NAS (NAFLD activity score) or the SAF (steatosis, inflammatory activity, fibrosis) score systems have been proposed to increase the reproducibility and homogeneity of liver biopsy results; these scores grade or quantify steatosis, inflammatory activity, and fibrosis [50,51].
7.1Recommendations- -
Liver biopsy should be considered in patients with NAFLD who are at increased risk of having NASH and fibrosis when noninvasive tests are unable to exclude advanced fibrosis.
- -
Liver biopsy should be considered in the differential diagnosis of patients with suspected NAFLD/NASH and other competing etiologies. It should also be considered in patients with known or suspected coexisting chronic liver diseases that cannot be assessed without this procedure.
- -
The pathology report should describe the presence of steatosis, inflammation, and/or the presence of NASH. The stage of severity should be described (ideally by proposed score systems). The amount of fibrosis should be mentioned.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement or agreement with minor comments.
8How should we follow subjects with NAFLD? Risk stratification periodsLiver fibrosis is undoubtedly the main predictor of morbidity and mortality in NAFLD and therefore it should be considered the most important factor that sets the tone in the prognosis and follow-up in these patients. In a biopsy-proven NAFLD study, when compared to controls, the risk of severe liver disease increased per stage of fibrosis (F0; HR: 1.9 to F4; HR: 104.9) and the time for the development of liver decompensation progressed from 22 to 26 years in F0 stage to 0.9 years in F4 stage [52]. A further meta-analysis comprising 4428 patients with NAFLD found that compared with no fibrosis (F0), cirrhotic patients (F4 stage) presented an increase in all-cause mortality (RR: 3.42), liver-related mortality (RR: 11.13), liver transplantation (RR: 5.42), and liver-related events (RR: 12.78), even after adjustment for confounders [53].
The interval of fibrosis reassessment is subject to intense debate. Some experts in the field have suggested that patients with mild fibrosis (F0–F1) can be reassessed every 12 months, using the suggested approach of stepwise non-invasive tests (Fig. 1) [39,54]. In patients with moderate to severe fibrosis (F2–3), reassessment can be performed with elastography or a liver biopsy can be considered, if not performed initially. Regarding the ideal timing, there is insufficient data to recommend a specific interval; it should be tailored to the individual risk factors of fibrosis progression [55]. Re-assessment using liver biopsy could be considered every 5 years in those patients with advanced fibrosis and adequate control of their disease. In those patients with risk factors such as advanced age, BMI≥30kg/m2, features of metabolic syndrome, the persistence of elevated transaminases despite an adequate medical control, detection of one of the polymorphisms associated with a worse prognosis of NAFLD (PNPLA3, TM6SF2, MBOAT7) or suspicious of progression to advanced disease, a liver biopsy could be done within a shorter interval.
However, fibrosis staging should only be one part of an extensive approach that must be designed individually for each patient at the moment of NAFLD diagnosis and in subsequent follow-up visits. Since NAFLD is associated with an increased risk of fatal and non-fatal cardiovascular events [56], an assessment of cardiovascular risk by an efficient and simple score system like the Framingham score represents a cost-effective tool that could be routinely performed in each patient for an early referral to a cardiologist [57]. Moreover, assessment of adequate control of other chronic degenerative diseases and alcohol consumption must be evaluated within follow-up for the increased risk that they represent for fibrosis progression and HCC development [58] (Fig. 1). Routine laboratory studies comprising complete blood count, blood chemistry, and liver function tests should be obtained in every follow-up visit; impedance measurements and imaging studies should be requested according to baseline fibrosis assessment and corresponding HCC screening recommendations.
8.1Recommendations- -
There is no consensus regarding the optimal interval for fibrosis reassessment. Periodic reassessment of liver fibrosis should be considered according to individual risk factors of progression.
- -
Assessment of cardiovascular risk should be performed in every follow-up visit.
Delphi consensus: Achieved on the second round – all experts expressed complete agreement or agreement with minor comments.
9Referral algorithms: when to refer to the hepatologists? When to refer to other specialties?Due to the burden that NAFLD imposes on health systems, it is necessary to design and implement patient referral algorithms. This is particularly important because just a minority of patients with NAFLD will require specialized care [59]. In practice, most patients with NAFLD are diagnosed by front-line healthcare providers, such as general practitioners, who should be able to stratify patients according to their cardiovascular and liver-related risk. To identify patients with a higher risk of liver fibrosis, several scores and algorithms were proposed over the last decade [39,60]. These methods should be inexpensive and easy to implement so that they can be extensively applied. On the other side, hepatologists should identify patients with NAFLD who require a specialized consultation due to their associated comorbidities.
9.1When should patients with NAFLD be referred to a hepatologist consultation?As mentioned, several scores were designed to identify patients with NAFLD at a higher risk of liver fibrosis. Non-invasive scores such as FIB-4 [61] and the NAFLD fibrosis scores [38] are the most attractive tools to stratify patients in the front-line. In every-day practice, various algorithms propose that front-line physicians should use the scores to rule-out advanced fibrosis with the objective to identify patients with NAFLD who do not need to be referred to a hepatologist consultation. Thus, in patients in whom advanced fibrosis cannot be ruled out by these scores (results compatible with indeterminate or high risk of advanced fibrosis), they should be referred to the specialist for further evaluation, including transient elastography and liver biopsy, when appropriate [39,62].
9.2When should Hepatologists refer patients with NAFLD to other specialist consultation?All patients with NAFLD should be screened at least for the following comorbidities: type 2 diabetes, obesity, dyslipidemia, hypertension, and cardiovascular disease. Additionally, when present, risk stratification is advised to decide which patients to refer for a specialist consultation.
Even though there are no universal recommendations, it is advisable to consider referral to a specialist in any of the following situations:
- -
Non-diabetic hyperglycemia (pre-diabetes) and diabetes [62].
- -
Obesity [62].
- -
Moderate to high cardiovascular risk [63].
- -
Severe dislipidemia (for example LDL cholesterol > 190mg/dL, triglycerides>500mg/dL), familial or genetic dyslipidemia and in cases of treatment-intolerance.
- -
Secondary, refractory, occult, or white coat hypertension.
- -
Clinical or biochemical findings that are compatible with chronic kidney disease (GFR<60ml/min/1.73m2) or obstructive sleep apnea.
- -
FIB-4 and/or NAFLD fibrosis score (ideally both) should be calculated in all patients with NAFLD at their initial assessment.
- -
Patients with indeterminate scores should be referred if they portray a high-risk profile (type 2 diabetes or metabolic syndrome and >50 years old).
- -
Patients with high results in at least one of the two scores should be referred to a hepatologist for further evaluation.
- -
Hepatologists should refer patients with significant or difficult-to-treat comorbidities to a specialized consultation.
Delphi consensus: Achieved on the second round – all experts expressed complete agreement or agreement with minor comments.
10Alcohol consumption and thresholds in NAFLDCurrently, the NAFLD definition requires the exclusion of significant alcohol consumption. This threshold has been set at 20g/per day for women and 30g/day in men, which can be simplified for patients as less than 2 and 3 daily drinks for women and men, respectively. This cut-off point has been adopted for patient inclusion in NAFLD treatment trials [64] due to classic studies that found that higher alcohol consumption over two years was related to a higher risk for liver disease [65]. However, the effect of moderate amounts of alcohol consumption (below 30g/daily) and health-related risk in the general population is controversial: there is data that reflects an association with a lower risk for metabolic syndrome and cardiovascular disease, even though these results have geographical disparities. On the other hand, recent data suggests that any amount of alcohol consumption increases the risk of cancer-related mortality and all-cause mortality [66]. In a recent longitudinal population-based cohort study, moderate alcohol intake (below the selected thresholds for NAFLD definition) was found to be related to incident liver disease, thus suggesting a safe amount of alcohol consumption regarding liver risk may not exist [67].
In NAFLD patients the effect of moderate alcohol consumption is also controversial. There are several observational studies dated back from 2001 that suggest a possible beneficial effect regarding disease progression and liver fibrosis in NAFLD patients with moderate-low alcohol intake in comparison with abstinent patients. However, the majority have a cross-sectional or retrospective design and consider present alcohol consumption, with scarce data regarding their alcohol history. Furthermore, selection bias should be considered (completely abstinent patients are more prone to having severe comorbidities or prior heavy alcohol use) and bias related to self-assessment methods to obtain consumption information [68]. Regarding the risk of hepatocarcinoma, a synergistic effect of alcohol and NAFLD has been suggested, indicating that alcohol may be an independent risk factor for HCC in NAFLD/NASH-cirrhosis [69]. When analyzing cardiovascular risk and NAFLD patients, despite evidence suggesting a protective role, a recent longitudinal cohort failed to find an association between alcohol and cardiovascular risk factors or subclinical markers of cardiovascular disease [70].
10.1Recommendations- -
Current data do not allow for recommendations of any amount of alcohol consumption to reduce cardiovascular or other health-related risks in NAFLD patients.
- -
The safest strategy in NAFLD patients is to restrain from any alcohol consumption. However, if patients choose to consume alcohol, it should be recommended below the threshold of 14 and 21 drinks per week for women and men, respectively.
- -
Patients with NASH and/or moderate to advanced fibrosis should abstain from alcohol consumption, due to their high risk for disease progression.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement or agreement with minor comments.
11Hepatocellular carcinoma screeningAlthough the incidence of hepatocellular carcinoma (HCC) in NAFLD patients has been increasing and it is the fastest-growing cause of HCC in liver transplant candidates [71], the surveillance strategies are markedly suboptimal. A study showed that NAFLD patients were less likely to receive HCC screening than chronic hepatitis C patients (HR 0.44, 95%CI 0.19–0.99, p<0.05) [72]. Thus, there is a need to improve the coverage of this intervention in high-risk NAFLD patients.
NAFLD has been related to an increased risk of HCC in the presence of cirrhosis. Although the annual incidence of HCC in NAFLD-related cirrhosis is lower than in that related to HCV, it is significantly higher than in controls. A study that followed 195 patients with NAFLD related cirrhosis during a median of 3.2 years found an annual incidence of HCC of 2.6% [58]. However, HCC has also been reported in NAFLD patients with liver fibrosis and even in the absence of fibrosis [73]. Thus, the need to delimit a population where HCC screening is cost-effective is warranted.
Based on cost-effectiveness studies it has been suggested that in a population with an annual incidence of ≥1.5% HCC screening is recommended assuming the availability of therapies and acceptable performance status and liver function. Therefore, HCC screening in NAFLD-related cirrhosis is mandatory. However, screening should not be performed if the treatment of HCC is not feasible or if it will not improve survival. Another study showed that, although higher than in controls, the risk of HCC in non-cirrhotic NAFLD patients is markedly lower than in cirrhotic patients (0.08/1000 person-years vs. 10.6/1000 person-years). Thus, the risk in non-cirrhotic NAFLD patients doesn't reach the minimum threshold to perform cost-effective HCC surveillance [74]. Notably, the authors found that the HCC risk in those patients without cirrhosis but with a high FIB-4 score result was 0.39/1000 person-years compared to 0.04/1000 person-years on those without cirrhosis and a persistently low FIB-4 result.
Importantly, obesity, tobacco, and alcohol consumption have all been associated with an increased risk of HCC in NAFLD patients. Thus, the recommendation of weight loss, increase physical activity, and to discontinue alcohol and tobacco consumption should be reinforced [73].
A proper strategy for effective HCC screening needs to be followed. An ultrasonography (US) performed every six months is the preferred method considering that it was employed in most studies and has an acceptable diagnostic yield [75]. Notably, increasing its frequency doesn’t improve the detection rate [76]. The use of alpha-fetoprotein in addition to the US does not add a clear benefit, thus its use is not mandatory [77].
A few observational studies have reported that the likelihood of inadequate ultrasound quality is significantly higher in overweight or obese patients. Thus, to employ an alternative radiologic method such as CT or MRI is recommendable if the liver cannot be properly evaluated using US [73]. Nonetheless, routinely use of CT or MRI as a screening method seems non-cost-effective [78].
11.1Recommendations- -
Universal HCC screening is not recommended in NAFLD patients. HCC must be performed in all patients with cirrhosis and should be considered in patients with advanced fibrosis due to NAFLD if curative or palliative therapies are available and without reduced short-term survival.
- -
HCC screening must be performed employing ultrasonography every six months with or without a simultaneous measurement of alpha-fetoprotein levels. Only if a proper liver visualization is not achieved employing ultrasonography, a different radiologic test can be employed (CT scan or MRI).
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement.
Management:
12Is diet effective for NAFLD treatment?Diet, as part of lifestyle interventions, is key in NAFLD treatment, although the effect of these interventions is often difficult to assess due to the lack of standardization of the study designs. It has been established that a decreased caloric intake and reductions of at least 5% of bodyweight achieve a significant reduction in intrahepatic lipid content; furthermore, a decrease of 7−10% of body weight or greater is ideal, because it has been associated with improvement in all the histological parameters (steatosis, inflammation, and fibrosis) in a higher percentage of subjects [62,79–81].
Experts recommendations suggest a caloric reduction between 500−1000kcal/day, or total intake between 1200−1800kcal/day, low in fat and carbohydrates and rich in fiber, avoiding fructose, especially if it derives from sugary drinks [62,79], which are highly consumed in Latin-America. Carbohydrates, especially fructose and/or sucrose added to beverages, foods, and desserts, have been shown to be related to the development of NAFLD, increasing liver triglyceride synthesis.
Studies have shown that the Mediterranean diet is beneficial independently of achieving weight loss. The Mediterranean diet is characterized by reduced carbohydrate intake, especially sugars and refined carbohydrates (40% of the calories vs. 50–60% in a typical low-fat diet), and increased monounsaturated and omega-3 fatty acid intake (40% of the calories as fat vs. up-to 30% in a typical low-fat diet), and has been shown to improve insulin resistance (IR) and steatosis assessed by MRI spectroscopy or elastography [81–84]. Polyunsaturated fatty acids, particularly omega-3 types, have been shown to improve insulin sensitivity and reduce intrahepatic triglyceride content, thus improving steatohepatitis [83].
Finally, regular coffee consumption, mainly due to its antioxidant effect, would have a beneficial role in both the general population and in patients with NASH, improving liver fibrosis. These effects on fibrosis are best seen in patients with low levels of insulin resistance [85,86]. Two or three cups of coffee per day are recommended.
12.1Recommendations- -
A reduction in calorie intake to 1200−1800kcal/day and the achievement of a weight reduction of 7−10% is beneficial in NAFLD management.
- -
A low-refined sugar diet based on the Mediterranean diet is beneficial in the management of NAFLD.
- -
The consumption of coffee in moderate amounts is recommended in patients with NAFLD.
Delphi consensus: Achieved on the first round of revision – 4 out of 5 experts expressed complete agreement.
13Is exercise effective for NAFLD treatment?Exercise has shown to be an effective intervention to reduce intrahepatic triglyceride content, independent of weight loss in patients with NAFLD [80,87]. A meta-analysis that included 28 randomized controlled trials, showed that exercise, independent of changes in diet, is associated with a significant reduction in intrahepatic triglyceride (IHTG) content (p<0.0001), and with a reduction in ALT (p<0.004) and AST (p=0.01) values [88]. Regular exercise not only modifies the IHTG but improves cardiovascular comorbidities and insulin-resistance specifically associated with NAFLD [89]. The data is less clear regarding the type, frequency, and intensity of exercise. There is consensus among experts that exercise should be prescribed in 3–5 sessions per week (150−200min/week) of moderate-intensity aerobic exercise associating sessions of resistance exercise [62]. Both aerobic and resistance exercise are effective in reducing hepatic steatosis, in sessions of at least three times a week for twelve weeks, although in resistance exercise, energy consumption and exercise intensity were lower (MET 3.5 vs. 4.8 in aerobic exercise) [87]. A resistance exercise program lasting 24 weeks showed similar benefits to those experienced by aerobic exercise in terms of IHTG, ALT levels, and insulin sensitivity [90].
A study compared the effect of 12 weeks of an exercise program on obese men with NAFLD, randomized into 3 groups (resistance exercise (n=20) vs. high-intensity interval aerobic exercise (n=21) vs. moderate-intensity aerobic exercise (n=20)). Besides clinical and biochemical parameters, they used MRI to assess abdominal fat distribution, in addition to controlled-attenuation parameter and spectroscopy to assess intrahepatic lipid content. The authors concluded that intrahepatic lipid content was similar in all three groups (−14.3% vs. −13.7% vs. −14.3%), with no specific changes in weight or visceral fat. Hepatic stiffness significantly improved only in the group randomized to high-intensity interval exercise (−16.8%) [91]. Recently, an 8-week high-intensity interval aerobic exercise showed a beneficial effect on IHTG, visceral lipids, and quality of life in diabetic obese patients with NAFLD [92].
13.1Recommendations- -
Regular exercise (aerobic, resistance, or combined) should be part of the first line of non-pharmacological treatment of NAFLD.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement.
14Are antioxidants, insulin-sensitizers, and lipid-lowering drugs effective for the treatment of NAFLD?Despite the imperative need for effective pharmacotherapy for NASH patients, there is no standard-of-care treatment currently available. Several treatment strategies have been evaluated, with different study populations and efficacy endpoints, and thus, with heterogeneous results.
14.1AntioxidantsVitamin E is a potent antioxidant that has been extensively studied for NAFLD. However, there are only a few randomized placebo-controlled trials addressing vitamin E alone for the treatment of NASH patients considering histological endpoints. The PIVENS trial assessed its efficacy in adult non-cirrhotic non-diabetic patients with NASH: vitamin E met the primary endpoint, defined as a two-point reduction in the NAFLD Activity Score, with improvement in ballooning and inflammation or steatosis, without worsening of fibrosis. However, it failed to improve fibrosis [93]. In NASH patients with type 2 diabetes, it failed to achieve a two-point reduction in the nonalcoholic fatty liver disease activity score without worsening of fibrosis, being only efficacious in reducing steatosis [94]. Finally, in the TONIC trial performed in non-diabetic non-cirrhotic children and adolescents, vitamin E was significantly associated with the resolution of NASH mainly due to improvement in hepatocellular ballooning but had no significant effects on steatosis, inflammation, or fibrosis [95]. There have been concerns regarding its long-term safety since few meta-analyses have found an association of long-term vitamin E treatment and higher all-cause mortality, risk of hemorrhagic stroke [96], and prostate cancer in males older than 50 [97]. These findings need to be confirmed in well-designed randomized controlled trials.
14.2Insulin sensitizersMetformin is a first-line agent for the treatment of type 2 diabetes mellitus, it inhibits hepatic gluconeogenesis, increases glucose uptake in skeletal muscle, and improves peripheral insulin sensitivity. In a recent meta-analysis addressing NAFLD treatment, metformin was found to be associated with a significant reduction in body mass index, serum aminotransferases levels, cholesterol, and fasting glucose. However, histological parameters such as ballooning, fibrosis, steatosis, or NAFLD histological score were not modified, and inflammation worsened [98].
Thiazolidinediones are ligands for the nuclear transcription factor PPAR-γ implicated in the regulation of glucose and lipid metabolism. Pioglitazone is a thiazolidinedione that has been extensively studied as a treatment option for NAFLD. In a 2011 meta-analysis that included 7 randomized trials involving thiazolidinediones in the treatment of diabetic and non-diabetic patients with NASH, pioglitazone showed a significant reduction in hepatic steatosis, lobular inflammation, and hepatocellular ballooning, with modest effects on fibrosis in diabetic and non-diabetic patients [99]. Regarding its safety, weight gain (mean, 4.4kg) is the main concern. Bone loss has been reported in women, and there are controversial findings regarding its role in risk for bladder cancer [100].
14.3Lipid-lowering agentsStatins are lipid-lowering agents that reduce cholesterol biosynthesis by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A(HMG-CoA) reductase. Even though these drugs are effective in reducing cardiovascular risk and mortality in patients with NAFLD, they are often under-prescribed due to concerns regarding their safety. However, data suggests statins are safe and well-tolerated in NAFLD and NASH patients with type 2 diabetes mellitus [101], including those with slightly elevated transaminases [102], and may even be beneficial in patients with chronic liver diseases [103]. Regarding its efficacy for NAFLD/NASH treatment, there are insufficient studies with histological endpoints to recommend their use [104].
Ezetimibe, an inhibitor of cholesterol absorption, has been related to the improvement of liver enzyme levels, but only reduced hepatocyte ballooning in randomized-controlled trials [105]. Finally, the supplementation with omega 3 polyunsaturated fatty acid may be related to beneficial changes in liver fat and transaminase levels, but further data with randomized control design and histological endpoints are needed [104].
14.4Recommendations- -
Vitamin E (in non-cirrhotic, non-diabetic patients) and pioglitazone (in non-cirrhotic patients with or without diabetes) have shown to be effective for the treatment of patients with histological diagnosis of NASH and may be used after discussing their risks and benefits. However, larger randomized controlled trials are needed before they can be firmly recommended.
- -
No evidence supports the efficacy of metformin or lipid-lowering agents for NAFLD/NASH treatment. However, they should be used to treat comorbidities.
Delphi consensus: Achieved on the first round of revision – 4 out of 5 experts expressed complete agreement or agreement with minor comments.
15Which are the drugs under development for the treatment of NAFLD?At the present time, more than 70 active studies are testing a myriad of drugs for the treatment of NAFLD (www.clinicaltrials.gov). However, to date, none of these drugs has been approved by the main regulatory agencies [106]. Although ongoing phase 3 clinical trials are expected to provide useful information to advance the field of NASH treatment in the near future, the majority of completed studies so far have yielded negative results [107]. The field is evolving rapidly and effective drug treatment for NASH is in sight but data is still lacking. Thus, to answer the question posed in this section we will focus on drugs currently in advanced stages of clinical testing (Table 2).
List of drugs for the treatment of NAFLD/NASH currently being evaluated in phase 3 clinical trials (as available by July 30th, 2020).
| Drug | Mechanism of action | ClinicalTrials.gov identifier | Interim results |
|---|---|---|---|
| Obeticholic acid | FXR agonist | NCT02548351 | Available, positive |
| Elafibranor | PPAR-α/δ agonist | NCT02704403 | Available, negative |
| Cenicriviroc | CCR2/CCR5 | NCT03028740 | Not available |
| Resmetirom | THR-ß agonist | NCT03900429 | Not available |
| Aramchol | SCD1 inhibitor | NCT04104321 | Not available |
Abbreviations: FXR, farnesoid X receptor; PPAR, peroxisome proliferator-activated receptor; CCR, C-C chemokine receptor; THR-ß, thyroid hormone receptor ß; SCD-1, Stearoyl-CoA desaturase 1.
Obeticholic acid (OA) is the first-in-class agonist of the farnesoid X receptor (FXR), which is an intracellular bile acid receptor involved in the regulation of lipogenesis and bile acid homeostasis. Experimental evidence shows that FXR agonism influences several pathways resulting in beneficial effects in NASH including a reduction in hepatic lipogenesis and amelioration of liver inflammation and fibrosis. A phase 2b trial (FLINT, NCT01265498) showed that OA improved the histological features of NASH with a statistically significant improvement in fibrosis. More recent data, from an interim analysis of an international phase 3 trial (REGENERATE, NCT0254835) that evaluated two doses of OA 10−25mg/day in adult patients with definite NASH and fibrosis stages F2–F3, or F1 with at least one accompanying comorbidity showed that while the co-primary endpoint of NASH resolution without worsening of fibrosis was not reached, all variables of the histologic NAFLD activity score improved [108]. The endpoint for the improvement in fibrosis was achieved by 37 (12%) patients in the placebo group, 55 (18%) in the OA 10-mg group (p=0.045), and 71 (23%) in the OA 25-mg group (p=0.0002). Thus, OA is a potentially effective drug for NASH treatment pending the long-term follow-up phase of this study. Other FXR agonists (i.e. tropifexor) have also been tested in phase 2 trials with promising results [109] and are entering phase 3 testing.
15.1.2ElafibranorElafibranor is a PPAR-α/δ agonist being tested in the RESOLVE-IT phase 3 clinical trial. Results of an interim analysis of data from 1070 patients with NASH and F2 and F3 fibrosis stage were recently released and showed that the predefined primary endpoint of NASH resolution without worsening of fibrosis was not met [110]. The response rate in those patients receiving the study drug was 19.2% compared to 14.7% for patients in the placebo arm. The secondary endpoint of at least one stage of liver fibrosis improvement was similar in patients receiving elafibranor and placebo (24.5% vs. 22.4%).
15.1.3Cenicriviroc (CVC)CVC is a CCR2/CCR5 receptor inhibitor that after a positive signal in a phase 2b study showing improvement in fibrosis and no worsening of NASH compared with placebo [111] is being tested in a large multicenter phase 3 study (AURORA trial) [112]. The final results of the study are still pending at the time of the writing of this document.
15.1.4ResmetiromResmetirom is an orally active, selective thyroid hormone receptor-β agonist (thyromimetic) that has shown beneficial effects in NASH in both preclinical and clinical studies. Of note, a phase 2b trial [113] showed that in addition to decreasing liver fat, the drug was associated with NASH histological resolution. Currently, resmetirom is being tested in a Phase 3 study (MAESTRO-NASH trial), which is expected to enroll 2000 patients with biopsy-proven NASH.
15.1.5AramcholAramchol is a fatty acid bile acid conjugate (arachidyl amido cholanoic acid) able to inhibit the stearoyl-CoA desaturase 1 (SCD-1) enzyme, which is a key enzyme in fatty acid metabolism. SCD-1 inhibition determines a reduction in hepatic lipogenesis and an increase in mitochondrial β‐oxidation of fatty acids, thus decreasing in liver fat content. Preliminary results of a phase 2b trial showed positive effects with NASH resolution without fibrosis worsening occurring more often (16.7%) in patients receiving the active drug compared to those receiving placebo (5.0%). Also, a ≥1 stage fibrosis reduction without NASH worsening was seen in 29.5% of aramchol treated patients compared with 17.5% of patients receiving placebo [114], a phase 3 trial is underway.
15.1.6Combination therapiesThe use of drug combinations attempting to tailor NAFLD/NASH treatment according to the predominant pathway involved, disease stage, and associated conditions (e.g. presence of type 2 diabetes) is being explored in several phase 2 trials. This approach is promising but more data is needed [115,116].
16Is bariatric (metabolic) surgery effective for the treatment of NAFLD?Bariatric surgery (BS) is an effective treatment for obesity. Regarding NAFLD/NASH, there is clinical and experimental evidence that BS is cost-effective and has beneficial effects, inducing histological resolution of NASH through weight loss-dependent and independent mechanisms, involving hormonal and bile acid metabolism changes [117]. Indeed, steatosis, inflammation, and fibrosis appear to improve or completely resolve in the majority of patients after BS–induced weight loss, as it was demonstrated in 2008 by a meta-analysis comprising 15 studies, including 4 Latin-American cohorts [118]. However, most data evaluating NAFLD and BS are derived from retrospective studies. A recent French study showed that BS provides a long-term resolution of NASH and regression of fibrosis. They observed the resolution of NASH in paired liver biopsies from 84% of patients 5 years after the procedure. Additionally, they concluded that the reduction of fibrosis is progressive, beginning during the first year and continuing through 5 years. Even before this study, some experts considered that BS should be offered as a treatment for NASH without the need for further clinical trials [117].
Some comprehensive or systematic reviews and meta-analysis have been published in the last few years [104,119–121]. The most recent one analyzed data from 32 studies comprising 3093 biopsy specimens, with a biopsy-confirmed resolution of steatosis in 66% of patients, inflammation in 50%, ballooning degeneration in 76%, and fibrosis in 40%. Mean NAFLD activity score (NAS) also presented a significant reduction (mean of −2.39 points). Moreover, the authors emphasized that the overall grading of recommendations and evidence quality was very low and concluded that randomized studies are still needed to better evaluate the effect of BS in this population [121], especially to consider NASH as a sole indication for metabolic surgery regardless of BMI.
Other issues deserve further discussion, such as concerns about BS in patients with advanced liver disease due to NAFLD, as well as the choice of the type of procedure. Currently, patients with compensated NASH cirrhosis (Child–Pugh A) may represent candidates for BS, especially those with MELD less than 10 and without significant portal hypertension. However, no clear recommendations can be made, due to the lack of solid data [117]. There is debate whether these patients should undergo Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG). Liver decompensation is more common after primarily malabsorptive procedures, such as jejunoileal bypass, that is no longer performed [117]. In a recent meta-analysis that compared RYGB and SG on NAFLD patients considering AST, ALT, NAS, and NAFLD fibrosis score as outcomes. The authors demonstrated that BS significantly improved these biochemical and histologic parameters but failed to indicate superiority between RYGB and SG in ameliorating NAFLD [120].
16.1Recommendations- -
Bariatric (metabolic) surgery should be considered in obese patients with NAFLD unresponsive to clinical and pharmacological management, as it is related to improvement in histological outcomes, including fibrosis. However, the recommendation should be weighted with the risk of complications.
- -
Additional randomized studies addressing BS in NAFLD are needed, as well as to define the best surgical technique in this population.
Delphi consensus: Achieved on the first round of revision – 4 out of 5 experts expressed complete agreement or agreement with minor comments.
17How should we manage comorbidities in NAFLD (hypertension, cardiovascular disease)?During the last decade, it has been observed that NAFLD leads to an increased cardiovascular risk with an acceleration of arteriosclerosis and events related to it, being the main cause of its morbidity and mortality [122]. The higher incidence of cardiovascular events in the NAFLD population could be explained by several mechanisms. Patients with NAFLD typically meet the diagnostic criteria for metabolic syndrome and therefore have multiple risk factors for cardiovascular disease [123]. Likewise, the presence of systemic inflammation in combination with metabolic abnormalities may act synergistically to increase cardiovascular risk in these patients. Pre-hypertension and hypertension are both significant risk factors for the development of NAFLD independent of other risk factors. Controlled blood pressure appears to be independently protective of NAFLD and the absence of hypertension also protects against moderate-to-severe hepatic fibrosis risk [124].
The estimation of cardiovascular risk is recommended and various easy-to-use tools are available for this purpose, such as the Framingham Score or the Atherosclerotic Cardiovascular Disease Risk Estimator of the American College of Cardiology [57,63,125], however, traditional scores have limitations. On the one hand, these predictive tools were not specifically developed for NAFLD patients. The performance of these scores is suboptimal because traditional cardiovascular risk factors do not fully explain the increased cardiovascular risk in patients with NAFLD, and current risk functions do not represent other contributing factors (i.e. inflammation). Thus, a multidisciplinary and step-wise approach is recommended. In an attempt to address these limitations, it has been proposed to add another predictive factor, such as the detection of subclinical atheromatosis. Indicators of early vascular atherosclerosis such as carotid atherosclerotic plaques diagnosed by ultrasound or coronary calcium score quantified by computed tomography are frequently altered in patients with NAFLD. Two meta-analyses showed that the patients with NAFLD had higher coronary calcium score and carotid plaque prevalence compared with controls, independent of traditional risk factors [126,127].
A considerable amount of literature has shown the association between NAFLD and cardiovascular disease. A recent meta-analysis showed an elevated risk of cardiovascular events in NAFLD patients compared to controls (RR 1.77; 95%CI 1.26–2.48, p<0.001) [128]. Statin utilization is the cornerstone of cardiovascular risk management. To determine whether a patient is a candidate for statin therapy, clinicians must first determine the patient’s risk of having a future cardiovascular event. The statin use has historically been hampered, in individuals with liver disease, owing to the fear of hepatotoxicity. However, studies suggest that statins are not only effective in reducing cardiovascular events but may also exert multiple beneficial effects on the liver [129].
17.1Recommendations- -
Risk scores should be used for the initial stratification of cardiovascular risk.
- -
NAFLD patients should get regular blood pressure measurement and lipids checks during clinical visits, with specific thresholds for intervention according to current guidelines.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement.
18How should we manage comorbidities in NAFLD (obesity, T2DM)?NAFLD is considered the hepatic manifestation of metabolic syndrome. Therapeutic principles for the management of patients with NAFLD, obesity, and T2DM are mainly based on the implementation of lifestyle optimization strategies aiming to achieve significant weight loss, which ideally should be planned in the setting of multidisciplinary teams. Currently, there is no particular drug approved for the treatment of NAFLD/NASH in patients with T2DM. Although, several approved diabetic medications hold promise for NAFLD/NASH treatment and several NASH-specific drugs are in evaluation in phase III RCTs.
The PIVENS trial (comparing vitamin E, pioglitazone, and placebo) did not include diabetic subjects. Later publications suggested benefit of vitamin E in an observational study [130] but limited benefit in a RCT in patients with diabetes [94]. Pioglitazone efficacy in NAFLD therapy has been evaluated in five randomized controlled trials (498 patients) with consistent results in resolution of NASH and improvement in ballooning or inflammation, while the improvement of fibrosis was reported in some of the studies [15]. Most of them included diabetic subjects, showing improvement in steatosis and fibrosis [131]. However, some safety issues have been raised with vitamin E and pioglitazone therapies.
From the standpoint of diabetes management, appropriate glycemic control is associated with a reduction in steatosis, a decrease in serum levels of aminotransferases, and eventually improvement of fibrosis. Interestingly, there are medications for the treatment of diabetes with potential efficacy for NAFLD/NASH. Metformin, although it hasn't been shown to be associated with a significant hepatic histological improvement, has shown to reduce the incidence of HCC of the diabetic population in basic studies [132]. Other anti-diabetic drugs have been associated with potentially beneficial effects in NAFLD. Among the most promising therapies, Liraglutide, a GLP-1 agonist, is of special interest. Liraglutide has demonstrated to induce a significant improvement in metabolic control and a reduction in cardiovascular events and deaths in diabetic subjects (HR 0.78 95%CI 0.66−0.93; N=9340, follow-up 5 years) [133] and diabetes prevention in subjects with prediabetes (HR: 0.21 95%CI 0.13−0.34, N=2254) [134]. Regarding NAFLD, a small (N=52) phase 2 trial in non-diabetic subjects [135] demonstrated a significant increase in NASH resolution using Liraglutide 1.8mg/d (39% vs. 9%, P=0.019). Exenatide, another GLP-1 analog, has demonstrated reducing weight and liver enzymes [136] (ongoing trial in NAFLD -NCT01208649-). Semaglutide (GLP-1 agonist administered once weekly) is currently being assessed in a phase III trial in NASH. Data on the effects of dipeptidyl peptidase 4 inhibitors (DPP4) in NAFLD shown conflicting results [137], thus further studies are needed. Sodium-glucose cotransporter2 (SGLT2) inhibitors have shown to have reno- and cardio-protective effects and are recommended as an add-on treatment when glycemic control is not achieved in diabetics. Empagliflozin has been shown to reduce cardiovascular deaths (HR 0.62 95%CI 0.49−0.77), and hospitalizations for heart failure (HR 0.65 95% CI 0.5−0.85) [138,139]. The E-LIFT study addressed the effect of the empagliflozin in T2DM patients with NAFLD showing that it was associated with a reduction in steatosis (assessed by MRI-PDFF) and in serum levels of aminotransferases [140]. Similar results were found with dapagliflozin [141] and other SGLT2 [142,143]. Regarding histological outcomes, only one very small cohort study (N=5) have reported improvement in steatosis and NAS score with canagliflozin [144]. Thus, although some promising data has been published, the effectiveness of this class of drugs for NASH treatment remains to be determined in future RCTs.
Appropriate treatment of dyslipidemia in the T2DM population is also very relevant and statins are effective and safe in subjects with NAFLD [145]. Finally, a combination approach with multiple drug targeting different pathways will likely represent the future of NASH therapeutics but more robust evidence for this approach is needed.
18.1Recommendations- -
Therapeutic principles for the management of patients with NAFLD and T2DM are mainly based on the implementation of lifestyle optimization strategies aiming to achieve significant weight loss, which ideally should be planned in the setting of multidisciplinary teams.
- -
Metformin should be considered to be a first-line option for the treatment of T2DM but with minimal benefit for treating NASH.
- -
Glucagon-like peptide-1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors are promising agents for the treatment of T2DM in NAFLD.
Delphi consensus: Achieved on the first round of revision – 4 out of 5 experts expressed complete agreement or agreement with minor comments.
19When is liver transplantation indicated in NAFLD?NASH-related cirrhosis is a growing indication for liver transplantation (LT) [146,147]. In western countries, the percentage of LT for hepatitis C has declined in the last decades, and NASH has become one of the most common indications. In a recent multicenter Latin America study, NASH-cirrhosis was the third etiology of underlying disease in HCC candidates for LT [148].
19.1Indication for LT in NAFLD patientsIndications for LT should be considered once a patient with NASH-cirrhosis has developed a complication such as ascites, hepatic encephalopathy, variceal hemorrhage, or hepatocellular dysfunction that results in a MELD-Na score ≥15 or Child–Pugh ≥7 points [146,147]. LT is recommended as the first-line option for NASH-cirrhosis and HCC within Milan criteria when the tumor is unsuitable for resection, in the absence of tumor vascular invasion and extrahepatic metastases. Patients beyond the Milan criteria can be considered for LT after successful downstaging with locoregional therapy [148].
19.2Comorbidities of NAFLD patients in the LT settingPatients with NASH-cirrhosis on the waiting list for LT have a high prevalence of metabolic comorbidities: obesity (53.4–68%), diabetes (49–72.9%), and arterial hypertension (37.5–75%) [149]. In a recent meta-analysis, the presence of NAFLD was associated with a 65% increased risk of developing fatal and nonfatal cardiovascular events over a median follow-up of almost 7 years. Based on this strong link, most societies suggest that NAFLD should be considered an independent risk factor for cardiovascular events. Unfortunately, defining the best approach to assess cardiovascular risk in LT candidates is a focus of intense and moving debate [56]. Obesity alone (BMI≥30kg/m2) does not constitute a contraindication for LT. However, in the presence of medical comorbidities, particularly concurrent diabetes, rigorous patient selection is recommended. The presence of obesity represents technical challenges and is associated with post-transplant complications, but the impact of obesity on long-term mortality after LT is debated [147]. Despite the presence of obesity, sarcopenia is the most common finding in NASH cirrhosis and is associated with higher mortality while waiting for LT as well as increased hospitalization rates in the post-transplant setting [150]. The prevalence of cardiovascular diseases in patients transplanted by NASH is high: coronary artery disease, 20%; congestive heart failure, 7%; stroke, 8% [151]. Cardiovascular mortality is responsible for a higher proportion of deaths among NASH patients compared to non-NASH transplant recipients. Despite this, NASH patients have shown to have similar overall post-transplant survival rates compared to patients without NASH. These results could be explained in part by the lower risk of graft failure compared with other indications, particularly hepatitis C in the pre-direct acting antiviral era [149]. NASH is an independent risk factor for LT renal dysfunction before and after LT, thus appropriate screening and management of kidney disease is recommended. In addition, NASH cirrhosis is the most rapidly growing indication for simultaneous liver-kidney transplantation in the United States and Europe [152].
19.3Recurrent and de novo NAFLD/NASH after LTRecurrent and de novo NAFLD occurs in over one-half of recipients as soon as 1year after LT. NASH recurs in most patients after LT (53% at 1year), whereas de novo NASH is less frequent (13% at 1year). NAFLD/NASH after LT is associated with metabolic risk factors; in a recent meta-analysis, post-LT body mass index and hyperlipidemia were the most consistent independent predictors of outcomes. The impact of recurrent or de novo NAFLD/NASH after LT on allograft and patient survival is unclear. In patients transplanted for NASH cirrhosis, immunosuppressive protocols with short-term and low dose steroids and calcineurin inhibitor minimization should be preferred [153].
19.4Recommendations- -
Indication for liver transplantation should be considered once a patient with NASH-related cirrhosis has developed an index complication, hepatocellular dysfunction (MELD-Na Score ≥15 or Child–Pugh ≥7), and/or hepatocellular carcinoma within Milan criteria unsuitable for resection in the absence of tumor vascular invasion and extrahepatic metastases.
- -
Liver transplant candidates with NASH-related cirrhosis should be considered at higher risk of developing cardiovascular events before and after transplantation. There is not enough evidence to support a different approach to the pre-liver transplant cardiovascular assessment.
- -
Liver transplantation in patients with NASH-related cirrhosis (w/o HCC) has a similar post-transplant survival as other liver transplant etiologies. NAFLD/NASH usually recurs after transplant but the impact of the recurrence on allograft and patient survival is unclear.
Delphi consensus: Achieved on the first round of revision – all experts expressed complete agreement or agreement with minor comments.
SummaryA summary of the recommendations is presented in Table 3.
Summary of recommendations.
| Definition and epidemiology |
| The prevalence of NAFLD ranges from 14.3% to 35.2% in Latin American population-based studies, but most of them were performed more than 10 years ago. |
| The variances in the prevalence of NAFLD/NASH may be related to differences in both genetic and environmental risk factors. As the frequency of obesity and metabolic syndrome continues to increase, further studies on NAFLD/NASH prevalence should be performed in the general population of Latin America. |
| Pathogenesis, genetics, and genetic testing |
| Carriers of the PNPLA3 I148M and the TM6SF2 E167K variants have a higher liver fat content and increased risk of NASH. |
| Genotyping might be considered in the Latin American population, however, it still has not proven to be able to guide therapy on an individual basis and is costly. Thus, its role in clinical practice is yet to be established. |
| How should we diagnose NAFLD? (screening, clinic, laboratory, and images) |
| NAFLD screening is not recommended in the general population. NAFLD screening is recommended for patients with repeatedly altered liver enzymes, features of metabolic syndrome, or obesity (BMI>30). In patients with a high-risk profile (type 2 diabetes or metabolic syndrome and >50 years old) NASH and liver fibrosis diagnosis should be undertaken. |
| The recommended method for initial screening for NAFLD in clinical practice is an abdominal ultrasound. |
| How should we use non-invasive assessment and images in NAFLD? (risk stratification) |
| NAFLD Fibrosis Score and FIB-4 are useful tools for the initial assessment of fibrosis in NAFLD patients |
| When NFS score and/or FIB-4 score are unable to exclude advanced fibrosis, an elastography based method is the suggested method in the risk stratification algorithm. Vibration controlled transient elastography is the best validated and available tool; other elastography techniques should be considered upon availability. |
| When non-invasive methods are unable to exclude significant fibrosis, liver biopsy should be considered. |
| When should we perform a liver biopsy in NAFLD? |
| Liver biopsy should be considered in patients with NAFLD who are at increased risk of having NASH and fibrosis when noninvasive tests are unable to exclude advanced fibrosis. |
| Liver biopsy should be considered in the differential diagnosis of patients with suspected NAFLD/NASH and other competing etiologies. It should also be considered in patients with known or suspected coexisting chronic liver diseases that cannot be assessed without this procedure. |
| The pathology report should describe the presence of steatosis, inflammation, and/or the presence of NASH. The stage of severity should be described (ideally by proposed score systems). The amount of fibrosis should be mentioned. |
| How should we follow subjects with NAFLD? Risk stratification periods |
| There is no consensus regarding the optimal interval for fibrosis reassessment. Periodic reassessment of liver fibrosis should be considered according to individual risk factors of progression. |
| Assessment of cardiovascular risk should be performed in every follow-up visit. |
| Referral algorithms: when to refer to the hepatologists? When to refer to other specialties? |
| FIB-4 and/or NAFLD fibrosis score (ideally both) should be calculated in all patients with NAFLD at their initial assessment. |
| Patients with indeterminate scores should be referred if they portray a high-risk profile (type 2 diabetes or metabolic syndrome and >50 years old). |
| Patients with high results in at least one of the two scores should be referred to a hepatologist for further evaluation. |
| Hepatologists should refer patients with significant or difficult-to-treat comorbidities to a specialized consultation. |
| Alcohol consumption and thresholds in NAFLD |
| Current data do not allow for recommendations of any amount of alcohol consumption to reduce cardiovascular or other health-related risks in NAFLD patients. |
| The safest strategy in NAFLD patients is to restrain from any alcohol consumption. However, if patients choose to consume alcohol it should be recommended below the threshold of 14 and 21 drinks per week for women and men, respectively. |
| Patients with NASH and/or moderate to advanced fibrosis should abstain from alcohol consumption, due to their high risk for disease progression. |
| Hepatocellular carcinoma screening |
| Universal HCC screening is not recommended in NAFLD patients. HCC must be performed in all patients with cirrhosis and should be considered in patients with advanced fibrosis due to NAFLD if curative or palliative therapies are available and without reduced short-term survival. |
| HCC screening must be performed employing ultrasonography every six months with or without a simultaneous measurement of alpha-fetoprotein levels. Only if a proper liver visualization is not achieved employing ultrasonography a different radiologic test can be employed (CT scan or MRI). |
| Is Diet effective for NAFLD treatment? |
| A reduction in calorie intake to 1200−1800kcal/day and the achievement of a weight reduction of 7−10% is beneficial in NAFLD management. |
| A low-refined sugar diet based on the Mediterranean diet is beneficial in the management of NAFLD. |
| The consumption of coffee in moderate amounts is recommended in patients with NAFLD. |
| Is exercise effective for NAFLD treatment? |
| Regular exercise (aerobic, resistance, or combined) should be part of the first line of non-pharmacological treatment of NAFLD. |
| Are antioxidants, insulin sensitizers, and lipid-lowering drugs effective for the treatment of NAFLD? |
| Vitamin E (in non-cirrhotic, non-diabetic patients) and pioglitazone (in non-cirrhotic patients with or without diabetes) have shown to be effective for the treatment of patients with histological diagnosis of NASH and may be used after discussing their risks and benefits. However, larger randomized controlled trials are needed before they can be firmly recommended. |
| No evidence supports the efficacy of metformin or lipid-lowering agents for NAFLD/NASH treatment. However, they should be used to treat comorbidities. |
| Is bariatric (metabolic) surgery effective for the treatment of NAFLD? |
| Bariatric (metabolic) surgery should be considered in obese patients with NAFLD unresponsive to clinical and pharmacological management, as it is related to improvement in histological outcomes, including fibrosis. However, the recommendation should be weighted with the risk of complications. |
| Additional randomized studies addressing BS in NAFLD are needed, as well as to define the best surgical technique in this population. |
| How should we manage comorbidities in NAFLD (hypertension, cardiovascular disease)? |
| Risk scores should be used for the initial stratification of cardiovascular risk. |
| NAFLD patients should get regular blood pressure measurement and lipids checks during clinical visits, with specific thresholds for intervention according to current guidelines. |
| How should we manage comorbidities in NAFLD (obesity, T2DM)? |
| Therapeutic principles for the management of patients with NAFLD and T2DM are mainly based on the implementation of lifestyle optimization strategies aiming to achieve significant weight loss, which ideally should be planned in the setting of multidisciplinary teams. |
| Metformin should be considered to be a first-line option for the treatment of T2DM but with minimal benefit for treating NASH. |
| Glucagon-like peptide-1 (GLP-1) agonists and Sodium-glucose cotransporter 2 (SGLT2) inhibitors are promising agents for the treatment of T2DM in NAFLD. |
| When is liver transplantation indicated in NAFLD? |
| Indication for liver transplantation should be considered once a patient with NASH-related cirrhosis has developed an index complication, hepatocellular dysfunction (MELD-Na Score≥15 or Child–Pugh ≥7), and/or hepatocellular carcinoma within Milan criteria unsuitable for resection in the absence of tumor vascular invasion and extrahepatic metastases. |
| Liver transplant candidates with NASH-related cirrhosis should be considered at higher risk of developing cardiovascular events before and after transplantation. There is not enough evidence to support a different approach to the pre-liver transplant cardiovascular assessment. |
| Liver transplantation in patients with NASH-related cirrhosis (w/o HCC) has a similar post-transplant survival as other liver transplant etiologies. NAFLD/NASH usually recurs after transplant but the impact of the recurrence on allograft and patient survival is unclear. |
The authors have no conflicts of interest to declare.